Complex Trophic Interactions in an Acidophilic Microbial Community
Abstract
1. Introduction
2. Materials and Methods
2.1. Organisms, Place of Origin and Culture Conditions
2.2. Experimental Set-Up
2.3. Statistical Analysis
3. Results
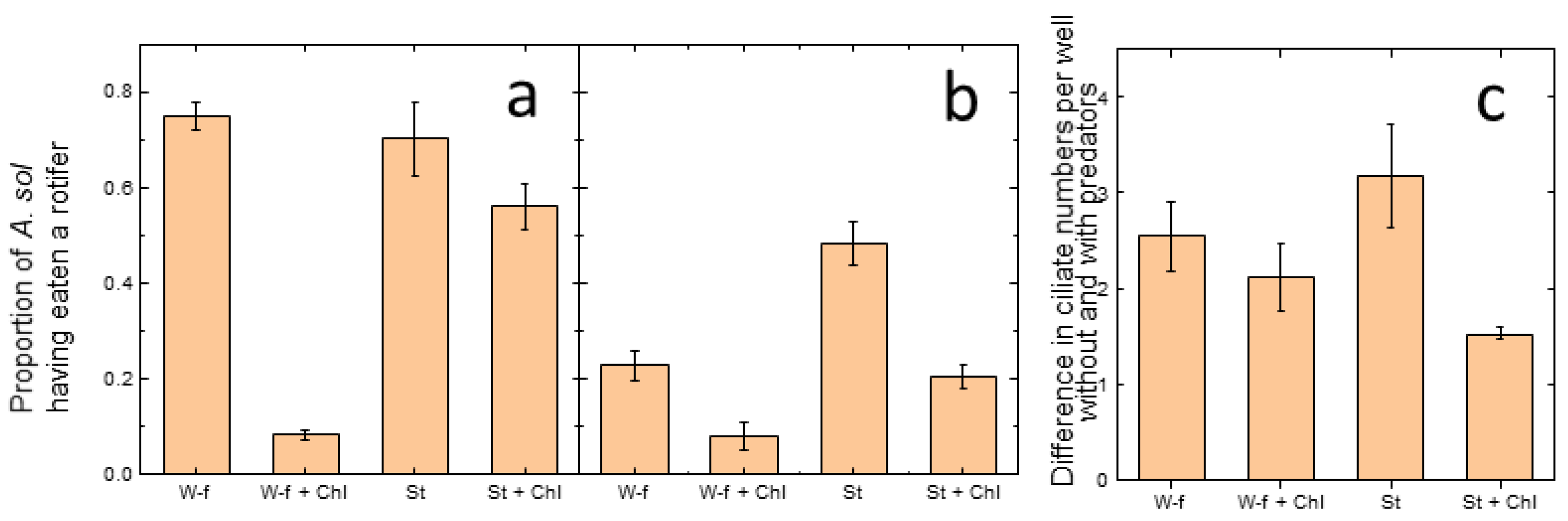
3.1. Micro-Scale Experiment
3.1.1. Rotifers
3.1.2. Ciliates
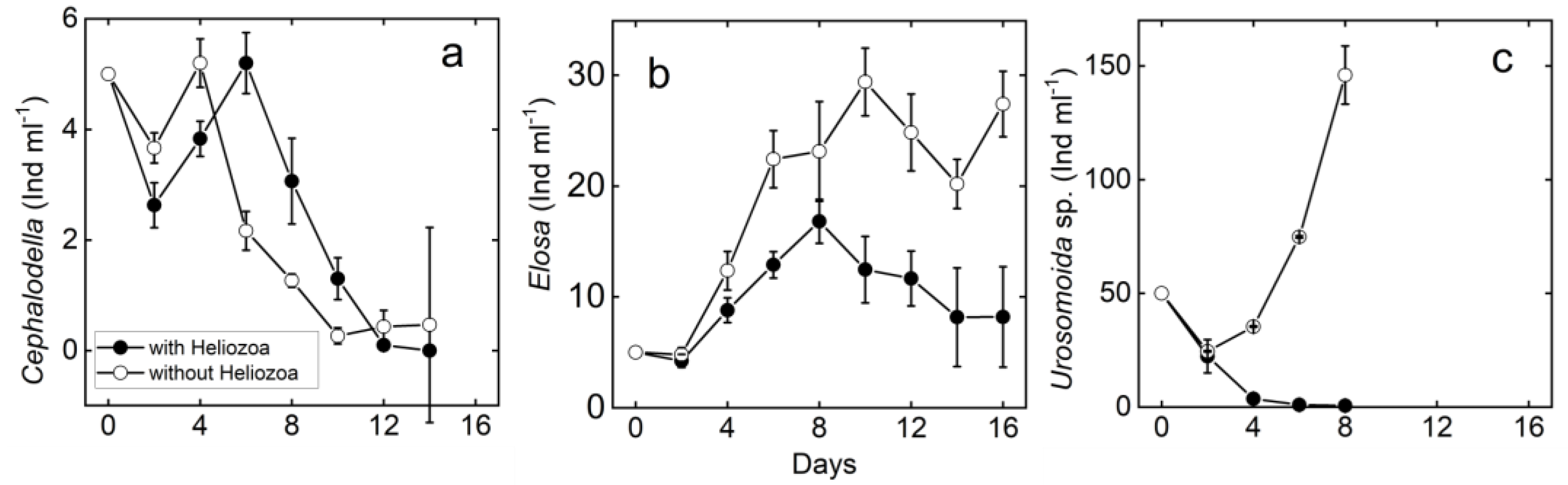
3.2. Meso-Scale Experiment
3.2.1. Rotifers
3.2.2. Ciliates
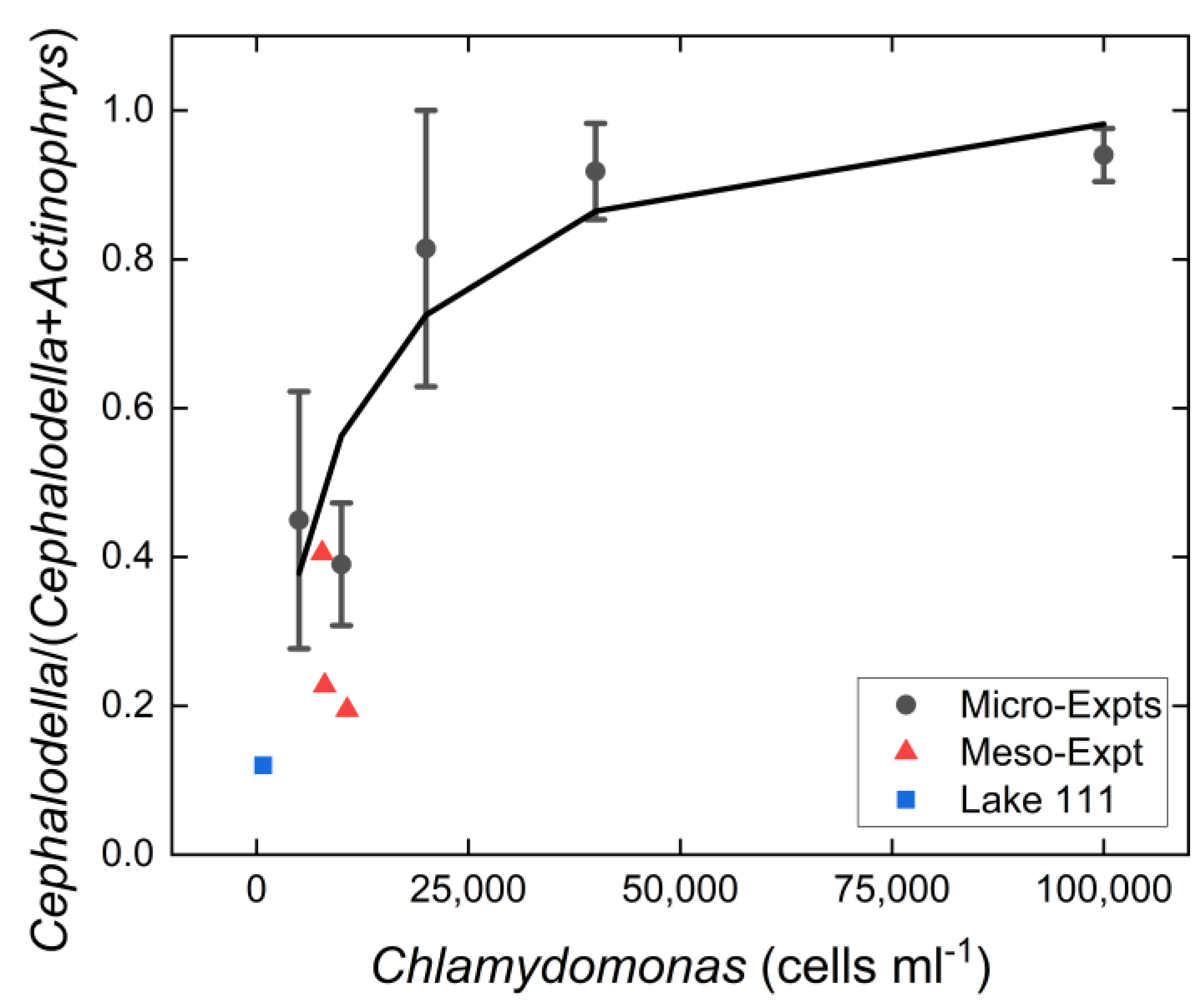
3.3. Resource Level Experiment
4. Discussion
4.1. Predation on Rotifers
4.2. Predation on Ciliates
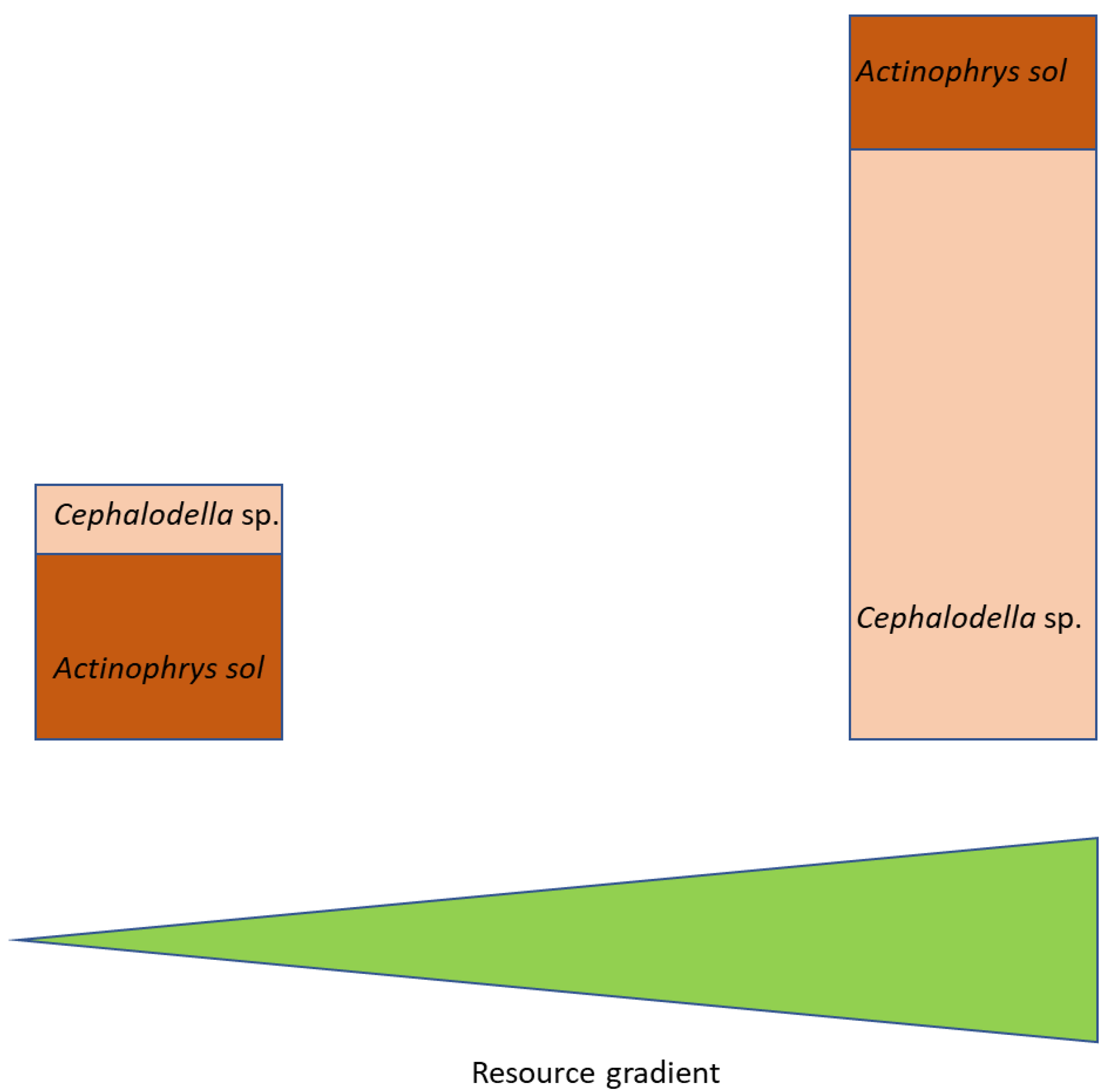
4.3. Food Web Implications
- (1)
- At low productivity, the intraguild predator can suppress the intraguild prey and simultaneously make use of the shared resource.
- (2)
- At high productivity, the growth of the intraguild predator is reduced due to clogging of the axiopods by too many small prey items. This overload releases the intraguild prey (here: Cephalodella sp.) from predation pressure, allowing it to grow to high abundances.
4.4. Comparison with Circum-Neutral Habitats
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bell, E.M. Life at Extremes–Environments, Organisms and Strategies for Survival; CABI: Wallingford, UK, 2012; 554p. [Google Scholar]
- Nixdorf, B.; Lessmann, D.; Deneke, R. Mining lakes in a disturbed landscape: Application of the EC Water Framework Directive and future management strategies. Ecol. Eng. 2005, 24, 67–73. [Google Scholar] [CrossRef]
- Pedrozo, F.; Kelly, L.; Diaz, M.; Temporetti, P.; Baffico, G.; Kringel, R.; Friese, K.; Mages, M.; Geller, W.; Woelfl, S. First results on the water chemistry, algae and trophic status of an Andean acidic lake system of volcanic origin in Patagonia (Lake Caviahue). Hydrobiologia 2001, 452, 129–137. [Google Scholar] [CrossRef]
