The Current Knowledge on the Pathogenesis of Tissue and Medical Device-Related Biofilm Infections
Abstract
:1. Introduction
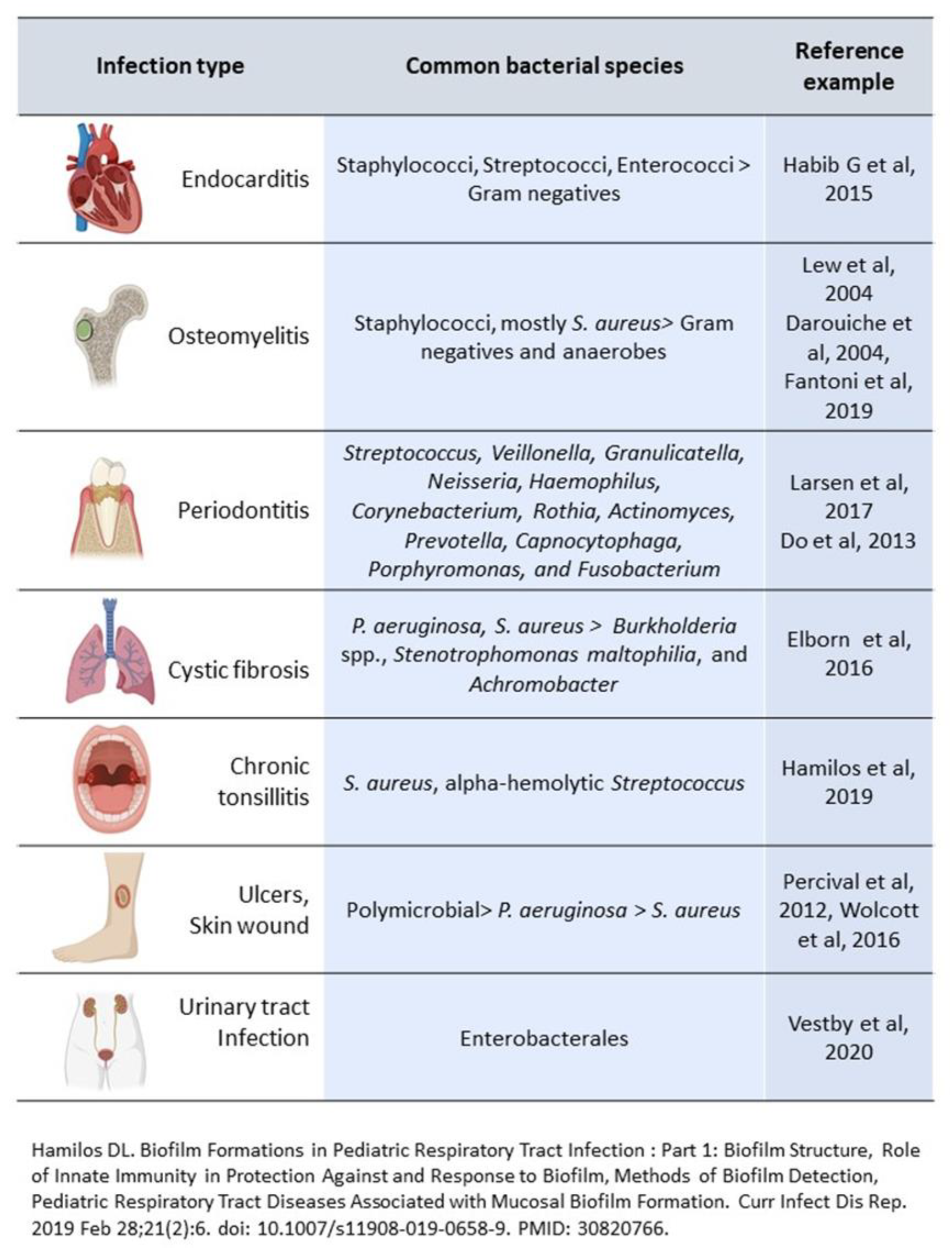
2. Biofilm and Chronic Infections in Tissues
2.1. Pathogenesis
2.2. Infective Endocarditis
2.3. Chronic Osteomyelitis
2.4. Chronic Nonhealing Wounds
2.5. Cystic Fibrosis
2.6. Dental Infections
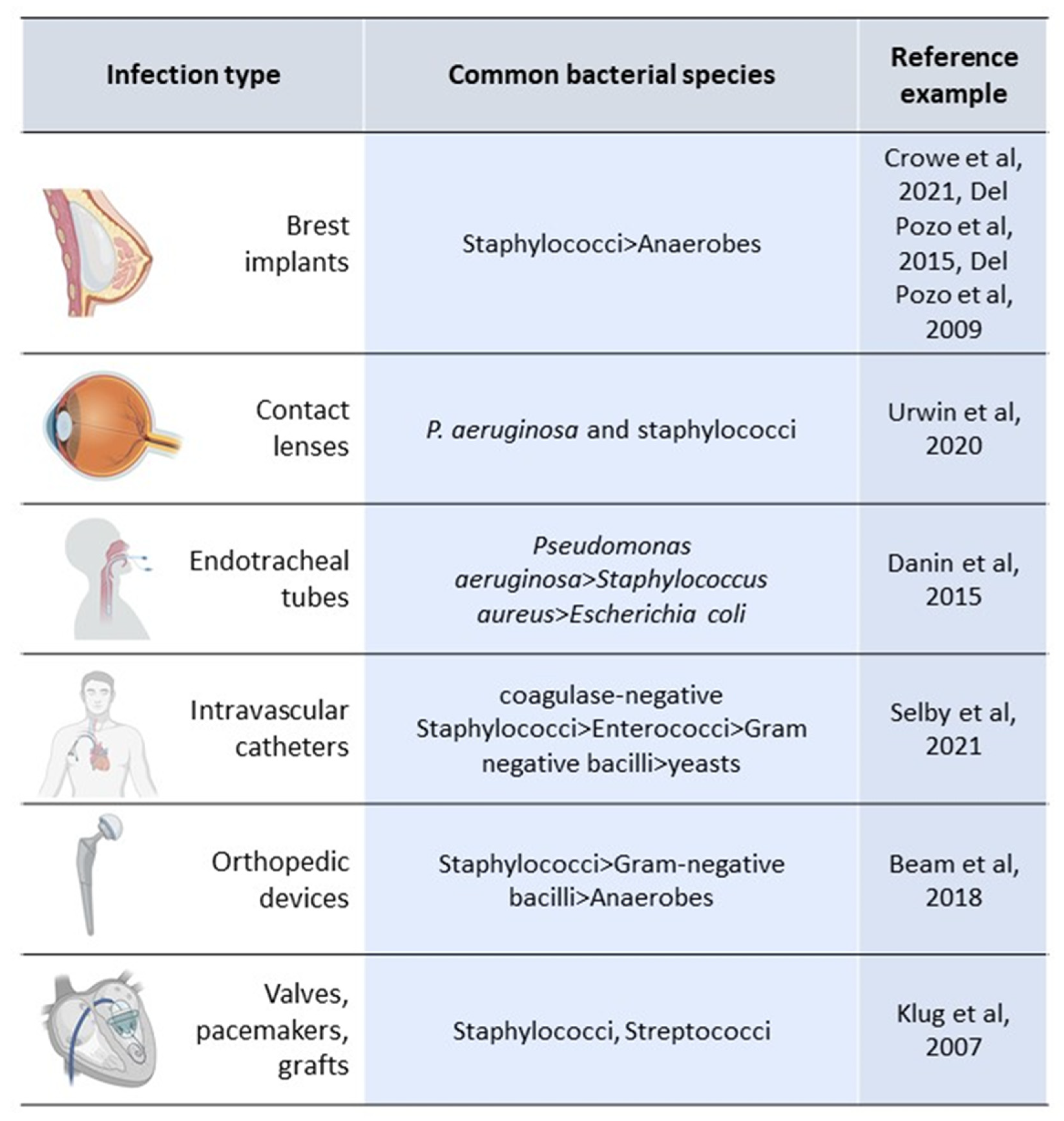
3. Biofilm and Device-Related Infections
3.1. Catheter-Related Bloodstream Infections (C-RBSI)
3.2. Ventilator-Associated Pneumonia (VAP)
3.3. Prosthetic Joint Infections (PJIs)
3.4. Prosthetic Heart Valve Infection
3.5. Breast Implant Infections (BII)
3.6. Contact Lens Infection
3.7. New Approaches for the Prevention and Management of BRDI
4. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Inform Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival Mechanisms of Clinically Relevant Microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [Green Version]
- Ciofu, O.; Tolker-Nielsen, T.; Jensen, P.; Wang, H.; Høiby, N. Antimicrobial resistance, respiratory tract infections and role of biofilms in lung infections in cystic fibrosis patients. Adv. Drug Deliv. Rev. 2015, 85, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, E.G.; Cavallo, I.; Sivori, F.; Marchesi, F.; Prignano, G.; Pimpinelli, F.; Sperduti, I.; Pelagalli, L.; Di Salvo, F.; Celesti, I.; et al. Biofilm Production by Carbapenem-Resistant Klebsiella pneumoniae Significantly Increases the Risk of Death in Oncological Patients. Front. Cell. Infect. Microbiol. 2020, 10, 561741. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, E.G.; Marchesi, F.; Cavallo, I.; Toma, L.; Sivori, F.; Papa, E.; Spadea, A.; Cafarella, G.; Terrenato, I.; Prignano, G.; et al. The Impact of Bacterial Biofilms on End-Organ Disease and Mortality in Patients with Hematologic Malignancies Developing a Bloodstream Infection. Microbiol. Spectr. 2021, 9, e0055021. [Google Scholar] [CrossRef] [PubMed]
- Tascini, C.; Sozio, E.; Corte, L.; Sbrana, F.; Scarparo, C.; Ripoli, A.; Bertolino, G.; Merelli, M.; Tagliaferri, E.; Corcione, A.; et al. The role of biofilm forming on mortality in patients with candidemia: A study derived from real world data. Infect. Dis. 2017, 50, 214–219. [Google Scholar] [CrossRef]
- Agarwal, A.; Kelkar, A.; Agarwal, A.G.; Jayaswal, D.; Schultz, C.; Jayaswal, A.; Goel, V.K.; Agarwal, A.K.; Gidvani, S. Implant Retention or Removal for Management of Surgical Site Infection after Spinal Surgery. Glob. Spine J. 2020, 10, 640–666. [Google Scholar] [CrossRef] [Green Version]
- Høiby, N.; Bjarnsholt, T.; Moser, C.; Bassi, G.L.; Coenye, T.; Donelli, G.; Hall-Stoodley, L.; Hola, V.; Imbert, C.; Kirketerp-Moller, K.; et al. ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clin. Microbiol. Infect. 2015, 21 (Suppl. S1), S1–S25. [Google Scholar] [CrossRef] [Green Version]
- Lebeaux, D.; Ghigo, J.-M.; Beloin, C. Biofilm-Related Infections: Bridging the Gap between Clinical Management and Fundamental Aspects of Recalcitrance toward Antibiotics. Microbiol. Mol. Biol. Rev. 2014, 78, 510–543. [Google Scholar] [CrossRef] [Green Version]
- Sharma, D.; Misba, L.; Khan, A.U. Antibiotics versus biofilm: An emerging battleground in microbial communities. Antimicrob. Resist. Infect. Control 2019, 8, 76. [Google Scholar] [CrossRef]
- Karygianni, L.; Ren, Z.; Koo, H. Thurnheer T. Biofilm Matrixome: Extracellular Components in Structured Microbial Communities. Trends Microbiol. 2020, 28, 668–681. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Serra, D.O.; Richter, A.M.; Hengge, R.; Stahlhut, S.G.; Chattopadhyay, S.; Kisiela, D.I.; Hvidtfeldt, K.; Clegg, S.; Struve, C.; Sokurenko, E.V.; et al. Cellulose as an Architectural Element in Spatially Structured Escherichia coli Biofilms. J. Bacteriol. 2013, 195, 5540–5554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewenza, S. Extracellular DNA-induced antimicrobial peptide resistance mechanisms in Pseudomonas aeruginosa. Front. Microbiol. 2013, 4, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okshevsky, M.; Regina, V.R.; Meyer, R.L. Extracellular DNA as a target for biofilm control. Curr. Opin. Biotechnol. 2015, 33, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Sivori, F.; Cavallo, I.; Kovacs, D.; Guembe, M.; Sperduti, I.; Truglio, M.; Pasqua, M.; Prignano, G.; Mastrofrancesco, A.; Toma, L.; et al. Role of Extracellular DNA in Dalbavancin Activity against Methicillin-Resistant Staphylococcus aureus (MRSA) Biofilms in Patients with Skin and Soft Tissue Infections. Microbiol. Spectr. 2022, 10, e0035122. [Google Scholar] [CrossRef] [PubMed]
