The Advances of Broad-Spectrum and Hot Anti-Coronavirus Drugs
Abstract
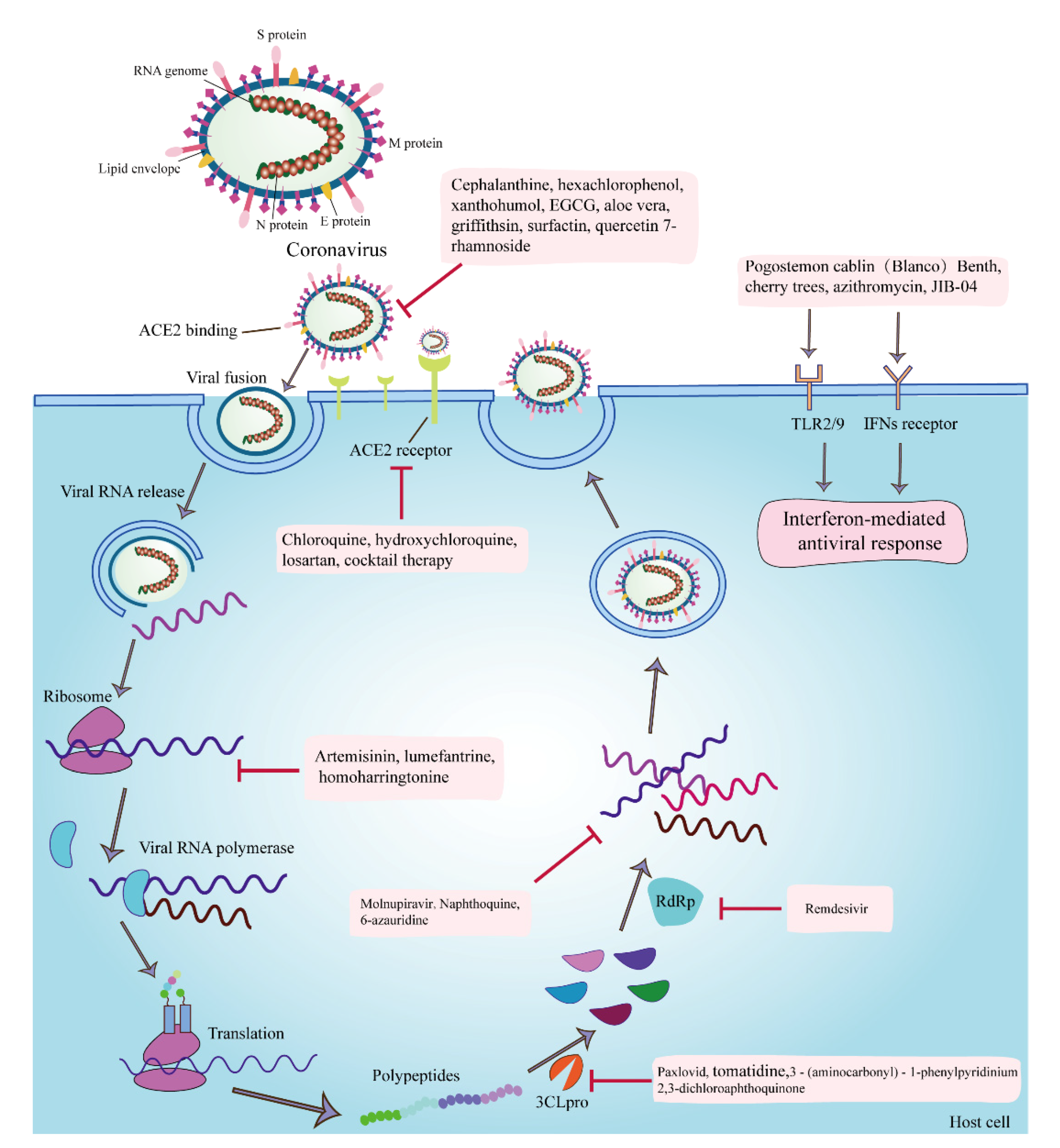
1. Introduction
2. Nucleoside Analogs
2.1. Remdesivir
2.2. Molnupiravir
| Antiviral Drugs | Mechanisms/Targets | Virus | IC50/EC50 Value | Reference |
|---|---|---|---|---|
| Remdesivir | RdRp and nucleoside components | SARS-CoV, MERS-CoV, MHV, PEDV, SARS-CoV-2 | EC50 = 0.74 μmol/L | [28,29,31] |
| Molnupiravir | Genomic RNA of the virus | SARS-CoV-2 | - | [33,34,35] |
3. Enzyme Inhibitors
3.1. Paxlovid
3.2. 3-(aminocarbonyl)-1-phenylpyridinium and 2,3-dichloroaphthoquinone
3.3. Hexachlorophenol
3.4. Xanthohumol
3.5. Tomatidine
| Antiviral Drugs | Mechanisms/Targets | Virus | IC50/EC50 Value | Reference |
|---|---|---|---|---|
| Paxlovid | 3CLpro and CYP3A4 | SARS-CoV-2, HIV-1 | - | [39,40] |
| 3-(aminocarbonyl)-1-phenylpyridinium; 2,3-dichloroaphthoquinone | Mpro (Cys144, Glu165, Gln191) | PEDV, FIPV | EC50 = 100 μM | [42] |
| Hexachlorophenol | Mpro and ATPase | SARS-CoV | - | [43] |
| Xanthohumol | Mpro | BVDV, HSV-1, HSV-2, RhV, PEDV, SARS-CoV-2 | IC50 = 1.53 µM and 7.51 µM | [44,45,46,47] |
| Tomatidine | 3CLpro | PEDV, TGEV, PRRSV, EMCV, SVA, PEDV | - | [51] |
4. Antimalarial Drugs
4.1. Chloroquine and Hydroxychloroquine
4.2. Naphthoquine
| Antiviral Drugs | Mechanisms/Targets | Virus | IC50/EC50 Value | Reference |
|---|---|---|---|---|
| Chloroquine | ACE2 receptor and proinflammatory cytokines | SARS-CoV-2 | - | [56] |
| Hydroxychloroquine | ||||
| NPQ | Affecting both entry and post-entry replication of the virus | HCoV-229E, HCoV-OC43, SARS-CoV-2 | IC50 = 2.05 ± 1.44, 5.83 ± 0.74, and 2.01 ± 0.38 μM, respectively | [61] |
5. Natural Antioxidants
5.1. (−)-epigallocatechin-3-gallate, Betulinic Acid, Ursolic Acid, Aescin, Lithocholic Acid, Nordihydroguaiaretic Acid, Caffeic Acid Phenethyl Ester, and Grape Seed Extract
5.2. Cherry Trees
5.3. Aloe Vera
| Antiviral Drugs | Mechanisms/Targets | Virus | IC50/EC50 Value | Reference |
|---|---|---|---|---|
| EGCG | Inhibiting viral attachment, entry, replication, and assembly | HIV, IAV, HBV, HCV, PRRSV, PCV2, PEDV | - | [69,70,71,72,73,74,75] |
| Betulinic acid, ursolic acid, aescin, lithocholic acid, nordihydroguaiaretic acid, caffeic acid phenethyl ester, and grape seed extract | Reducing virus-induced oxygen species production | PEDV | - | [76] |
| Cherry trees (phenolic compounds) | Inhibiting DPPH hydroxyl radical scavenging activity, reducing power capacity, and SOD-like activity | PEDV | - | [79] |
| Aloe vera (anthraquinones) | Antiviral genes, viral enzymes, and proteins | IAV, PPMV-1, HSV-1, PEDV | - | [82,83,84] |
6. Traditional Chinese Medicine (TCM)
6.1. Artemisinin and Lumefantrine
6.2. Puerarin and Quercetin 7-Rhamnoside
6.3. Cepharanthine
6.4. Pogostemon cablin (Blanco) Benth
6.5. Cimicifuga rhizoma, Meliae cortex, Coptidis rhizoma, Phellodendron cortex, and Sophira subsprata Radix
| Antiviral Drugs | Mechanisms/Targets | Virus | IC50/EC50 Value | Reference |
|---|---|---|---|---|
| Artemisinin and lumefantrine | Early proteins | SARS-CoV-2, HCV | - | [94,95,96,97,98] |
| PR | - | HIV-1, HBV, HRSV, PEDV | - | [102,103,104,105,106] |
| Q7R | Initial stage of infection | PEDV | IC50 = 0.014 μg/mL | [107] |
| CEP | S protein | SARS-CoV-2, HIV-1, SARS-CoV | EC50 = 0.98 μM | [108,109,110,111,112] |
| Pogostemon cablin (Blanco) Benth. | Enhanced antioxidant activity | PEDV, IV, CV, RSV, HSV, HAdVs | - | [116,117,118] |
| Cimicifuga rhizoma, Meliae cortex, Coptidis rhizoma, Phellodendron cortex, and Sophira subsprata Radix | - | MHV, VSV, PEDV | EC50 = 2.0 to 27.5 μg/mL | [119] |
7. Other Potential Antiviral Agents
7.1. Azithromycin
7.2. Losartan
7.3. Trichlormethiazide, D-(þ)-Biotin and Glutathione
7.4. Griffithsin
7.5. Surfactin
7.6. Carbazole Alkaloids
7.7. Exosomes
7.8. 6-Azauridine
7.9. Homoharringtonine
7.10. ZnO
7.11. JIB-04
7.12. Cocktail Therapy for Coronavirus
7.13. Interferon
| Antiviral Drugs | Mechanisms/Targets | Virus | IC50/EC50 Value | Reference |
|---|---|---|---|---|
| AZM | Inducing type I interferon immune responses | SARS-CoV-2 | - | [120,122] |
| Losartan | ACE2 | SARS-CoV-2 | - | [125,126,127,130] |
| Trichlormethiazide, D- (þ) Biotin, GSH | N protein | PEDV | Its concentrations of 0.094, 0.094, and 1.5 mg/mL, respectively | [128,129,130,131,132] |
| Griffithsin | Preventing viral attachment to host cells and disrupting cell-to-cell transmission | HIV, SARS-CoV, MERS-COV, HCV, HSV-2, JEV, PEDV, HPV | - | [135,136,137,138,139,140,141,144] |
| Surfactin and SLP5 | Reducing the rate of viral fusion with the cell membrane and hindering the lamellar phase lipids to form negative curvatures | PRV, PPV, NDV, IBDV, HSV-1, HSV-2, TGEV, PEDV | - | [146,147,148,149] |
| Carbazole alkaloids | - | HIV, HCV, CV, HSV, PEDV | - | [152,153,154,155,156,157,158] |
| Exosomes | C3, C6, and CFB complexes | PEDV | - | [159] |
| 6-azauridine | Inhibiting viral RNA synthesis | HCoV-NL63, FMDV, KFDV | - | [160,161,162] |
| Homoharringtonine | Asparagine and thymidine | MHV, BCoV-L9, and HECoV-4408 | - | [163] |
| ZnO | Increasing total superoxide dismutase activity | PEDV | - | [166] |
| JIB-04 | Promoting methylation of histone H3 on lysine 9 (H3K9) and lysine 27 (H3K27), and initiating host antiviral responses | SARS-CoV-2, TGEV | - | [167] |
| Cocktail therapy (CBS, BBS, NAC) | PLpro, Mpro, Hel, and ACE2 | SARS-CoV-2, MERS-CoV, HCoV-229E, SARS-CoV-2α Variant (b.1.1.7) | - | [168] |
| IFNs | Promoting the expression of antiviral proteins (2,5-oligoadenylate synthetase, protein kinases, and phosphodiesterases) | HBV, HCV, herpes virus, HCoV, SARS-CoV-2 | - | [170,171,172,174] |
8. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farsani, S.M.; Dijkman, R.; Jebbink, M.F.; Goossens, H.; Ieven, M.; Deijs, M.; Molenkamp, R.; van der Hoek, L. The first complete genome sequences of clinical isolates of human coronavirus 229E. Virus Genes 2012, 45, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, Z.; Li, M.; Wang, X. Comparative Review of SARS-CoV-2, SARS-CoV, MERS-CoV, and Influenza A Respiratory Viruses. Front. Immunol. 2020, 11, 552909. [Google Scholar] [CrossRef] [PubMed]