- Bowers, K.J.; Wiegel, J. Temperature and pH optima of extremely halophilic archaea: A mini-review. Extremophiles 2011, 15, 119–128. [Google Scholar] [CrossRef]
- Amaral-Zettler, L.A.; Gómez, F.; Zettler, E.; Keenan, B.G.; Amils, R.; Sogin, M.L. Eukaryotic diversity in Spain’s River of Fire. Nature 2002, 417, 137. [Google Scholar] [CrossRef]
- Amaral-Zettler, L.A. Eukaryotic diversity at pH extremes. Front. Microbiol. 2013, 3, 441. [Google Scholar] [CrossRef]
- Packroff, G.; Woelfl, S. A review on the occurrence and taxonomy of heterotrophic protists in extreme acidic environments of pH values ≤ 3. Hydrobiolgia 2000, 433, 153–156. [Google Scholar] [CrossRef]
- Bedogni, G.L.; Massello, F.L.; Giaveno, A.; Donati, E.R.; Urbieta, M.S. A deeper look into the biodiversity of extremely acidic Copahue volcano-RioAgrio region system in Nequén, Argentina. Microorganisms 2019, 8, 58. [Google Scholar] [CrossRef]
- Mesa, V.; Gallego, J.L.R.; González-Gil, R.; Lauga, B.; Sánchez, J.; Méndez-Garcia, C.; Peláez, A.I. Bacterial, archaeal, and eukaryotic diversity across microhabitats in an acid mine drainage. Front. Microbiol. 2017, 8, 1756. [Google Scholar] [CrossRef]
- Kamjunke, N.; Gaedke, U.; Tittel, J.; Weithoff, G.; Bell, E.M. Strong vertical differences in the plankton composition of an extremely acidic lake. Arch. Hydrobiol. 2004, 161, 289–306. [Google Scholar] [CrossRef]
- Aguilera, A.; Souza-Egipsy, V.; Gómez, F.; Amils, R. Development and structure of eukaryotic biofilms in an extreme acidic environment, Río Tinto (SW Spain). Microb. Ecol. 2007, 53, 294–305. [Google Scholar] [CrossRef]