- Wilton, M.; Charron-Mazenod, L.; Moore, R.; Lewenza, S. Extracellular DNA Acidifies Biofilms and Induces Aminoglycoside Resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2016, 60, 544–553. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, S.; Bhargava, A. Biofilms and human health. Biotechnol. Lett. 2016, 38, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Hall-Stoodley, L.; Stoodley, P. Evolving concepts in biofilm infections. Cell. Microbiol. 2009, 11, 1034–1043. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S. Antimicrobial Tolerance in Biofilms. Microbiol. Spectr. 2015, 3, 3. [Google Scholar] [CrossRef] [Green Version]
- Paharik, A.E.; Horswill, A.R. The Staphylococcal Biofilm: Adhesins, Regulation, and Host Response. Microbiol. Spectr. 2016, 4, 529–566. [Google Scholar] [CrossRef] [Green Version]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial Biofilm and its Role in the Pathogenesis of Disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.P.; Del Zotti, F. 2015 ESC Guidelines for the management of infective endocarditis. The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). G. Ital. Cardiol. 2016, 17, 277–319. [Google Scholar]
- Darouiche, R.O. Treatment of infections associated with surgical implants. N. Engl. J. Med. 2004, 350, 1422–1429. [Google Scholar] [CrossRef] [PubMed]
- Lew, D.P.; Waldvogel, F.A. Osteomyelitis. Lancet 2004, 364, 369–379. [Google Scholar] [CrossRef]
- Fantoni, M.; Taccari, F.; Giovannenze, F. Systemic antibiotic treatment of chronic osteomyelitis in adults. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 258–270. [Google Scholar]
- Percival, S.L.; Hill, K.E.; Williams, D.W.; Hooper, S.J.; Thomas, D.W.; Costerton, J.W. A review of the scientific evidence for biofilms in wounds. Wound Repair Regen. 2012, 20, 647–657. [Google Scholar] [CrossRef]
- Wolcott, R.D.; Hanson, J.D.; Rees, E.J.; Koenig, L.D.; Phillips, C.D.; Wolcott, R.A.; Cox, S.B.; White, J.S. Analysis of the chronic wound microbiota of 2963 patients by 16S rDNA pyrosequencing. Wound Repair Regen. 2016, 24, 163–174. [Google Scholar] [CrossRef]
- Elborn, J.S. Cystic fibrosis. Lancet 2016, 388, 2519–2531. [Google Scholar] [CrossRef]
- Larsen, T.; Fiehn, N.E. Dental biofilm infections—An update. APMIS 2017, 125, 376–384. [Google Scholar] [CrossRef]
- Hamilos, D.L. Biofilm Formations in Pediatric Respiratory Tract Infection: Part 1: Biofilm Structure, Role of Innate Immunity in Protection Against and Response to Biofilm, Methods of Biofilm Detection, Pediatric Respiratory Tract Diseases Associated with Mucosal Biofilm Formation. Curr. Infect. Dis. Rep. 2019, 21, 6. [Google Scholar]
- Libraty, D.H.; Patkar, C.; Torres, B. Staphylococcus aureus Reactivation Osteomyelitis after 75 Years. N. Engl. J. Med. 2012, 366, 481–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliva, A.; Stefani, S.; Venditti, M.; Di Domenico, E.G. Biofilm-Related Infections in Gram-Positive Bacteria and the Potential Role of the Long-Acting Agent Dalbavancin. Front. Microbiol. 2021, 12, 749685. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial Biofilms: A Common Cause of Persistent Infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [Green Version]
- Marrie, T.J.; Cooper, J.H.; Costerton, J.W. Ultrastructure of cardiac bacterial vegetations on native valves with emphasis on alterations in bacterial morphology following antibiotic treatment. Can. J. Cardiol. 1987, 3, 275–280. [Google Scholar] [PubMed]