- Gerdts, V.; Zakhartchouk, A. Vaccines for porcine epidemic diarrhea virus and other swine coronaviruses. Vet. Microbiol. 2017, 206, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Sheehy, L.M. Considerations for Postacute Rehabilitation for Survivors of COVID-19. JMIR Public Health Surveill. 2020, 6, e19462. [Google Scholar] [CrossRef] [PubMed]
- Turlewicz-Podbielska, H.; Pomorska-Mol, M. Porcine Coronaviruses: Overview of the State of the Art. Virol. Sin. 2021, 36, 833–851. [Google Scholar] [CrossRef]
- Tang, Q.; Song, Y.; Shi, M.; Cheng, Y.; Zhang, W.; Xia, X.Q. Inferring the hosts of coronavirus using dual statistical models based on nucleotide composition. Sci. Rep. 2015, 5, 17155. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- Li, G.; Fan, Y.; Lai, Y.; Han, T.; Li, Z.; Zhou, P.; Pan, P.; Wang, W.; Hu, D.; Liu, X.; et al. Coronavirus infections and immune responses. J. Med. Virol. 2020, 92, 424–432. [Google Scholar] [CrossRef]
- Chujo, T.; Ishibashi, K.; Miyashita, S.; Ishikawa, M. Functions of the 5′- and 3′-untranslated regions of tobamovirus RNA. Virus Res. 2015, 206, 82–89. [Google Scholar] [CrossRef]
- Kothandan, R.; Uthayasooriyan, P.; Vairamani, S. Search for RNA aptamers against non-structural protein of SARS-CoV-2: Design using molecular dynamics approach. Beni Suef Univ. J. Basic Appl. Sci. 2021, 10, 64. [Google Scholar] [CrossRef]
- Hatmal, M.M.; Alshaer, W.; Al-Hatamleh, M.; Hatmal, M.; Smadi, O.; Taha, M.O.; Oweida, A.J.; Boer, J.C.; Mohamud, R.; Plebanski, M. Comprehensive Structural and Molecular Comparison of Spike Proteins of SARS-CoV-2, SARS-CoV and MERS-CoV, and Their Interactions with ACE2. Cells 2020, 9, 2638. [Google Scholar] [CrossRef]
- Bos, R.; Rutten, L.; van der Lubbe, J.; Bakkers, M.; Hardenberg, G.; Wegmann, F.; Zuijdgeest, D.; de Wilde, A.H.; Koornneef, A.; Verwilligen, A.; et al. Ad26 vector-based COVID-19 vaccine encoding a prefusion-stabilized SARS-CoV-2 Spike immunogen induces potent humoral and cellular immune responses. Npj Vaccines 2020, 5, 91. [Google Scholar] [CrossRef]
- Li, F. Structure, Function, and Evolution of Coronavirus Spike Proteins. Annu. Rev. Virol. 2016, 3, 237–261. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Moore, M.J.; Vasilieva, N.; Sui, J.; Wong, S.K.; Berne, M.A.; Somasundaran, M.; Sullivan, J.L.; Luzuriaga, K.; Greenough, T.C.; et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003, 426, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Hartenian, E.; Nandakumar, D.; Lari, A.; Ly, M.; Tucker, J.M.; Glaunsinger, B.A. The molecular virology of coronaviruses. J. Biol. Chem. 2020, 295, 12910–12934. [Google Scholar] [CrossRef] [PubMed]
- Pavithra, V.; Sasha, M.D.; Lisa, A.L.; Kelly, A.D.; Brenda, G.H. Coronavirus envelope (E) protein remains at the site of assembly. Virology 2015, 478, 75–85. [Google Scholar]
- Nieto-Torres, J.L.; Dediego, M.L.; Alvarez, E.; Jimenez-Guardeno, J.M.; Regla-Nava, J.A.; Llorente, M.; Kremer, L.; Shuo, S.; Enjuanes, L. Subcellular location and topology of severe acute respiratory syndrome coronavirus envelope protein. Virology 2011, 415, 69–82. [Google Scholar] [CrossRef]
- Dewald, S.; Burtram, C.F. Coronavirus envelope protein: Current knowledge. Virol. J. 2019, 16, 69. [Google Scholar]
- Travis, R.R.; Carolyn, E.M. The Coronavirus E Protein: Assembly and Beyond. Viruses 2012, 4, 363–382. [Google Scholar]
- Boopathi, S.; Poma, A.B.; Kolandaivel, P. Novel 2019 coronavirus structure, mechanism of action, antiviral drug promises and rule out against its treatment. J. Biomol. Struct. Dyn. 2021, 39, 3409–3418. [Google Scholar] [CrossRef]
- Wang, H.; Yang, P.; Liu, K.; Guo, F.; Zhang, Y.; Zhang, G.; Jiang, C. SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway. Cell Res. 2008, 18, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Ou, X.; Liu, Y.; Lei, X.; Li, P.; Mi, D.; Ren, L.; Guo, L.; Guo, R.; Chen, T.; Hu, J.; et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020, 11, 1620. [Google Scholar] [CrossRef] [PubMed]
- Morse, J.S.; Lalonde, T.; Xu, S.; Liu, W. Learning from the Past: Possible Urgent Prevention and Treatment Options for Severe Acute Respiratory Infections Caused by 2019-nCoV. ChemRxiv 2020. [Google Scholar] [CrossRef]
- Bhise, N.S.; Elsayed, A.H.; Cao, X.; Pounds, S.; Lamba, J.K. MicroRNAs Mediated Regulation of Expression of Nucleoside Analog Pathway Genes in Acute Myeloid Leukemia. Genes 2019, 10, 319. [Google Scholar] [CrossRef]
- Tsuda, M.; Terada, K.; Ooka, M.; Kobayashi, K.; Sasanuma, H.; Fujisawa, R.; Tsurimoto, T.; Yamamoto, J.; Iwai, S.; Kadoda, K.; et al. The dominant role of proofreading exonuclease activity of replicative polymerase epsilon in cellular tolerance to cytarabine (Ara-C). Oncotarget 2017, 8, 33457–33474. [Google Scholar] [CrossRef] [PubMed]
- Eyer, L.; Nencka, R.; de Clercq, E.; Seley-Radtke, K.; Ruzek, D. Nucleoside analogs as a rich source of antiviral agents active against arthropod-borne flaviviruses. Antivir. Chem. Chemother. 2018, 26, 1631086509. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.J.; Won, J.J.; Graham, R.L.; Dinnon, K.R.; Sims, A.C.; Feng, J.Y.; Cihlar, T.; Denison, M.R.; Baric, R.S.; Sheahan, T.P. Broad spectrum antiviral remdesivir inhibits human endemic and zoonotic deltacoronaviruses with a highly divergent RNA dependent RNA polymerase. Antivir. Res. 2019, 169, 104541. [Google Scholar] [CrossRef]
- Agostini, M.L.; Andres, E.L.; Sims, A.C.; Graham, R.L.; Sheahan, T.P.; Lu, X.; Smith, E.C.; Case, J.B.; Feng, J.Y.; Jordan, R.; et al. Coronavirus Susceptibility to the Antiviral Remdesivir (GS-5734) Is Mediated by the Viral Polymerase and the Proofreading Exoribonuclease. mBio 2018, 9, e00221-18. [Google Scholar] [CrossRef]
- Xie, Y.; Guo, X.; Hu, T.; Wei, D.; Ma, X.; Wu, J.; Huang, B.; Shen, J. Significant Inhibition of Porcine Epidemic Diarrhea Virus In Vitro by Remdesivir, Its Parent Nucleoside and beta-D-N(4)-hydroxycytidine. Virol. Sin. 2021, 36, 997–1005. [Google Scholar] [CrossRef]
- Xu, Y.; Barauskas, O.; Kim, C.; Babusis, D.; Murakami, E.; Kornyeyev, D.; Lee, G.; Stepan, G.; Perron, M.; Bannister, R.; et al. Off-Target In Vitro Profiling Demonstrates that Remdesivir Is a Highly Selective Antiviral Agent. Antimicrob. Agents Chemother. 2021, 65, e02237-20. [Google Scholar] [CrossRef]
