Abstract
Staphylococcus haemolyticus (S. haemolyticus) constitutes the main part of the human skin microbiota. It is widespread in hospitals and among medical staff, resulting in being an emerging microbe causing nosocomial infections. S. haemolyticus, especially strains that cause nosocomial infections, are more resistant to antibiotics than other coagulase-negative Staphylococci. There is clear evidence that the resistance genes can be acquired by other Staphylococcus species through S. haemolyticus. Severe infections are recorded with S. haemolyticus such as meningitis, endocarditis, prosthetic joint infections, bacteremia, septicemia, peritonitis, and otitis, especially in immunocompromised patients. In addition, S. haemolyticus species were detected in dogs, breed kennels, and food animals. The main feature of pathogenic S. haemolyticus isolates is the formation of a biofilm which is involved in catheter-associated infections and other nosocomial infections. Besides the biofilm formation, S. haemolyticus secretes other factors for bacterial adherence and invasion such as enterotoxins, hemolysins, and fibronectin-binding proteins. In this review, we give updates on the clinical infections associated with S. haemolyticus, highlighting the antibiotic resistance patterns of these isolates, and the virulence factors associated with the disease development.
1. Introduction
Coagulase-negative Staphylococci (CoNS) constitute the main microbiota of the skin. These pathogens were underestimated and a distinct species identification was not included in many microbiology laboratories [1]. Only the coagulase-positive S. aureus was considered pathogenic and therefore gained great interest and thoroughly analyzed in different studies. In the late 1960s, one of the CoNS, S. saprophyticus, was observed in patients with urinary tract infections (UTIs) [2]. Later, the first CoNS infections were identified in the 1970s in patients with invasive and indwelling medical devices [3,4]. The advances in diagnostic protocols and molecular techniques enabled more accurate identification of the other species in the genus Staphylococci [5]. Scientists have observed increasing numbers of CoNS infections. In the USA between 1980 and 1989, CoNS causing nosocomial bacteremia increased from 9 to 27% [6]. Staphylococci species are phylogenetically a very coherent group. The average nucleotide identity values of S. aureus versus CoNS such as S. epidermidis and S. haemolyticus is approximately 75%, showing their close genetic association [7,8].
S. haemolyticus is a part of skin microflora and one of the main species of CoNS [9]. This species accounts for 10–20% of clinical CoNS infections [10] and is the second-highest species of CoNS in frequency and importance among isolates from clinical infections [11]. There are several clinical infections recorded with S. haemolyticus including bacteremia, meningitis, eye infections, skin infections, peritonitis, urinary tract infections, and male genital dysfunction [12,13]. Furthermore, S. haemolyticus strains were isolated from dogs and dogs’ owners suggesting a possibility of zoonotic transmission [14]. A characteristic feature of S. haemolyticus is the formation of biofilms, which are crucial for the development of infections [15]. Furthermore, S. haemolyticus produces several toxins and invasive enzymes that help in bacterial pathogenesis by changing the host immune responses and inducing damage in the host cells [15].
S. haemolyticus is an emerging pathogen causing nosocomial infections. The factors which cause the survival and spread of S. haemolyticus in hospitals are not well defined [16]. The genome of S. haemolyticus causing hospital infections is characterized by the abundance of insertion sequences, and resistance to several antibiotics [17,18].
In the absence of appropriate diagnosis and management of infections caused by S. haemolyticus, resistant strains of this pathogen can spread to other hospital settings, and probably to the community [17].
In this review, we give updates on the clinical infections associated with S. haemolyticus, antibiotic resistance patterns of these isolates, and the virulence factors associated with the disease development.
2. Clinical Infections Associated with S. haemolyticus
There are several clinical manifestations recorded with S. haemolyticus infections such as bloodstream infections, ocular infections, epididymo-orchitis, chronic prostatitis, UTI, etc. Furthermore, it is an important pathogen associated with hospital-acquired infections (Figure 1). Other complications were also recorded especially in immunocompromised patients such as septicemia, peritonitis, and otitis [12,13].
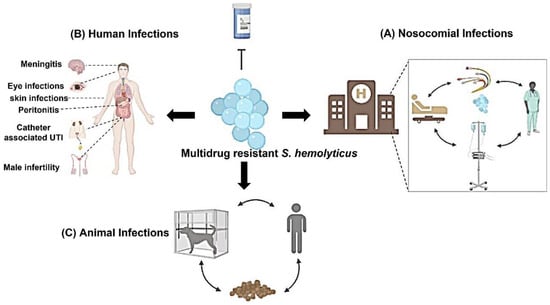
Figure 1.
Infections associated with S. haemolyticus: Several infections are associated with S. haemolyticus isolates. S. haemolyticus causes nosocomial infections that can be spread among health care personnel, medical devices, catheters, and patients. In addition, several clinical human infections are recorded with S. haemolyticus such as eye infections, bacteremia, UTIs, male infertility, etc. Human infections are considered nosocomial infections if the infections are acquired in the hospitals. Moreover, S. haemolyticus infect animals such as dogs and infection can spread throughout the animal, owner, kennel breed, and animal food.