- Holland, T.L.; Baddour, L.M.; Bayer, A.S.; Hoen, B.; Miro, J.M.; Fowler, V.G., Jr. Infective endocarditis. Nat. Rev. Dis. Primers 2016, 2, 16059. [Google Scholar] [CrossRef] [PubMed]
- Brady, R.A.; Leid, J.G.; Calhoun, J.H.; Costerton, J.W.; Shirtliff, M.E. Osteomyelitis and the role of biofilms in chronic infection. FEMS Immunol. Med. Microbiol. 2008, 52, 13–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haesler, E.; Swanson, T.; Ousey, K.; Carville, K. Clinical indicators of wound infection and biofilm: Reaching international consensus. J. Wound Care 2019, 28, s4–s12. [Google Scholar] [CrossRef]
- Attinger, C.; Wolcott, R. Clinically Addressing Biofilm in Chronic Wounds. Adv. Wound Care 2012, 1, 127–132. [Google Scholar] [CrossRef] [Green Version]
- Cantin, A.M.; Hartl, D.; Konstan, M.W.; Chmiel, J.F. Inflammation in cystic fibrosis lung disease: Pathogenesis and therapy. J. Cyst. Fibros. 2015, 14, 419–430. [Google Scholar] [CrossRef] [Green Version]
- Worlitzsch, D.; Tarran, R.; Ulrich, M.; Schwab, U.; Cekici, A.; Meyer, K.C.; Birrer, P.; Bellon, G.; Berger, J.; Weiss, T.; et al. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J. Clin. Investig. 2002, 109, 317–325. [Google Scholar] [CrossRef]
- Yoon, S.S.; Hennigan, R.F.; Hilliard, G.M.; Ochsner, U.A.; Parvatiyar, K.; Kamani, M.C.; Allen, H.L.; DeKievit, T.R.; Gardner, P.R.; Schwab, U.; et al. Pseudomonas aeruginosa Anaerobic Respiration in Biofilms: Relationships to Cystic Fibrosis Pathogenesis. Dev. Cell 2002, 3, 593–603. [Google Scholar] [CrossRef] [Green Version]
- Ciofu, O.; Tolker-Nielsen, T. Tolerance and Resistance of Pseudomonas aeruginosa Biofilms to Antimicrobial Agents-How P. aeruginosa Can Escape Antibiotics. Front. Microbiol. 2019, 10, 913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welp, A.; Bomberger, J.M. Bacterial Community Interactions During Chronic Respiratory Disease. Front. Cell. Infect. Microbiol. 2020, 10, 213. [Google Scholar] [CrossRef] [PubMed]
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.; Yu, W.H.; Lakshmanan, A.; Wade, W.G. The human oral microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar] [CrossRef] [Green Version]
- Do, T.; Devine, D.; Marsh, P.D. Oral biofilms: Molecular analysis, challenges, and future prospects in dental diagnostics. Clin. Cosmet. Investig. Dent. 2013, 5, 11–19. [Google Scholar]
- Olsen, I.; van Winkelhoff, A.J. Acute focal infections of dental origin. Periodontology 2000 2014, 65, 178–189. [Google Scholar] [CrossRef]
- Vogkou, C.T.; Vlachogiannis, N.I.; Palaiodimos, L.; Kousoulis, A.A. The causative agents in infective endocarditis: A systematic review comprising 33,214 cases. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 1227–1245. [Google Scholar] [CrossRef]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. JCMA 2018, 81, 7–11. [Google Scholar] [CrossRef]
- Saeed, K.; McLaren, A.C.; Schwarz, E.M.; Antoci, V.; Arnold, W.V.; Chen, A.F.; Clauss, M.; Esteban, J.; Gant, V.; Hendershot, E.; et al. 2018 international consensus meeting on musculoskeletal infection: Summary from the biofilm workgroup and consensus on biofilm related musculoskeletal infections. J. Orthop. Res. 2019, 37, 1007–1017. [Google Scholar] [CrossRef]
- Song, F.; Koo, H.; Ren, D. Effects of Material Properties on Bacterial Adhesion and Biofilm Formation. J. Dent. Res. 2015, 94, 1027–1034. [Google Scholar] [CrossRef]
- Pal, Z.; Urban, E.; Dosa, E.; Nagy, E. Biofilm formation on intrauterine devices in relation to duration of use. J. Med. Microbiol. 2005, 54, 1199–1203. [Google Scholar] [CrossRef] [PubMed]
- Khelissa, S.O.; Jama, C.; Abdallah, M.; Boukherroub, R.; Faille, C.; Chihib, N.E. Effect of incubation duration, growth temperature, and abiotic surface type on cell surface properties, adhesion and pathogenicity of biofilm-detached Staphylococcus aureus cells. AMB Express 2017, 7, 191. [Google Scholar] [CrossRef] [PubMed]