- Rubin, D.; Chan-Tack, K.; Farley, J.; Sherwat, A. FDA Approval of Remdesivir—A Step in the Right Direction. N. Engl. J. Med. 2020, 383, 2598–2600. [Google Scholar] [CrossRef]
- De Clercq, E. Remdesivir: Quo vadis? BioChem. Pharm. 2021, 193, 114800. [Google Scholar] [CrossRef] [PubMed]
- Wahl, A.; Gralinski, L.E.; Johnson, C.E.; Yao, W.; Kovarova, M.; Dinnon, K.R.; Liu, H.; Madden, V.J.; Krzystek, H.M.; De, C.; et al. SARS-CoV-2 infection is effectively treated and prevented by EIDD-2801. Nature 2021, 591, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Cave, J.A.; Phizackerley, D. Molnupiravir: Evidence by press release. Drug Bull. 2022, 60, 2. [Google Scholar] [CrossRef] [PubMed]
- Kabinger, F.; Stiller, C.; Schmitzova, J.; Dienemann, C.; Kokic, G.; Hillen, H.S.; Hobartner, C.; Cramer, P. Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis. Nat. Struct. Mol. Biol. 2021, 28, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Singh, A.; Singh, R.; Misra, A. Molnupiravir in COVID-19: A systematic review of literature. Diabetes Metab. Syndr. 2021, 15, 102329. [Google Scholar] [CrossRef]
- Su, C.T.; Koh, D.W.; Gan, S.K. Reviewing HIV-1 Gag Mutations in Protease Inhibitors Resistance: Insights for Possible Novel Gag Inhibitor Designs. Molecules 2019, 24, 3243. [Google Scholar] [CrossRef]
- Nocentini, A.; Capasso, C.; Supuran, C.T. Perspectives on the design and discovery of alpha-ketoamide inhibitors for the treatment of novel coronavirus: Where do we stand and where do we go? Expert Opin. Drug Discov. 2022, 1–11. [Google Scholar] [CrossRef]
- Hammond, J.; Leister-Tebbe, H.; Gardner, A.; Abreu, P.; Bao, W.; Wisemandle, W.; Baniecki, M.; Hendrick, V.M.; Damle, B.; Simon-Campos, A.; et al. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19. N. Engl. J. Med. 2022, 386, 1397–1408. [Google Scholar] [CrossRef]
- Heskin, J.; Pallett, S.; Mughal, N.; Davies, G.W.; Moore, L.; Rayment, M.; Jones, R. Caution required with use of ritonavir-boosted PF-07321332 in COVID-19 management. Lancet 2022, 399, 21–22. [Google Scholar] [CrossRef]
- Wen, W.; Chen, C.; Tang, J.; Wang, C.; Zhou, M.; Cheng, Y.; Zhou, X.; Wu, Q.; Zhang, X.; Feng, Z.; et al. Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-19a meta-analysis. Ann. Med. 2022, 54, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Lei, Y.; Ye, G.; Sun, L.; Fang, L.; Xiao, S.; Fu, Z.F.; Yin, P.; Song, Y.; Peng, G. Identification of two antiviral inhibitors targeting 3C-like serine/3C-like protease of porcine reproductive and respiratory syndrome virus and porcine epidemic diarrhea virus. Vet. Microbiol. 2018, 213, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Sattar, S.A.; Raphael, R.A.; Lochnan, H.; Springthorpe, V.S. Rotavirus inactivation by chemical disinfectants and antiseptics used in hospitals. Can. J. Microbiol. 1983, 29, 1464–1469. [Google Scholar] [CrossRef] [PubMed]
- Hongming, L.; Qinmei, L.; Zhongmei, W.; Haihua, F.; Xuming, D.; Xinxin, C. Xanthohumol ameliorates lipopolysaccharide (LPS)-induced acute lung injury via induction of AMPK/GSK3β-Nrf2 signal axis. Redox Biol. 2017, 12, 311–324. [Google Scholar]
- Chen, X.; Li, Z.; Hong, H.; Wang, N.; Chen, J.; Lu, S.; Zhang, H.; Zhang, X.; Bei, C. Xanthohumol suppresses inflammation in chondrocytes and ameliorates osteoarthritis in mice. Biomed. Pharm. 2021, 137, 111238. [Google Scholar] [CrossRef]
- Torrens-Mas, M.; Alorda-Clara, M.; Martinez-Vigara, M.; Roca, P.; Sastre-Serra, J.; Oliver, J.; Pons, D.G. Xanthohumol reduces inflammation and cell metabolism in HT29 primary colon cancer cells. Int. J. Food Sci. Nutr. 2022, 73, 471–479. [Google Scholar] [CrossRef]
- Lin, Y.; Zang, R.; Ma, Y.; Wang, Z.; Li, L.; Ding, S.; Zhang, R.; Wei, Z.; Yang, J.; Wang, X. Xanthohumol Is a Potent Pan-Inhibitor of Coronaviruses Targeting Main Protease. Int. J. Mol. Sci. 2021, 22, 12134. [Google Scholar] [CrossRef]
- Jiang, Q.W.; Chen, M.W.; Cheng, K.J.; Yu, P.Z.; Wei, X.; Shi, Z. Therapeutic Potential of Steroidal Alkaloids in Cancer and Other Diseases. Med. Res. Rev. 2016, 36, 119–143. [Google Scholar] [CrossRef]
- Ahsan, A.; Zheng, Y.; Ma, S.; Liu, M.; Cao, M.; Li, Y.; Zheng, W.; Zhou, X.; Xin, M.; Hu, W.W.; et al. Tomatidine protects against ischemic neuronal injury by improving lysosomal function. Eur. J. Pharm. 2020, 882, 173280. [Google Scholar] [CrossRef]
- Chiu, F.L.; Lin, J.K. Tomatidine inhibits iNOS and COX-2 through suppression of NF-kappaB and JNK pathways in LPS-stimulated mouse macrophages. FEBS Lett. 2008, 582, 2407–2412. [Google Scholar] [CrossRef]
- Wang, P.; Bai, J.; Liu, X.; Wang, M.; Wang, X.; Jiang, P. Tomatidine inhibits porcine epidemic diarrhea virus replication by targeting 3CL protease. Vet. Res. 2020, 51, 136. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Sahi, P.K. Malaria: An Update. Indian J. Pediatr. 2017, 84, 521–528. [Google Scholar] [CrossRef]
- Sinha, N.; Balayla, G. Hydroxychloroquine and COVID-19. Postgrad. Med. J. 2020, 96, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Martinez, G.P.; Zabaleta, M.E.; Di Giulio, C.; Charris, J.E.; Mijares, M.R. The Role of Chloroquine and Hydroxychloroquine in Immune Regulation and Diseases. Curr. Pharm. Des. 2020, 26, 4467–4485. [Google Scholar] [CrossRef] [PubMed]
- Devaux, C.A.; Rolain, J.M.; Colson, P.; Raoult, D. New insights on the antiviral effects of chloroquine against coronavirus: What to expect for COVID-19? Int. J. Antimicrob. Agents 2020, 55, 105938. [Google Scholar] [CrossRef]
- Ratiani, L.; Gegechkory, S.; Machavariani, K.; Shotadze, T.; Sanikidze, T.; Intskirveli, N. The peculiarity of Covid-19 genome and the coronavirus RNA translation process as apotential target for etiotropic medicationswith adenine and other nucleotide analogues (review). Georgian Med. News 2021, 310, 119–124. [Google Scholar]
- Singh, T.U.; Parida, S.; Lingaraju, M.C.; Kesavan, M.; Kumar, D.; Singh, R.K. Drug repurposing approach to fight COVID-19. Pharm. Rep. 2020, 72, 1479–1508. [Google Scholar] [CrossRef]