- Aguilera, A. Eukaryotic organisms in extreme environments, the Río Tinto case. Life 2013, 3, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Weithoff, G.; Moser, M.; Kamjunke, N.; Gaedke, U.; Weisse, T. Lake morphometry and wind exposure may shape the plankton community structure in acidic mining lakes. Limnologica 2010, 40, 161–166. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Weisse, T.; Berendonk, T.; Kamjunke, N.; Moser, M.; Scheffel, U.; Stadler, P.; Weithoff, G. Significant habitat effects influence protist fitness: Evidence for local adaptation from acidic mining lakes. Ecosphere 2011, 2, 134. [Google Scholar] [CrossRef]
- Packroff, G. Protozooplankton in acidic mining lakes with special respect to ciliates. Hydrobiologia 2000, 433, 157–166. [Google Scholar] [CrossRef]
- Bell, E.M.; Weithoff, G. Benthic recruitment of zooplankton in an acidic lake. J. Exp. Mar. Biol. Ecol. 2003, 285–286, 205–219. [Google Scholar] [CrossRef]
- Weisse, T.; Moser, M.; Scheffel, U.; Stadler, P.; Berendonk, T.; Weithoff, G.; Berger, H. Systematics and species-specific response to pH of Oxytricha acidotolerans sp. nov. and Urosomoida sp. (Ciliophora, Hypotricha) from acid mining lakes. Eur. J. Protistol. 2013, 49, 255–271. [Google Scholar] [CrossRef]
- Deneke, R. Review of rotifers and crustaceans in highly acidic environments of pH values ≤ 3. Hydrobiologia 2000, 433, 167–172. [Google Scholar] [CrossRef]
- Hrdinka, T.; Šobr, M.; Fott, J.; Nedbalová, L. The unique environment of the most acidified permanently meromictic lake in the Czech Republic. Limnologica 2013, 43, 417–423. [Google Scholar] [CrossRef]