- Bjarnsholt, T. The role of bacterial biofilms in chronic infections. APMIS 2013, 121, 1–58. [Google Scholar] [CrossRef] [PubMed]
- Rumbaugh, K.P.; Trivedi, U.; Watters, C.; Burton-Chellew, M.N.; Diggle, S.P.; West, S.A. Kin selection, quorum sensing and virulence in pathogenic bacteria. Proc. Biol. Sci. 2012, 279, 3584–3588. [Google Scholar] [CrossRef]
- Selby, L.M.; Rupp, M.E.; Cawcutt, K.A. Prevention of Central-Line Associated Bloodstream Infections: 2021 Update. Infect. Dis. Clin. N. Am. 2021, 35, 841–856. [Google Scholar] [CrossRef]
- Danin, P.-E.; Girou, E.; Legrand, P.; Louis, B.; Fodil, R.; Christov, C.; Devaquet, J.; Isabey, D.; Brochard, L. Description and Microbiology of Endotracheal Tube Biofilm in Mechanically Ventilated Subjects. Respir. Care 2014, 60, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Beam, E.; Osmon, D. Prosthetic Joint Infection Update. Infect. Dis. Clin. N. Am. 2018, 32, 843–859. [Google Scholar] [CrossRef]
- Klug, D.; Balde, M.; Pavin, D.; Hidden-Lucet, F.; Clementy, J.; Sadoul, N.; Rey, J.L.; Lande, G.; Lazarus, A.; Victor, J.; et al. Risk factors related to infections of implanted pacemakers and cardioverter-defibrillators: Results of a large prospective study. Circulation 2007, 116, 1349–1355. [Google Scholar] [CrossRef]
- Del Pozo, J.L.; Tran, N.V.; Petty, P.M.; Johnson, C.H.; Walsh, M.F.; Bite, U.; Clay, R.P.; Mandrekar, J.N.; Piper, K.E.; Steckelberg, J.M.; et al. Pilot study of association of bacteria on breast implants with capsular contracture. J. Clin. Microbiol. 2009, 47, 1333–1337. [Google Scholar] [CrossRef] [Green Version]
- Crowe, S.A.; Simister, R.L.; Spence, J.S.; Kenward, P.A.; Van Slyke, A.C.; Lennox, P.; Carr, N. Microbial community compositions in breast implant biofilms associated with contracted capsules. PLoS ONE 2021, 16, e0249261. [Google Scholar] [CrossRef]
- del Pozo, J.L.; Auba, C. Role of biofilms in breast implant associated infections and capsular contracture. Adv. Exp. Med. Biol. 2015, 831, 53–67. [Google Scholar] [PubMed]
- Urwin, L.; Okurowska, K.; Crowther, G.; Roy, S.; Garg, P.; Karunakaran, E.; MacNeil, S.; Partridge, L.J.; Green, L.R.; Monk, P.N. Corneal Infection Models: Tools to Investigate the Role of Biofilms in Bacterial Keratitis. Cells 2020, 9, 2450. [Google Scholar] [CrossRef] [PubMed]
- Chaves, F.; Garnacho-Montero, J.; Del Pozo, J.L.; Bouza, E.; Capdevila, J.A.; de Cueto, M.; Dominguez, M.A.; Esteban, J.; Fernandez-Hidalgo, N.; Sampedro, M.F.; et al. Diagnosis and treatment of catheter-related bloodstream infection: Clinical guidelines of the Spanish Society of Infectious Diseases and Clinical Microbiology and (SEIMC) and the Spanish Society of Spanish Society of Intensive and Critical Care Medicine and Coronary Units (SEMICYUC). Med. Intensiva 2018, 42, 5–36. [Google Scholar] [PubMed]
- Timsit, J.-F.; Rupp, M.; Bouza, E.; Chopra, V.; Kärpänen, T.; Laupland, K.; Lisboa, T.; Mermel, L.; Mimoz, O.; Parienti, J.-J.; et al. A state of the art review on optimal practices to prevent, recognize, and manage complications associated with intravascular devices in the critically ill. Intensiv. Care Med. 2018, 44, 742–759. [Google Scholar] [CrossRef] [Green Version]
- Zampieri, F.G.; De Oliveira, N.E.; Nassar, A.P., Jr.; Manoel, A.L.D.O.; Grion, C.; Lacerda, F.; Maia, I.; Thompson, M.; Giancursi, T.S.; Martins, P.D.A.; et al. Bundle of Coated Devices to Reduce Nosocomial Infections in the Intensive Care Unit. CRITIC Pilot Randomized Controlled Trial. Ann. Am. Thorac. Soc. 2020, 17, 1257–1263. [Google Scholar] [CrossRef]
- Longo, R.; Llorens, M.; Goetz, C.; Platini, C.; Eid, N.; Sellies, J.; Ouamara, N.; Quétin, P. Taurolidine/Citrate Lock Therapy for Primary Prevention of Catheter-Related Infections in Cancer Patients: Results of a Prospective, Randomized, Phase IV Trial (ATAPAC). Oncology 2017, 93, 99–105. [Google Scholar] [CrossRef]
- Rubia, M.; Cordero, A.; Pérez-Granda, M.J.; Cercenado, E.; Pascual, C.; Muñoz, P.; Guembe, M. In Vitro Study to Evaluate the Bioactivity of Freezing a Heparin-Based Dalbavancin Lock Solution. Antimicrob. Agents Chemother. 2020, 64, e01495-20. [Google Scholar] [CrossRef]