- Perez, J.; Roustit, M.; Lepelley, M.; Revol, B.; Cracowski, J.L.; Khouri, C. Reported Adverse Drug Reactions Associated with the Use of Hydroxychloroquine and Chloroquine during the COVID-19 Pandemic. Ann. Intern. Med. 2021, 174, 878–880. [Google Scholar] [CrossRef]
- Tandon, V.K.; Kumar, S. Recent development on naphthoquinone derivatives and their therapeutic applications as anticancer agents. Expert Opin. Pat. 2013, 23, 1087–1108. [Google Scholar] [CrossRef]
- Moore, B.R.; Laman, M.; Salman, S.; Batty, K.T.; Page-Sharp, M.; Hombhanje, F.; Manning, L.; Davis, T.M. Naphthoquine: An Emerging Candidate for Artemisinin Combination Therapy. Drugs 2016, 76, 789–804. [Google Scholar] [CrossRef]
- Song, Y.; Deng, Y.; Wang, H.; Bei, Z.; Gu, H.; Zhao, H.; Wang, H.; Zhang, D.; Xu, L.; Wang, B.; et al. Naphthoquine: A Potent Broad-Spectrum Anti-Coronavirus Drug In Vitro. Molecules 2022, 27, 712. [Google Scholar] [CrossRef] [PubMed]
- Vincent, M.J.; Bergeron, E.; Benjannet, S.; Erickson, B.R.; Rollin, P.E.; Ksiazek, T.G.; Seidah, N.G.; Nichol, S.T. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J. 2005, 2, 69. [Google Scholar] [CrossRef] [PubMed]
- Neha, K.; Haider, M.R.; Pathak, A.; Yar, M.S. Medicinal prospects of antioxidants: A review. Eur. J. Med. Chem. 2019, 178, 687–704. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q. Why natural antioxidants are readily recognized by biological systems? 3D architecture plays a role! Food Chem. 2022, 380, 132143. [Google Scholar] [CrossRef]
- Mut-Salud, N.; Alvarez, P.J.; Garrido, J.M.; Carrasco, E.; Aranega, A.; Rodriguez-Serrano, F. Antioxidant Intake and Antitumor Therapy: Toward Nutritional Recommendations for Optimal Results. Oxid. Med. Cell. Longev. 2016, 2016, 6719534. [Google Scholar] [CrossRef]
- Yang, H.; Landis-Piwowar, K.; Chan, T.H.; Dou, Q.P. Green tea polyphenols as proteasome inhibitors: Implication in chemoprevention. Curr. Cancer Drug Targets 2011, 11, 296–306. [Google Scholar] [CrossRef]
- Singh, M.; Singh, R.; Bhui, K.; Tyagi, S.; Mahmood, Z.; Shukla, Y. Tea polyphenols induce apoptosis through mitochondrial pathway and by inhibiting nuclear factor-kappaB and Akt activation in human cervical cancer cells. Oncol. Res. 2011, 19, 245–257. [Google Scholar] [CrossRef]
- Onoda, C.; Kuribayashi, K.; Nirasawa, S.; Tsuji, N.; Tanaka, M.; Kobayashi, D.; Watanabe, N. (-)-Epigallocatechin-3-gallate induces apoptosis in gastric cancer cell lines by down-regulating survivin expression. Int. J. Oncol. 2011, 38, 1403–1408. [Google Scholar] [CrossRef][Green Version]
- Fassina, G.; Buffa, A.; Benelli, R.; Varnier, O.E.; Noonan, D.M.; Albini, A. Polyphenolic antioxidant (-)-epigallocatechin-3-gallate from green tea as a candidate anti-HIV agent. AIDS 2002, 16, 939–941. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Honda, M.; Ikigai, H.; Hara, Y.; Shimamura, T. Inhibitory effects of (-)-epigallocatechin gallate on the life cycle of human immunodeficiency virus type 1 (HIV-1). Antivir. Res. 2002, 53, 19–34. [Google Scholar] [CrossRef]
- Ge, M.; Xiao, Y.; Chen, H.; Luo, F.; Du, G.; Zeng, F. Multiple antiviral approaches of (-)-epigallocatechin-3-gallate (EGCG) against porcine reproductive and respiratory syndrome virus infection in vitro. Antivir. Res. 2018, 158, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Song, D.; Wang, S.; Dai, Y.; Zhou, J.; Gu, J. Antiviral Effect of Epigallocatechin Gallate via Impairing Porcine Circovirus Type 2 Attachment to Host Cell Receptor. Viruses 2020, 12, 176. [Google Scholar] [CrossRef] [PubMed]
- Jariwalla, R.J.; Roomi, M.W.; Gangapurkar, B.; Kalinovsky, T.; Niedzwiecki, A.; Rath, M. Suppression of influenza A virus nuclear antigen production and neuraminidase activity by a nutrient mixture containing ascorbic acid, green tea extract and amino acids. Biofactors 2007, 31, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Calland, N.; Albecka, A.; Belouzard, S.; Wachowski, C.; Duverlie, G.; Descamps, V.; Hober, D.; Dubuisson, J.; Rouille, Y.; Seron, K. (-)-Epigallocatechin-3-gallate is a new inhibitor of hepatitis C virus entry. Hepatology 2012, 55, 720–729. [Google Scholar] [CrossRef]
- Huan, C.; Xu, W.; Ni, B.; Guo, T.; Pan, H.; Jiang, L.; Li, L.; Yao, J.; Gao, S. Epigallocatechin-3-Gallate, the Main Polyphenol in Green Tea, Inhibits Porcine Epidemic Diarrhea Virus In Vitro. Front. Pharm. 2021, 12, 628526. [Google Scholar] [CrossRef]
- Li, W.; Zhang, M.; Zheng, H.; Zhou, P.; Liu, Z.; Jongkaewwattana, A.; Luo, R.; He, Q. Construction of a Recombinant Porcine Epidemic Diarrhea Virus Encoding Nanoluciferase for High-Throughput Screening of Natural Antiviral Products. Viruses 2021, 13, 1866. [Google Scholar] [CrossRef]
- Piccolella, S.; Fiorentino, A.; Pacifico, S.; D’Abrosca, B.; Uzzo, P.; Monaco, P. Antioxidant properties of sour cherries (Prunus cerasus L.): Role of colorless phytochemicals from the methanolic extract of ripe fruits. J. Agric. Food Chem. 2008, 56, 1928–1935. [Google Scholar] [CrossRef]
- Heinonen, I.M.; Meyer, A.S.; Frankel, E.N. Antioxidant activity of berry phenolics on human low-density lipoprotein and liposome oxidation. J. Agric. Food Chem. 1998, 46, 4107–4112. [Google Scholar] [CrossRef]
- Yook, H.S.; Kim, K.H.; Park, J.E.; Shin, H.J. Antioxidative and antiviral properties of flowering cherry fruits (Prunus serrulata L. var. spontanea). Am. J. Chin. Med. 2010, 38, 937–948. [Google Scholar] [CrossRef]
- Zhang, L.B.; Man, Z.T.; Li, W.; Zhang, W.; Wang, X.Q.; Sun, S. Calcitonin protects chondrocytes from lipopolysaccharide-induced apoptosis and inflammatory response through MAPK/Wnt/NF-kappaB pathways. Mol. Immunol. 2017, 87, 249–257. [Google Scholar] [CrossRef]
- Li, X.; Mei, W.; Huang, Z.; Zhang, L.; Zhang, L.; Xu, B.; Shi, X.; Xiao, Y.; Ma, Z.; Liao, T.; et al. Casticin suppresses monoiodoacetic acid-induced knee osteoarthritis through inhibiting HIF-1alpha/NLRP3 inflammasome signaling. Int. Immunopharmacol. 2020, 86, 106745. [Google Scholar] [CrossRef] [PubMed]
- Ching-Tai, H.; Chen-Yiu, H.; Yu-Chia, H.; Chia-Shiang, C.; Arul, B.V.; Yueh-Chia, H.; Yu-Lin, H.; Ting-An, C.; Tse-Ching, C.; Chun-Yen, L.; et al. Effect of aloin on viral neuraminidase and hemagglutinin-specific T cell immunity in acute influenza. Phytomedicine 2019, 64, 152904. [Google Scholar]