- Weithoff, G.; Seiferth, J.; Neumann, C.; Weisse, T. Living on the edge: Reproduction, dispersal potential, maternal effects and local adaptation in aquatic, extremophilic invertebrates. Aquat. Sci. 2019, 81, 40. [Google Scholar] [CrossRef]
- Canganella, F.; Wiegel, J. Extremophiles: From abyssal to terrestrial ecosystems and possibly beyond. Naturwissenschaften 2011, 98, 253–279. [Google Scholar] [CrossRef]
- Dhakar, K.; Pandey, A. Wide pH range tolerance in extremophiles: Towards understanding an important phenomenon for future biotechnology. Appl. Microbiol. Biotechnol. 2016, 100, 2499–2510. [Google Scholar] [CrossRef] [PubMed]
- Spijkerman, E.; Weithoff, G. Acidic Environments. In Life at Extremes–Environments, Organisms and Strategies for Survival; Bell, E.M., Ed.; CABI: Wallingford, UK, 2012; pp. 364–379. [Google Scholar]
- Schmidtke, A.; Bell, E.M.; Weithoff, G. Potential grazing impact of the mixotrophic flagellate Ochromonas sp. (Chrysophyceae) on bacteria in an extremely acidic lake. J. Plankton Res. 2006, 28, 991–1001. [Google Scholar] [CrossRef]
- Bell, E.M.; Weithoff, G.; Gaedke, U. Temporal dynamics and growth of Actinophrys sol (Sarcodina: Heliozoa), the top predator in an extremely acidic lake. Freshw. Biol. 2006, 51, 1149–1161. [Google Scholar] [CrossRef]
- Weithoff, G. On the ecology of the rotifer Cephalodella hoodi isolated from an extremely acidic lake. Freshw. Biol. 2005, 50, 1464–1473. [Google Scholar] [CrossRef]
- Weithoff, G. Vertical niche separation of two consumers in an extreme habitat. Oecologia 2004, 139, 594–603. [Google Scholar] [CrossRef]
- Tittel, J.; Bissinger, V.; Gaedke, U.; Kamjunke, N. Inorganic carbon limitation and mixotrophic growth of Chlamydomonas from an acidic mining lake. Protist 2005, 156, 63–75. [Google Scholar] [CrossRef]