- Alonso, B.; Pérez-Granda, M.; Rodríguez-Huerta, A.; Rodríguez, C.; Bouza, E.; Guembe, M. The optimal ethanol lock therapy regimen for treatment of biofilm-associated catheter infections: An in-vitro study. J. Hosp. Infect. 2018, 100, e187–e195. [Google Scholar] [CrossRef]
- Ashcraft, M.; Douglass, M.; Garren, M.; Mondal, A. Nitric Oxide-Releasing Lock Solution for the Prevention of Catheter-Related Infection and Thrombosis. ACS Appl. Bio Mater. 2022, 5, 1519–1527. [Google Scholar] [CrossRef]
- Mansouri, M.D.; Ramanthan, V.; Mansouri, D.L.; Hull, R.A. In vitro activities of N-acetyl cysteine and levofloxacin as a catheter lock therapy against catheter-associated infections. J. Appl. Microbiol. 2022, 132, 3915–3924. [Google Scholar] [CrossRef]
- American Thoracic Society, & Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am. J. Respir. Crit. Care Med. 2005, 171, 388–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandecandelaere, I.; Coenye, T. Microbial composition and antibiotic resistance of biofilms recovered from endotracheal tubes of mechanically ventilated patients. Adv. Exp. Med. Biol. 2015, 830, 137–155. [Google Scholar] [PubMed]
- De Souza, P.R.; De Andrade, D.; Cabral, D.B.; Watanabe, E. Endotracheal tube biofilm and ventilator-associated pneumonia with mechanical ventilation. Microsc. Res. Tech. 2014, 77, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Dsouza, R.; Spillman, D.R., Jr.; Barkalifa, R.; Monroy, G.L.; Chaney, E.J.; White, K.C.; Boppart, S.A. In vivo detection of endotracheal tube biofilms in intubated critical care patients using catheter-based optical coherence tomography. J. Biophotonics 2019, 12, e201800307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tincu, R.C.; Cobilinschi, C.; Tincu, I.F.; Macovei, R.A. Efficacy of Noble Metal–alloy Endotracheal Tubes in Ventilator-associated Pneumonia Prevention: A Randomized Clinical Trial. Balk. Med. J. 2022, 39, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Granda, M.J.; Alonso, B.; Zavala, R.; Latorre, M.C.; Hortal, J.; Samaniego, R.; Bouza, E.; Muñoz, P.; Guembe, M. Selective digestive decontamination solution used as “lock therapy” prevents and eradicates bacterial biofilm in an in vitro bench-top model. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 44. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Merchán, E.C.; Davidson, D.J.; Liddle, A.D. Recent Strategies to Combat Infections from Biofilm-Forming Bacteria on Orthopaedic Implants. Int. J. Mol. Sci. 2021, 22, 10243. [Google Scholar] [CrossRef]
- Antony, S.; Farran, Y. Prosthetic Joint and Orthopedic Device Related Infections. The Role of Biofilm in the Pathogenesis and Treatment. Infect. Disord. Drug Targets 2016, 16, 22–27. [Google Scholar] [CrossRef]
- Schoenmakers, J.W.A.; Heuker, M.; López-Álvarez, M.; Nagengast, W.B.; van Dam, G.M.; van Dijl, J.M. Image-guided in situ detection of bacterial biofilms in a human prosthetic knee infection model: A feasibility study for clinical diagnosis of prosthetic joint infections. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 757–767. [Google Scholar] [CrossRef]
- Malizos, K.; Blauth, M.; Danita, A.; Capuano, N.; Mezzoprete, R.; Logoluso, N.; Drago, L.; Romanò, C.L. Fast-resorbable antibiotic-loaded hydrogel coating to reduce post-surgical infection after internal osteosynthesis: A multicenter randomized controlled trial. J. Orthop. Traumatol. 2017, 18, 159–169. [Google Scholar] [CrossRef] [Green Version]
- López, T., II; Vaquero-Martín, J.; Torres-Suárez, A.I.; Navarro-García, F.; Fraguas-Sánchez, A.I.; León-Román, V.E.; Sanz-Ruiz, P. The tale of microencapsulated rifampicin: Is it useful for the treatment of periprosthetic joint infection? Int. Orthop. 2022, 46, 677–685. [Google Scholar] [CrossRef] [PubMed]
- López-Torres, I.I.; Sanz-Ruíz, P.; Navarro-García, F.; León-Román, V.E.; Vaquero-Martín, J. Experimental reproduction of periprosthetic joint infection: Developing a representative animal model. Knee 2020, 27, 1106–1112. [Google Scholar] [CrossRef] [PubMed]