- Li, S.W.; Yang, T.C.; Lai, C.C.; Huang, S.H.; Liao, J.M.; Wan, L.; Lin, Y.J.; Lin, C.W. Antiviral activity of aloe-emodin against influenza A virus via galectin-3 up-regulation. Eur. J. Pharm. 2014, 738, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Zhichao, X.; Yuan, L.; Peng, P.; Yufang, L.; Meiyan, H.; Yehuan, M.; Chunyi, X.; Yongchang, C. Aloe extract inhibits porcine epidemic diarrhea virus in vitro and in vivo. Vet. Microbiol. 2020, 249, 108849. [Google Scholar]
- Chang, W.T.; Toh, H.S.; Liao, C.T.; Yu, W.L. Cardiac Involvement of COVID-19: A Comprehensive Review. Am. J. Med. Sci. 2021, 361, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, C.; Shi, L.; Xue, Q. Beware of Steroid-Induced Avascular Necrosis of the Femoral Head in the Treatment of COVID-19-Experience and Lessons from the SARS Epidemic. Drug Des. Dev. 2021, 15, 983–995. [Google Scholar] [CrossRef]
- Li, Y.M.; Wang, S.X.; Gao, H.S.; Wang, J.G.; Wei, C.S.; Chen, L.M.; Hui, W.L.; Yuan, S.L.; Jiao, Z.S.; Yang, Z.; et al. Factors of avascular necrosis of femoral head and osteoporosis in SARS patients’ convalescence. Zhonghua Yi Xue Za Zhi 2004, 84, 1348–1353. [Google Scholar]
- Jordan, P.C.; Stevens, S.K.; Deval, J. Nucleosides for the treatment of respiratory RNA virus infections. Antivir. Chem. Chemother. 2018, 26, 1631083325. [Google Scholar] [CrossRef]
- Muhammad, Z.U.; Ashraf, U.K.; Ruqayya, A.; Hina, R.; Sidra, K.; Muhammad, N.; Hussain, A.; Yeong, S.K.; Salman, K. Attenuation of inflammatory pain by puerarin in animal model of inflammation through inhibition of pro-inflammatory mediators. Int. Immunopharmacol. 2018, 61, 306–316. [Google Scholar]
- Wu, C.Y.; Jan, J.T.; Ma, S.H.; Kuo, C.J.; Juan, H.F.; Cheng, Y.S.; Hsu, H.H.; Huang, H.C.; Wu, D.; Brik, A.; et al. Small molecules targeting severe acute respiratory syndrome human coronavirus. Proc. Natl. Acad. Sci. USA 2004, 101, 10012–10017. [Google Scholar] [CrossRef]
- Cinatl, J.; Morgenstern, B.; Bauer, G.; Chandra, P.; Rabenau, H.; Doerr, H. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet 2003, 361, 2045–2046. [Google Scholar] [CrossRef]
- Wang, J.; Xu, C.; Lun, Z.R.; Meshnick, S.R. Unpacking ‘Artemisinin Resistance’. Trends Pharm. Sci. 2017, 38, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Nosten, F.; White, N.J. Artemisinin-based combination treatment of falciparum malaria. Am. J. Trop. Med. Hyg. 2007, 77, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Hu, H.; Li, Y.; Wang, X.; Xu, M.; Liu, J.; Zhang, H.; Yan, Y.; Zhao, L.; Li, W.; et al. Anti-SARS-CoV-2 Potential of Artemisinins In Vitro. ACS Infect. Dis. 2020, 6, 2524–2531. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Park, K.; Cai, H.; Kapoor, A.; Forman, M.; Mott, B.; Posner, G.H.; Arav-Boger, R. Artemisinin-derived dimer diphenyl phosphate is an irreversible inhibitor of human cytomegalovirus replication. Antimicrob. Agents Chemother. 2012, 56, 3508–3515. [Google Scholar] [CrossRef]
- Efferth, T.; Marschall, M.; Wang, X.; Huong, S.; Hauber, I.; Olbrich, A.; Kronschnabl, M.; Stamminger, T.; Huang, E. Antiviral activity of artesunate towards wild-type, recombinant, and ganciclovir-resistant human cytomegaloviruses. J. Mol. Med. 2002, 80, 233–242. [Google Scholar] [CrossRef]
- Wohlfarth, C.; Efferth, T. Natural products as promising drug candidates for the treatment of hepatitis B and C. Acta Pharm. Sin. 2009, 30, 25–30. [Google Scholar] [CrossRef]
- Obeid, S.; Alen, J.; Nguyen, V.H.; Pham, V.C.; Meuleman, P.; Pannecouque, C.; Le Thanh, N.; Neyts, J.; Dehaen, W.; Paeshuyse, J. Artemisinin analogues as potent inhibitors of in vitro hepatitis C virus replication. PLoS ONE 2013, 8, e81783. [Google Scholar]
- Xiu-Juan, Y.; Ji-Ai, Y.; Yu-Feng, X.; Zhi-Feng, W.; Yu-Bin, L.; Mei, L.; Carlos, F.; Yue, D. Puerarin exerts antipyretic effect on lipopolysaccharide-induced fever in rats involving inhibition of pyrogen production from macrophages. J. Ethnopharmacol. 2012, 141, 322–330. [Google Scholar]
- Xiao, C.; Li, J.; Dong, X.; He, X.; Niu, X.; Liu, C.; Zhong, G.; Bauer, R.; Yang, D.; Lu, A. Anti-oxidative and TNF-alpha suppressive activities of puerarin derivative (4AC) in RAW264.7 cells and collagen-induced arthritic rats. Eur. J. Pharm. 2011, 666, 242–250. [Google Scholar] [CrossRef]
- Yang, X.; Hu, W.; Zhang, Q.; Wang, Y.; Sun, L. Puerarin inhibits C-reactive protein expression via suppression of nuclear factor kappaB activation in lipopolysaccharide-induced peripheral blood mononuclear cells of patients with stable angina pectoris. Basic Clin. Pharm. 2010, 107, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, F.; Ma, Y.; Li, H.; Ju, X.; Xu, J. Effect of Puerarin, Baicalin and Berberine Hydrochloride on the Regulation of IPEC-J2 Cells Infected with Enterotoxigenic Escherichia coli. Evid.-Based Complement. Altern. Med. 2019, 2019, 7438593. [Google Scholar] [CrossRef] [PubMed]
- Mediouni, S.; Jablonski, J.A.; Tsuda, S.; Richard, A.; Kessing, C.; Andrade, M.V.; Biswas, A.; Even, Y.; Tellinghuisen, T.; Choe, H.; et al. Potent suppression of HIV-1 cell attachment by Kudzu root extract. Retrovirology 2018, 15, 64. [Google Scholar] [CrossRef]
- Romero, M.R.; Efferth, T.; Serrano, M.A.; Castano, B.; Macias, R.I.; Briz, O.; Marin, J.J. Effect of artemisinin/artesunate as inhibitors of hepatitis B virus production in an “in vitro” replicative system. Antivir. Res. 2005, 68, 75–83. [Google Scholar] [CrossRef]
- Lin, T.J.; Yeh, C.F.; Wang, K.C.; Chiang, L.C.; Tsai, J.J.; Chang, J.S. Water extract of Pueraria lobata Ohwi has anti-viral activity against human respiratory syncytial virus in human respiratory tract cell lines. Kaohsiung J. Med. Sci. 2013, 29, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Zhang, Q.; Yi, D.; Wu, T.; Chen, H.; Guo, S.; Li, S.; Ji, C.; Wang, L.; Zhao, D.; et al. Quantitative Proteomic Analysis Reveals Antiviral and Anti-inflammatory Effects of Puerarin in Piglets Infected With Porcine Epidemic Diarrhea Virus. Front. Immunol. 2020, 11, 169. [Google Scholar] [CrossRef]