- Spijkerman, E. Inorganic carbon acquisition by Chlamydomonas acidophila across a pH range. Can. J. Bot. 2005, 83, 872–878. [Google Scholar] [CrossRef]
- Bissinger, V.; Jander, J.; Tittel, J. A new medium free of organic carbon to cultivate organisms from extremely acidic mining lakes (pH 2.7). Acta Hydroch. Hydrob. 2000, 28, 310–312. [Google Scholar] [CrossRef]
- Weithoff, G. Dietary restriction in two rotifer species: The effect of the length of food deprivation on life span and reproduction. Oecologia 2007, 153, 303–308. [Google Scholar] [CrossRef]
- Patterson, D.J.; Hausmann, K. Feeding by Actinophrys sol (Protista, Heliozoa). 1. Light microscopy. Microbios 1981, 31, 39–55. [Google Scholar]
- Grebecki, A.; Hausmann, K. Motor behaviour of prey during first steps of food capture by Actinophrys sol. Acta Protozool. 1993, 32, 157–164. [Google Scholar]
- Suzaki, T.; Shigenaka, Y.; Watanabe, S.; Toyohara, A. Food capture and ingestion in the large heliozoan Echinosphaerium nucleofilum. J. Cell Sci. 1980, 42, 61–79. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, M.; Hausmann, K.; Suzaki, T. Food capture and adhesion by the heliozoon Actinophrys sol. Protoplasma 1998, 203, 130–137. [Google Scholar] [CrossRef]
- Hausmann, K.; Patterson, D.J. Pseudopod formation and membrane production during prey capture in by a heliozoan (Feeding by Actinophrys. II). Cell Motil. 1982, 2, 9–24. [Google Scholar] [CrossRef]
- Weithoff, G.; Wacker, A. The mode of nutrition of mixotrophic flagellates determines the food quality for their consumers. Funct. Ecol. 2007, 21, 1092–1098. [Google Scholar] [CrossRef]