- Litzler, P.-Y.; Benard, L.; Barbier-Frebourg, N.; Vilain, S.; Jouenne, T.; Beucher, E.; Bunel, C.; Lemeland, J.-F.; Bessou, J.-P. Biofilm formation on pyrolytic carbon heart valves: Influence of surface free energy, roughness, and bacterial species. J. Thorac. Cardiovasc. Surg. 2007, 134, 1025–1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Cueto-López, M.; Del Pozo-León, J.L.; de Luna, F.F.A.; Marin-Arriaza, M. Microbiological diagnosis of medical device-associated infections. Enferm. Infecc. Y Microbiol. Clin. 2016, 34, 655–660. [Google Scholar] [CrossRef]
- Lauten, A.; Martinović, M.; Kursawe, L.; Kikhney, J.; Affeld, K.; Kertzscher, U.; Falk, V.; Moter, A. Bacterial biofilms in infective endocarditis: An in vitro model to investigate emerging technologies of antimicrobial cardiovascular device coatings. Clin. Res. Cardiol. 2020, 110, 323–331. [Google Scholar] [CrossRef]
- Kursawe, L.; Kikhney, J.; Affeld, K.; Kertzscher, U.; Falk, V.; Moter, A. An update on recent developments in the prevention and treatment of Staphylococcus aureus biofilms. Clin. Res. Cardiol. 2019, 309, 1–12. [Google Scholar]
- Fernández-Ibarburu, B.; Díaz-Navarro, M.; Ibarra, G.; Rivera, A.; Hafian, R.; Irigoyen, A.; Carrillo, R.; Pérez-Cano, R.; Muñoz, P.; García-Ruano, A.; et al. Efficacy of Povidone Iodine Against Microbial Biofilms in Breast Implants with Different Textures: Results From an in vitro Study. Front. Microbiol. 2022, 13, 868347. [Google Scholar] [CrossRef]
- Drinane, J.J.; Chowdhry, T.; Pham, T.H.; Ritter, E. Examining the Role of Antimicrobial Irrigation and Capsular Contracture: A Systematic Review and Meta-analysis. Ann. Plast. Surg. 2017, 79, 107–114. [Google Scholar] [CrossRef]
- Galdiero, M.; Larocca, F.; Iovene, M.R.; Francesca, M.; Pieretti, G.; D’Oriano, V.; Franci, G.; Ferraro, G.; d’Andrea, F.; Francesco, N.G. Microbial Evaluation in Capsular Contracture of Breast Implants. Plast. Reconstr. Surg. 2018, 141, 23–30. [Google Scholar] [CrossRef]
- Ngaage, L.M.; Elegbede, A.; Brao, K.; Chopra, K.; Gowda, A.U.; Nam, A.J.; Ernst, R.K.; Shirtliff, M.E.; Harro, J.; Rasko, Y.M. The Efficacy of Breast Implant Irrigant Solutions: A Comparative Analysis Using an In Vitro Model. Plast. Reconstr. Surg. 2020, 146, 301–308. [Google Scholar] [CrossRef]
- Barnea, Y.; Hammond, D.C.; Geffen, Y.; Navon-Venezia, S.; Goldberg, K. Plasma Activation of a Breast Implant Shell in Conjunction with Antibacterial Irrigants Enhances Antibacterial Activity. Aesthetic Surg. J. 2018, 38, 1188–1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleiszig, S.M.; Evans, D.J. Pathogenesis of contact lens-associated microbial keratitis. Optom. Vis. Sci. 2010, 87, 225–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vengayil, S.; Vanathi, M.; Dada, T.; Kai, S.; Panda, A. Filtering bleb-induced giant papillary conjunctivitis. Contact Lens Anterior Eye 2008, 31, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Lyczak, J.B.; Cannon, C.L.; Pier, G.B. Establishment of Pseudomonas aeruginosa infection: Lessons from a versatile opportunist. Microbes Infect. 2000, 2, 1051–1060. [Google Scholar] [CrossRef]
- Willcox, M.D.P.; Holden, B.A. Contact Lens Related Corneal Infections. Biosci. Rep. 2001, 21, 445–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bispo, P.J.M.; Haas, W.; Gilmore, M.S. Biofilms in Infections of the Eye. Pathogens 2015, 4, 111–136. [Google Scholar] [CrossRef]
- Garg, P. Diagnosis of microbial keratitis. Br. J. Ophthalmol. 2010, 94, 961–962. [Google Scholar] [CrossRef]
- Fleiszig, S.M. The Glenn A. Fry award lecture 2005. The pathogenesis of contact lens-related keratitis. Optom. Vis. Sci. 2006, 83, 866–873. [Google Scholar] [CrossRef]
- Tuft, S.; Burton, M. Focus Autumn 2013—The Royal College of Ophthalmologists. Microbial keratitis. Available online: www.rcophth.ac.uk/core/core_picker/download.asp?id=1826 (accessed on 15 May 2022).