- Choi, H.J.; Kim, J.H.; Lee, C.H.; Ahn, Y.J.; Song, J.H.; Baek, S.H.; Kwon, D.H. Antiviral activity of quercetin 7-rhamnoside against porcine epidemic diarrhea virus. Antivir. Res. 2009, 81, 77–81. [Google Scholar] [CrossRef]
- Rogosnitzky, M.; Okediji, P.; Koman, I. Cepharanthine: A review of the antiviral potential of a Japanese-approved alopecia drug in COVID-19. Pharm. Rep. 2020, 72, 1509–1516. [Google Scholar] [CrossRef]
- Zhang, C.H.; Wang, Y.F.; Liu, X.J.; Lu, J.H.; Qian, C.W.; Wan, Z.Y.; Yan, X.G.; Zheng, H.Y.; Zhang, M.Y.; Xiong, S.; et al. Antiviral activity of cepharanthine against severe acute respiratory syndrome coronavirus in vitro. Chin. Med. J. 2005, 118, 493–496. [Google Scholar]
- Fan, H.; Wang, L.; Liu, W.; An, X.; Liu, Z.; He, X.; Song, L.; Tong, Y. Repurposing of clinically approved drugs for treatment of coronavirus disease 2019 in a 2019-novel coronavirus-related coronavirus model. Chin. Med. J. 2020, 133, 1051–1056. [Google Scholar] [CrossRef]
- Hijikata, A.; Shionyu-Mitsuyama, C.; Nakae, S.; Shionyu, M.; Ota, M.; Kanaya, S.; Hirokawa, T.; Nakajima, S.; Watashi, K.; Shirai, T. Evaluating cepharanthine analogues as natural drugs against SARS-CoV-2. FEBS Open Bio 2022, 12, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, H.; Watashi, K.; Saso, W.; Shionoya, K.; Iwanami, S.; Hirokawa, T.; Shirai, T.; Kanaya, S.; Ito, Y.; Kim, K.S.; et al. Potential anti-COVID-19 agents, cepharanthine and nelfinavir, and their usage for combination treatment. iScience 2021, 24, 102367. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, L.; Hu, Q.; Wang, C. New natural furfural derivatives from the leaves and stems of Pogostemon cablin. Nat. Prod. Res. 2021, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Wang, Q.; Chang, R.; Aboragah, A.; Loor, J.J.; Xu, C. Network Pharmacology-Based Analysis of Pogostemon cablin (Blanco) Benth Beneficial Effects to Alleviate Nonalcoholic Fatty Liver Disease in Mice. Front. Pharm. 2021, 12, 789430. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, G.; Li, Q.; He, L.; Zhang, Y.; Wang, Y.; He, H. Identification and characterization of virulence-attenuated mutants in Ralstonia solanacearum as potential biocontrol agents against bacterial wilt of Pogostemon cablin. Microb. Pathog. 2020, 147, 104418. [Google Scholar] [CrossRef]
- Liu, F.; Cao, W.; Deng, C.; Wu, Z.; Zeng, G.; Zhou, Y. Polyphenolic glycosides isolated from Pogostemon cablin (Blanco) Benth. as novel influenza neuraminidase inhibitors. Chem. Cent. J. 2016, 10, 51. [Google Scholar] [CrossRef][Green Version]
- Li, Y.C.; Peng, S.Z.; Chen, H.M.; Zhang, F.X.; Xu, P.P.; Xie, J.H.; He, J.J.; Chen, J.N.; Lai, X.P.; Su, Z.R. Oral administration of patchouli alcohol isolated from Pogostemonis Herba augments protection against influenza viral infection in mice. Int. Immunopharmacol. 2012, 12, 294–301. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, Q.; Li, S.; Li, C.; Liao, S.; Yang, X.; Zhou, R.; Zhu, Y.; Teng, L.; Chen, H.; et al. Antiviral activity against porcine epidemic diarrhea virus of Pogostemon cablin polysaccharide. J. Ethnopharmacol. 2020, 259, 113009. [Google Scholar] [CrossRef]
- Kim, H.Y.; Shin, H.S.; Park, H.; Kim, Y.C.; Yun, Y.G.; Park, S.; Shin, H.J.; Kim, K. In vitro inhibition of coronavirus replications by the traditionally used medicinal herbal extracts, Cimicifuga rhizoma, Meliae cortex, Coptidis rhizoma, and Phellodendron cortex. J. Clin. Virol. 2008, 41, 122–128. [Google Scholar] [CrossRef]
- Mansour, B.S.; Salem, N.A.; Kader, G.A.; Abdel-Alrahman, G.; Mahmoud, O.M. Protective effect of Rosuvastatin on Azithromycin induced cardiotoxicity in a rat model. Life Sci. 2021, 269, 119099. [Google Scholar] [CrossRef]
- Shinuan, Z.; Xiaobin, M.; Qingyuan, H.; Nanfeng, L.; Lingbin, Z.; Xinying, J.; Xuemin, G. Spiramycin and azithromycin, safe for administration to children, exert antiviral activity against enterovirus A71 in vitro and in vivo. Int. J. Antimicrob. Agents 2019, 53, 362–369. [Google Scholar]
- Echeverría, E.D.; Martin, O.C.; Navarrete, R.M.E.; De Antonio, C.M.; Ferrández, O.; Horcajada, J.P.; Grau, S. Azithromycin in the treatment of COVID-19: A review. Expert Rev. Anti-Infect. 2020, 19, 147–163. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, Y.; Xiao, Y.; Gao, Q.; Gao, L.; Zhang, W.; Xin, X.; Chen, K.; Srivastava, U.; Ginjupalli, V.; et al. Arrhythmogenic mechanisms of interleukin-6 combination with hydroxychloroquine and azithromycin in inflammatory diseases. Sci. Rep. 2022, 12, 1075. [Google Scholar] [CrossRef] [PubMed]
- Montnach, J.; Baró, I.; Charpentier, F.; De Waard, M.; Loussouarn, G. Modelling sudden cardiac death risks factors in patients with coronavirus disease of 2019: The hydroxychloroquine and azithromycin case. Europace 2021, 23, 1124–1136. [Google Scholar] [CrossRef] [PubMed]
- Esmail, Z.N.; Loewen, P.S. Losartan as an alternative to ACE inhibitors in patients with renal dysfunction. Ann. Pharm. 1998, 32, 1096–1098. [Google Scholar] [CrossRef]
- Weisblum, Y.; Schmidt, F.; Zhang, F.; DaSilva, J.; Poston, D.; Lorenzi, J.C.; Muecksch, F.; Rutkowska, M.; Hoffmann, H.H.; Michailidis, E.; et al. Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants. eLife 2020, 9, e61312. [Google Scholar] [CrossRef]
- Kim, M.D.; Baumlin, N.; Yoshida, M.; Polineni, D.; Salathe, S.F.; David, J.K.; Peloquin, C.A.; Wanner, A.; Dennis, J.S.; Sailland, J.; et al. Losartan Rescues Inflammation-related Mucociliary Dysfunction in Relevant Models of Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 2020, 201, 313–324. [Google Scholar] [CrossRef]
- Su, M.; Shi, D.; Xing, X.; Qi, S.; Yang, D.; Zhang, J.; Han, Y.; Zhu, Q.; Sun, H.; Wang, X.; et al. Coronavirus Porcine Epidemic Diarrhea Virus Nucleocapsid Protein Interacts with p53 To Induce Cell Cycle Arrest in S-Phase and Promotes Viral Replication. J. Virol. 2021, 95, e18721. [Google Scholar] [CrossRef]
- Tian, Y.; Jiang, W.; Gao, N.; Zhang, J.; Chen, W.; Fan, D.; Zhou, D.; An, J. Inhibitory effects of glutathione on dengue virus production. Biochem. Biophys. Res. Commun. 2010, 397, 420–424. [Google Scholar] [CrossRef]