- Hartwig, M.; Weithoff, G.; Wacker, A. Changes in the competitive abilities of two rotifers feeding on mixotrophic flagellates. J. Plankton Res. 2010, 32, 1727–1731. [Google Scholar] [CrossRef]
- Wacker, A.; Weithoff, G. Carbon assimilation mode in mixotrophs and the fatty acid composition of their consumers–three rotifers. Freshw. Biol. 2009, 54, 2189–2199. [Google Scholar] [CrossRef]
- Méndez-Garcia, C.; Peláez, A.I.; Mesa, V.; Sánchez, J.; Golyshina, O.V.; Ferrer, M. Microbial diversity and metabolic networks in acid mine drainage habitats. Front. Microbiol. 2015, 6, 475. [Google Scholar] [CrossRef]
- Diehl, S.; Feissl, M. Effects of enrichment on three-level food chains with omnivory. Am. Nat. 2000, 155, 200–218. [Google Scholar] [CrossRef]
- Diehl, S.; Feissl, M. Intraguild prey suffers from enrichment of their resources: A microcosms experiment with ciliates. Ecology 2001, 82, 2977–2983. [Google Scholar] [CrossRef]
- Beaver, J.; Crisman, T. The role of ciliated protozoa in pelagic freshwater ecosystems. Microb. Ecol. 1989, 17, 111–136. [Google Scholar] [CrossRef] [PubMed]
- Müller, H. The relative importance of different ciliate taxa in the pelagic food web of Lake Constance. Microb. Ecol. 1989, 18, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Lischke, B.; Weithoff, G.; Wickham, S.A.; Attermeyer, K.; Grossart, H.-P.; Scharnweber, K.; Hilt, S.; Gaedke, U. Large biomass of small feeders: Ciliates may dominate herbivory in eutrophic Lakes. J. Plankton Res. 2015, 38, 2–15. [Google Scholar] [CrossRef]
- Azam, F.; Fenchel, T.; Field, J.G.; Gray, J.S.; Meyer-Reil, L.A.; Thingstad, F. The ecological role of water-column microbes in the sea. Mar. Ecol. Progr. Ser. 1983, 10, 257–263. [Google Scholar] [CrossRef]