- Tam, A.L.C.; Côté, E.; Saldanha, M.; Lichtinger, A.; Slomovic, A.R. Bacterial Keratitis in Toronto: A 16-Year Review of the Microorganisms Isolated and the Resistance Patterns Observed. Cornea 2017, 36, 1528–1534. [Google Scholar] [CrossRef]
- Chang, V.S.; Dhaliwal, D.K.; Raju, L.; Kowalski, R.P. Antibiotic Resistance in the Treatment of Staphylococcus aureus Keratitis: A 20-Year Review. Cornea 2015, 34, 698–703. [Google Scholar] [CrossRef] [Green Version]
- Saffari, M.; Karami, S.; Firoozeh, F.; Sehat, M. Evaluation of biofilm-specific antimicrobial resistance genes in Pseudomonas aeruginosa isolates in Farabi Hospital. J. Med. Microbiol. 2017, 66, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Heidari, H.; Hadadi, M.; Ebrahim-Saraie, H.S.; Mirzaei, A.; Taji, A.; Hosseini, S.; Motamedifar, M. Characterization of virulence factors, antimicrobial resistance patterns and biofilm formation of Pseudomonas aeruginosa and Staphylococcus spp. strains isolated from corneal infection. J. Fr. d’Ophtalmol. 2018, 41, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Saraswathi, P.; Beuerman, R.W. Corneal Biofilms: From Planktonic to Microcolony Formation in an Experimental Keratitis Infection with Pseudomonas aeruginosa. Ocul. Surf. 2015, 13, 331–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zegans, M.E.; DiGiandomenico, A.; Ray, K.; Naimie, A.; Keller, A.E.; Stover, C.K.; Lalitha, P.; Srinivasan, M.; Acharya, N.R.; Lietman, T.M. Association of Biofilm Formation, Psl Exopolysaccharide Expression, and Clinical Outcomes in Pseudomonas aeruginosa Keratitis: Analysis of Isolates in the Steroids for Corneal Ulcers Trial. JAMA Ophthalmol. 2016, 134, 383–389. [Google Scholar] [CrossRef] [Green Version]
- Dave, A.; Samarth, A.; Karolia, R.; Sharma, S.; Karunakaran, E.; Partridge, L.; MacNeil, S.; Monk, P.N.; Garg, P.; Roy, S. Characterization of Ocular Clinical Isolates of Pseudomonas aeruginosa from Non-Contact Lens Related Keratitis Patients from South India. Microorganisms 2020, 8, 260. [Google Scholar] [CrossRef] [Green Version]
- Jian, H.J.; Li, Y.J.; Unnikrishnan, B.; Huang, Y.F.; Luo, L.J.; Ma, D.H.K.; Harroun, S.G.; Chang, H.T.; Lin, H.J. Highly adhesive carbon quantum dots from biogenic amines for prevention of biofilm formation. Chem. Engl. J. 2020, 386, 123913. [Google Scholar] [CrossRef]
- Li, J.; Ma, X.; Zhao, L.; Li, Y.; Zhou, Q.; Du, X. Extended Contact Lens Wear Promotes Corneal Norepinephrine Secretion and Pseudomonas aeruginosa Infection in Mice. Investig. Opthalmol. Vis. Sci. 2020, 61, 17. [Google Scholar] [CrossRef]
- Dong, Q.; Brulc, J.M.; Iovieno, A.; Bates, B.; Garoutte, A.; Miller, D.; Revanna, K.V.; Gao, X.; Antonopoulos, D.A.; Slepak, V.Z.; et al. Diversity of bacteria at healthy human conjunctiva. Investig. Opthalmol. Vis. Sci. 2011, 52, 5408–5413. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Holland, M.J.; Makalo, P.; Joof, H.; Roberts, C.H.; Mabey, D.C.; Bailey, R.L.; Burton, M.J.; Weinstock, G.M.; Burr, S.E. The conjunctival microbiome in health and trachomatous disease: A case control study. Genome Med. 2014, 6, 99. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Oh, D.H.; Jung, J.Y.; Kim, J.C.; Jeon, C.O. Comparative Ocular Microbial Communities in Humans with and without Blepharitis. Investig. Opthalmol. Vis. Sci. 2012, 53, 5585–5593. [Google Scholar] [CrossRef] [Green Version]
- Busscher, H.; Ploeg, R.; Van Der Mei, H. SnapShot: Biofilms and Biomaterials, Mechanisms of Medical Device Related Infections. Biomaterials 2009, 30, 4247–4248. [Google Scholar] [PubMed]
- Scialla, S.; Martuscelli, G.; Nappi, F.; Singh, S.S.A.; Iervolino, A.; Larobina, D.; Ambrosio, L.; Raucci, M.G. Trends in Managing Cardiac and Orthopaedic Device-Associated Infections by Using Therapeutic Biomaterials. Polymers 2021, 13, 1556. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Ding, Y.; Tao, B.; Yuan, Z.; Yang, Y.; Xu, K.; Li, X.; Liu, P.; Cai, K. Surface modification of titanium substrate via combining photothermal therapy and quorum-sensing-inhibition strategy for improving osseointegration and treating biofilm-associated bacterial infection. Bioact. Mater. 2022, 18, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Strehmel, J.; Overhage, J. Potential Application of Antimicrobial Peptides in the Treatment of Bacterial Biofilm Infections. Curr. Pharm. Des. 2014, 21, 67–84. [Google Scholar]