- Basu, M.; Courtney, S.C.; Brinton, M.A. Arsenite-induced stress granule formation is inhibited by elevated levels of reduced glutathione in West Nile virus-infected cells. PLoS Pathog. 2017, 13, e1006240. [Google Scholar] [CrossRef]
- Laughhunn, A.; Huang, Y.S.; Vanlandingham, D.L.; Lanteri, M.C.; Stassinopoulos, A. Inactivation of chikungunya virus in blood components treated with amotosalen/ultraviolet A light or amustaline/glutathione. Transfusion 2018, 58, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Shanmugaraj, B.; Siriwattananon, K.; Wangkanont, K.; Phoolcharoen, W. Perspectives on monoclonal antibody therapy as potential therapeutic intervention for Coronavirus disease-19 (COVID-19). Asian Pac. J. Allergy Immunol. 2020, 38, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Sinha, I.; Calcagnotto, A.; Trushin, N.; Haley, J.S.; Schell, T.D.; Richie, J.J. Oral supplementation with liposomal glutathione elevates body stores of glutathione and markers of immune function. Eur. J. Clin. Nutr. 2018, 72, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Minooei, F.; Fried, J.R.; Fuqua, J.L.; Palmer, K.E.; Steinbach-Rankins, J.M. In vitro Study on Synergistic Interactions Between Free and Encapsulated Q-Griffithsin and Antiretrovirals Against HIV-1 Infection. Int. J. Nanomed. 2021, 16, 1189–1206. [Google Scholar] [CrossRef]
- Ziolkowska, N.E.; O’Keefe, B.R.; Mori, T.; Zhu, C.; Giomarelli, B.; Vojdani, F.; Palmer, K.E.; McMahon, J.B.; Wlodawer, A. Domain-swapped structure of the potent antiviral protein griffithsin and its mode of carbohydrate binding. Structure 2006, 14, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Millet, J.K.; Seron, K.; Labitt, R.N.; Danneels, A.; Palmer, K.E.; Whittaker, G.R.; Dubuisson, J.; Belouzard, S. Middle East respiratory syndrome coronavirus infection is inhibited by griffithsin. Antivir. Res. 2016, 133, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Takebe, Y.; Saucedo, C.J.; Lund, G.; Uenishi, R.; Hase, S.; Tsuchiura, T.; Kneteman, N.; Ramessar, K.; Tyrrell, D.L.; Shirakura, M.; et al. Antiviral lectins from red and blue-green algae show potent in vitro and in vivo activity against hepatitis C virus. PLoS ONE 2013, 8, e64449. [Google Scholar] [CrossRef] [PubMed]
- Nixon, B.; Stefanidou, M.; Mesquita, P.M.; Fakioglu, E.; Segarra, T.; Rohan, L.; Halford, W.; Palmer, K.E.; Herold, B.C. Griffithsin protects mice from genital herpes by preventing cell-to-cell spread. J. Virol. 2013, 87, 6257–6269. [Google Scholar] [CrossRef]
- Hassan, Z.A.I.; Chen, L.; Fengjuan, W.; Xiang, M. Griffithsin binds to the glycosylated proteins (E and prM) of Japanese encephalitis virus and inhibit its infection. Virus Res. 2016, 215, 50–54. [Google Scholar]
- Ishag, H.Z.; Li, C.; Huang, L.; Sun, M.X.; Wang, F.; Ni, B.; Malik, T.; Chen, P.Y.; Mao, X. Griffithsin inhibits Japanese encephalitis virus infection in vitro and in vivo. Arch. Virol. 2013, 158, 349–358. [Google Scholar] [CrossRef]
- Levendosky, K.; Mizenina, O.; Martinelli, E.; Jean-Pierre, N.; Kizima, L.; Rodriguez, A.; Kleinbeck, K.; Bonnaire, T.; Robbiani, M.; Zydowsky, T.M.; et al. Griffithsin and Carrageenan Combination To Target Herpes Simplex Virus 2 and Human Papillomavirus. Antimicrob. Agents Chemother. 2015, 59, 7290–7298. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Chandran, D.; Singh, D.D.; Vijayan, M. Multiplicity of carbohydrate-binding sites in beta-prism fold lectins: Occurrence and possible evolutionary implications. J. Biosci. 2007, 32, 1089–1110. [Google Scholar] [CrossRef] [PubMed]
- Fuqua, J.L.; Wanga, V.; Palmer, K.E. Improving the large scale purification of the HIV microbicide, griffithsin. BMC Biotechnol. 2015, 15, 12. [Google Scholar] [CrossRef]
- Li, L.; Yu, X.; Zhang, H.; Cheng, H.; Hou, L.; Zheng, Q.; Hou, J. In vitro antiviral activity of Griffithsin against porcine epidemic diarrhea virus. Virus Genes 2019, 55, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Tsan, P.; Volpon, L.; Besson, F.; Lancelin, J.M. Structure and dynamics of surfactin studied by NMR in micellar media. J. Am. Chem. Soc. 2007, 129, 1968–1977. [Google Scholar] [CrossRef] [PubMed]
- Dirk, V.; Muhsin, Ö.; Joachim, V.; Roza, M.K.; Georg, P. Mechanism of Inactivation of Enveloped Viruses by the Biosurfactant Surfactin from Bacillus subtilis. Biologicals 1997, 25, 289–297. [Google Scholar]
- Wang, X.; Hu, W.; Zhu, L.; Yang, Q. Bacillus subtilis and surfactin inhibit the transmissible gastroenteritis virus from entering the intestinal epithelial cells. Biosci. Rep. 2017, 37, BSR20170082. [Google Scholar] [CrossRef]
- Lvfeng, Y.; Shuai, Z.; Yongheng, W.; Yuchen, L.; Xiaoqing, W.; Qian, Y. Surfactin Inhibits Membrane Fusion during Invasion of Epithelial Cells by Enveloped Viruses. J. Virol. 2018, 92, e00809-18. [Google Scholar]
- Yuan, L.; Zhang, S.; Peng, J.; Li, Y.; Yang, Q. Synthetic surfactin analogues have improved anti-PEDV properties. PLoS ONE 2019, 14, e215227. [Google Scholar] [CrossRef]
- Knolker, H.J.; Reddy, K.R. Isolation and synthesis of biologically active carbazole alkaloids. Chem. Rev. 2002, 102, 4303–4427. [Google Scholar] [CrossRef]
- Schmidt, A.W.; Reddy, K.R.; Knolker, H.J. Occurrence, biogenesis, and synthesis of biologically active carbazole alkaloids. Chem. Rev. 2012, 112, 3193–3328. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Mizutani, T.C.; Nomura, N.; Takakura, T.; Kitamura, Y.; Miura, H.; Nishizawa, M.; Tatsumi, M.; Yamamoto, N.; Sugiura, W. A novel small molecular weight compound with a carbazole structure that demonstrates potent human immunodeficiency virus type-1 integrase inhibitory activity. Antivir. Chem. Chemother. 2005, 16, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Wang, X.Y.; Zhou, Y.P.; Lu, R.; Chen, C.H.; Zhang, M.H.; Cheng, Y.Y.; Morris-Natschke, S.L.; Lee, K.H.; Wang, Y.S. Carbazole Alkaloids from Clausena anisum-olens: Isolation, Characterization, and Anti-HIV Evaluation. Molecules 2019, 25, 99. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, A.; Kremb, S.; Bader, T.M.; Helfer, M.; Schmitt-Kopplin, P.; Gerwick, W.H.; Brack-Werner, R.; Voolstra, C.R. Alkaloids from the Sponge Stylissa carteri Present Prospective Scaffolds for the Inhibition of Human Immunodeficiency Virus 1 (HIV-1). Mar. Drugs 2016, 14, 28. [Google Scholar] [CrossRef] [PubMed]