- Pomeroy, L.R. The Ocean’s Food Web, A Changing Paradigm. BioScience 1974, 24, 499–504. [Google Scholar] [CrossRef]
- Zimmermann, U.; Müller, H.; Weisse, T. Seasonal and spatial variability of planktonic heliozoa in Lake Constance. Aquat. Microb. Ecol. 1996, 11, 21–29. [Google Scholar] [CrossRef][Green Version]
- Pierce, R.W.; Coats, D.W. The feeding ecology of Actinophrys sol (Sarcodina: Heliozoa) in Chesapeake Bay. J. Eukaryot. Microbiol. 1999, 46, 451–457. [Google Scholar] [CrossRef]
- Woelfl, S. Limnology of sulphur-acidic lignite mining lakes. Biological properties: Plankton structure of an extreme habitat. Verh. Internat. Verein. Limnol. 2000, 27, 2904–2907. [Google Scholar] [CrossRef]
- Arndt, H. A critical review of the importance of rhizopods (naked and testate amoebae) and actinopods (Heliozoa) in lake plankton. Mar. Microb. Food Webs 1993, 7, 3–29. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weithoff, G.; Bell, E.M. Complex Trophic Interactions in an Acidophilic Microbial Community. Microorganisms 2022, 10, 1340. https://doi.org/10.3390/microorganisms10071340
Weithoff G, Bell EM. Complex Trophic Interactions in an Acidophilic Microbial Community. Microorganisms. 2022; 10(7):1340. https://doi.org/10.3390/microorganisms10071340
Chicago/Turabian StyleWeithoff, Guntram, and Elanor M. Bell. 2022. "Complex Trophic Interactions in an Acidophilic Microbial Community" Microorganisms 10, no. 7: 1340. https://doi.org/10.3390/microorganisms10071340
APA StyleWeithoff, G., & Bell, E. M. (2022). Complex Trophic Interactions in an Acidophilic Microbial Community. Microorganisms, 10(7), 1340. https://doi.org/10.3390/microorganisms10071340