- Latorre, M.C.; Pérez-Granda, M.J.; Savage, P.B.; Alonso, B.; Martín-Rabadán, P.; Samaniego, R.; Bouza, E.; Muñoz, P.; Guembe, M. Endotracheal tubes coated with a broad-spectrum antibacterial ceragenin reduce bacterial biofilm in an in vitro bench top model. J. Antimicrob. Chemother. 2021, 76, 1168–1173. [Google Scholar] [CrossRef]
- Hawas, S.; Verderosa, A.D.; Totsika, M. Combination Therapies for Biofilm Inhibition and Eradication: A Comparative Review of Laboratory and Preclinical Studies. Front. Cell. Infect. Microbiol. 2022, 12, 850030. [Google Scholar] [CrossRef]
- Chang, C.; Yu, X.; Guo, W.; Guo, C.; Guo, X.; Li, Q.; Zhu, Y. Bacteriophage-Mediated Control of Biofilm: A Promising New Dawn for the Future. Front. Microbiol. 2022, 13, 825–828. [Google Scholar] [CrossRef]
- Cano, A.; Ettcheto, M.; Espina, M.; López-Machado, A.; Cajal, Y.; Rabanal, F.; Sánchez-López, E.; Camins, A.; García, M.L.; Souto, E.B. State-of-the-art polymeric nanoparticles as promising therapeutic tools against human bacterial infections. J. Nanobiotechnol. 2020, 18, 156. [Google Scholar] [CrossRef]
- Mallick, S.; Nag, M.; Lahiri, D.; Pandit, S.; Sarkar, T.; Pati, S.; Nirmal, N.P.; Edinur, H.A.; Kari, Z.A.; Zain, M.R.A.M.; et al. Engineered Nanotechnology: An Effective Therapeutic Platform for the Chronic Cutaneous Wound. Nanomaterials 2022, 12, 778. [Google Scholar] [CrossRef]
- Khan, S.S.; Ullah, I.; Ullah, S.; An, R.; Xu, H.; Nie, K.; Liu, C.; Liu, L. Recent Advances in the Surface Functionalization of Nanomaterials for Antimicrobial Applications. Materials 2021, 14, 6932. [Google Scholar] [CrossRef]
- Nag, M.; Lahiri, D.; Sarkar, T.; Ghosh, S.; Dey, A.; Edinur, H.A.; Pati, S.; Ray, R.R. Microbial Fabrication of Nanomaterial and Its Role in Disintegration of Exopolymeric Matrices of Biofilm. Front. Chem. 2021, 9, 690590. [Google Scholar] [CrossRef] [PubMed]
- Berra, L.; Coppadoro, A.; Bittner, E.A.; Kolobow, T.; Laquerriere, P.; Pohlmann, J.R.; Bramati, S.; Moss, J.; Pesenti, A. A clinical assessment of the Mucus Shaver: A device to keep the endotracheal tube free from secretions. Crit. Care Med. 2012, 40, 119–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ichioka, Y.; Derks, J.; Dahlén, G.; Berglundh, T.; Larsson, L. Mechanical removal of biofilm on titanium discs: An in vitro study. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 110, 1044–1055. [Google Scholar] [CrossRef] [PubMed]
- Kamionka, J.; Matthes, R.; Holtfreter, B.; Pink, C.; Schlüter, R.; von Woedtke, T.; Kocher, T.; Jablonowski, L. Efficiency of cold atmospheric plasma, cleaning powders and their combination for biofilm removal on two different titanium implant surfaces. Clin. Oral Investig. 2022, 26, 3179–3187. [Google Scholar] [CrossRef]
- Martinez, R.M.; Bowen, T.R.; Foltzer, M.A. Prosthetic Device Infections. Microbiol. Spectr. 2016, 4, 711–733. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S.; Bjarnsholt, T. Risk factors for chronic biofilm-related infection associated with implanted medical devices. Clin. Microbiol. Infect. 2020, 26, 1034–1038. [Google Scholar] [CrossRef]
- Guzmán-Soto, I.; McTiernan, C.; Gonzalez-Gomez, M.; Ross, A.; Gupta, K.; Suuronen, E.J.; Mah, T.-F.; Griffith, M.; Alarcon, E.I. Mimicking biofilm formation and development: Recent progress in in vitro and in vivo biofilm models. iScience 2021, 24, 102443. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Domenico, E.G.; Oliva, A.; Guembe, M. The Current Knowledge on the Pathogenesis of Tissue and Medical Device-Related Biofilm Infections. Microorganisms 2022, 10, 1259. https://doi.org/10.3390/microorganisms10071259
Di Domenico EG, Oliva A, Guembe M. The Current Knowledge on the Pathogenesis of Tissue and Medical Device-Related Biofilm Infections. Microorganisms. 2022; 10(7):1259. https://doi.org/10.3390/microorganisms10071259
Chicago/Turabian StyleDi Domenico, Enea Gino, Alessandra Oliva, and María Guembe. 2022. "The Current Knowledge on the Pathogenesis of Tissue and Medical Device-Related Biofilm Infections" Microorganisms 10, no. 7: 1259. https://doi.org/10.3390/microorganisms10071259
APA StyleDi Domenico, E. G., Oliva, A., & Guembe, M. (2022). The Current Knowledge on the Pathogenesis of Tissue and Medical Device-Related Biofilm Infections. Microorganisms, 10(7), 1259. https://doi.org/10.3390/microorganisms10071259