- Kang, I.J.; Wang, L.W.; Hsu, S.J.; Lee, C.C.; Lee, Y.C.; Wu, Y.S.; Yueh, A.; Wang, J.C.; Hsu, T.A.; Chao, Y.S.; et al. Design and efficient synthesis of novel arylthiourea derivatives as potent hepatitis C virus inhibitors. Bioorg. Med. Chem. Lett. 2009, 19, 6063–6068. [Google Scholar] [CrossRef]
- Pan, Q.M.; Li, Y.H.; Hua, J.; Huang, F.P.; Wang, H.S.; Liang, D. Antiviral Matrine-Type Alkaloids from the Rhizomes of Sophora tonkinensis. J. Nat. Prod. 2015, 78, 1683–1688. [Google Scholar] [CrossRef]
- Ren, Z.; Zhang, C.H.; Wang, L.J.; Cui, Y.X.; Qi, R.B.; Yang, C.R.; Zhang, Y.J.; Wei, X.Y.; Lu, D.X.; Wang, Y.F. In vitro anti-viral activity of the total alkaloids from Tripterygium hypoglaucum against herpes simplex virus type 1. Virol. Sin. 2010, 25, 107–114. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, J.; Wei, X.; Hua, H.; Hu, R.; Ding, N.; Zhang, J.; Song, D.; Ye, Y.; Tang, Y.; et al. Antiviral Activities of Carbazole Derivatives against Porcine Epidemic Diarrhea Virus In Vitro. Viruses 2021, 13, 2527. [Google Scholar] [CrossRef]
- Chen, J.; Jin, L.; Yan, M.; Yang, Z.; Wang, H.; Geng, S.; Gong, Z.; Liu, G. Serum Exosomes from Newborn Piglets Restrict Porcine Epidemic Diarrhea Virus Infection. J. Proteome Res. 2019, 18, 1939–1947. [Google Scholar] [CrossRef]
- Pyrc, K.; Bosch, B.J.; Berkhout, B.; Jebbink, M.F.; Dijkman, R.; Rottier, P.; van der Hoek, L. Inhibition of human coronavirus NL63 infection at early stages of the replication cycle. Antimicrob. Agents Chemother. 2006, 50, 2000–2008. [Google Scholar] [CrossRef]
- Kim, S.M.; Park, J.H.; Lee, K.N.; Kim, S.K.; Ko, Y.J.; Lee, H.S.; Cho, I.S. Enhanced inhibition of foot-and-mouth disease virus by combinations of porcine interferon-alpha and antiviral agents. Antivir. Res. 2012, 96, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Seguin, S.P.; Ireland, A.W.; Gupta, T.; Wright, C.M.; Miyata, Y.; Wipf, P.; Pipas, J.M.; Gestwicki, J.E.; Brodsky, J.L. A screen for modulators of large T antigen’s ATPase activity uncovers novel inhibitors of Simian Virus 40 and BK virus replication. Antivir. Res. 2012, 96, 70–81. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cao, J.; Forrest, J.C.; Zhang, X. A screen of the NIH Clinical Collection small molecule library identifies potential anti-coronavirus drugs. Antivir. Res. 2015, 114, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wessels, I.; Maywald, M.; Rink, L. Zinc as a Gatekeeper of Immune Function. Nutrients 2017, 9, 1286. [Google Scholar] [CrossRef]
- Mishra, P.K.; Mishra, H.; Ekielski, A.; Talegaonkar, S.; Vaidya, B. Zinc oxide nanoparticles: A promising nanomaterial for biomedical applications. Drug Discov. Today 2017, 22, 1825–1834. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, T.; Li, S.; Meng, Y.; Tan, Z.; Wu, M.; Yi, D.; Wang, L.; Zhao, D.; Hou, Y. Protective Effect of Zinc Oxide and Its Association with Neutrophil Degranulation in Piglets Infected with Porcine Epidemic Diarrhea Virus. Oxid. Med. Cell. Longev. 2021, 2021, 3055810. [Google Scholar] [CrossRef]
- Son, J.; Huang, S.; Zeng, Q.; Bricker, T.L.; Case, J.B.; Zhou, J.; Zang, R.; Liu, Z.; Chang, X.; Darling, T.L.; et al. JIB-04 Has Broad-Spectrum Antiviral Activity and Inhibits SARS-CoV-2 Replication and Coronavirus Pathogenesis. mBio 2022, 13, e337721. [Google Scholar] [CrossRef]
- Wang, R.; Chan, J.F.; Wang, S.; Li, H.; Zhao, J.; Ip, T.K.; Zuo, Z.; Yuen, K.Y.; Yuan, S.; Sun, H. Orally administered bismuth drug together with N-acetyl cysteine as a broad-spectrum anti-coronavirus cocktail therapy. Chem. Sci. 2022, 13, 2238–2248. [Google Scholar] [CrossRef]
- Wang, E.D.; Rahgozar, P. The Pathogenesis and Treatment of the Stiff Finger. Clin. Plast. Surg. 2019, 46, 339–345. [Google Scholar] [CrossRef]
- Fu, Y.; Cheng, Y.; Wu, Y. Understanding SARS-CoV-2-Mediated Inflammatory Responses: From Mechanisms to Potential Therapeutic Tools. Virol. Sin. 2020, 35, 266–271. [Google Scholar] [CrossRef]
- Yang, Y.; Cui, X.; Wei, H.; Guo, C.; Zhang, Y. Potential Anti-Coronavirus Agents and the Pharmacologic Mechanisms. Drug Des. Dev. Ther. 2021, 15, 1213–1223. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; He, M.; Wong, K.; Lum, C.T.; Poon, L.L.M.; Peng, Y.; Guan, Y.; Lin, M.C.M.; Kung, H. Potent inhibition of SARS-associated coronavirus (SCOV) infection and replication by type I interferons (IFN-alpha/beta) but not by type II interferon (IFN-gamma). J. Interferon Cytokine Res. 2004, 24, 388–390. [Google Scholar] [CrossRef] [PubMed]
- Cinatl, J.; Morgenstern, B.; Bauer, G.; Chandra, P.; Rabenau, H.; Doerr, H.W. Treatment of SARS with human interferons. Lancet 2003, 362, 293–294. [Google Scholar] [CrossRef]
- Monk, P.D.; Marsden, R.J.; Tear, V.J.; Brookes, J.; Batten, T.N.; Mankowski, M.; Gabbay, F.J.; Davies, D.E.; Holgate, S.T.; Ho, L.P.; et al. Safety and efficacy of inhaled nebulised interferon beta-1a (SNG001) for treatment of SARS-CoV-2 infection: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Respir. Med. 2021, 9, 196–206. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, S.; Li, Y.; Zhu, W.; Luo, Z.; Wu, K.; Li, X.; Fang, Y.; Qin, Y.; Chen, W.; Li, Z.; et al. The Advances of Broad-Spectrum and Hot Anti-Coronavirus Drugs. Microorganisms 2022, 10, 1294. https://doi.org/10.3390/microorganisms10071294
Zeng S, Li Y, Zhu W, Luo Z, Wu K, Li X, Fang Y, Qin Y, Chen W, Li Z, et al. The Advances of Broad-Spectrum and Hot Anti-Coronavirus Drugs. Microorganisms. 2022; 10(7):1294. https://doi.org/10.3390/microorganisms10071294
Chicago/Turabian StyleZeng, Sen, Yuwan Li, Wenhui Zhu, Zipeng Luo, Keke Wu, Xiaowen Li, Yiqi Fang, Yuwei Qin, Wenxian Chen, Zhaoyao Li, and et al. 2022. "The Advances of Broad-Spectrum and Hot Anti-Coronavirus Drugs" Microorganisms 10, no. 7: 1294. https://doi.org/10.3390/microorganisms10071294
APA StyleZeng, S., Li, Y., Zhu, W., Luo, Z., Wu, K., Li, X., Fang, Y., Qin, Y., Chen, W., Li, Z., Zou, L., Liu, X., Yi, L., & Fan, S. (2022). The Advances of Broad-Spectrum and Hot Anti-Coronavirus Drugs. Microorganisms, 10(7), 1294. https://doi.org/10.3390/microorganisms10071294