2.1. Bloodstream Infections (BSIs)
S.aureus and several species of CoNS such as S. hominis, S. haemolyticus and S. epidermidis cause BSIs in cancer patients [18]. S. haemolyticus causes bacteremia following the central catheter-related bloodborne infection [19,20], and it causes septicemia among neutropenic patients in intensive care units (ICU) [21] and renal dialysis catheter-related sepsis (CRS) [22]. S. haemolyticus causing BSIs are highly resistant to antibiotics, some isolates such as methicillin-resistant S. haemolyticus (MRSH) can cause severe complications and death [23,24]. The antimicrobial agents effective against multidrug-resistant S. haemolyticus are limited. Vancomycin and daptomycin are not good options, but a prolonged course of linezolid could be the best therapy [19].
2.2. Eyes Infections
Previously, the implication of CoNS in the pathogenesis of a corneal ulcer was ignored because these organisms are part of normal flora and ubiquitous. However, improvements in diagnosis and identification have revealed that CoNS are an important cause of infected corneal ulcers. Among CoNS, S. haemolyticus is the second most prevalent species causing eye infections [25,26]. Makki et al. reported that 36% of the ocular infections were caused by S. haemolyticus [27]. Likewise, Wong et al. reported that S. haemolyticus causes endophthalmitis mainly post-operation [28]. In the previous study, the authors described a case of endophthalmitis following femtosecond cataract surgery caused by S. haemolyticus that was associated with a progressive infection and severe inflammation on the first day following the operation [28]. Interestingly, S. haemolyticus isolates of multilocus sequence type 25 (ST25) which belong to clonal complex 1 (CC1) are reported as a causative agent of keratitis in India and Europe [29,30]. Moreover, S. haemolyticus, together with S. epidermidis, were also isolated from intraocular lenses [31,32]. S. haemolyticus isolates that cause ocular infection have intercellular adhesion (ica) properties due to the formation of biofilm which is composed of the extracellular DNA (eDNA) and protein [33]. The presence of biofilm and expression of quorum sensing are the main features of S. haemolyticus isolates causing ocular infections [33].
2.3. Nosocomial Infection
CoNS are common skin commensals that start to colonize the body surfaces very early in life. After 48 h of birth, about 100% of infants acquire CoNS during passage through the birth canal or by contacting nursery personnel [34]. The most common colonizing species are S. epidermidis, S.warneri, and S. haemolyticus [35]. S. haemolyticus, together with S. epidermidis and S. hominis, were the prevalent staphylococci species detected in surfaces that are touched at a high frequency in the community and hospitals in London [36]. Similarly, S. haemolyticus and S. epidermidis were the most common CoNS isolates (34% and 27%, respectively) detected in different hospital wards in Iran [37]. Genotyping studies showed that S. haemolyticus, also S. epidermidis, colonizing the GIT of newborns are responsible for late-onset sepsis in the preterm neonates [38]. Moreover, Perdreau-Remington et al. reported that a single clone of S. haemolyticus showed widespread dissemination among the hands of medical personnel and in different places and wards [39]. Collectively, the previous findings suggest the dissemination of S. haemolyticus in hospitals which explains the nosocomial infection associated with S. haemolyticus.
S. haemolyticus is an important causative pathogen of hospital-acquired infections, particularly in a neonatal ICU [40]. The presence of venous catheters or medical devices increases the risk of infections [40,41]. In addition, an outbreak caused by S. haemolyticus was recorded in an Italian intensive care unit [42]. The nosocomial isolates of S. haemolyticus showed the highest level of resistance to antibiotics among most members of the CoNS [43]. During hospitalization, an increase in the rate of antibiotic resistance is observed, particularly in the methicillin-resistant strains of S. haemolyticus [39]. Moreover, vancomycin and teicoplanin-resistant S. haemolyticus strain was recorded in a patient with myelogenous leukemia who suffered from a septic episode during the cytostatic course [44]. Furthermore, S. haemolyticus isolates that caused an outbreak in Italy were resistant to linezolid [42]. These multidrug-resistant skin colonizing bacteria are not only at risk for the emergence and spread of nosocomial infections but can also infect healthcare personnel and patient visitors [45].
Furthermore, S. haemolyticus can resist disinfection. Molecular typing of infections caused by MRSH collected over a 3 year period from the neonatal ICU revealed that S. haemolyticus can survive in disinfectant solutions which consequently can act as a reservoir for infecting the newborns, pointing to the importance of testing the ability of disinfectants in neonatal ICUs against these pathogens [9].
The factors that affect the survival and spread of multi-drug resistant S. haemolyticus isolates in hospitals are not completely known. Bouchami and colleagues reported that the insertion sequence transposition (mainly IS1272) and chromosomal rearrangement and recombination processes in S. haemolyticus is one strategy that helps in the bacterial evolution, adaptation, pathogenesis, and survival in the hospitals, hence causing nosocomial infections [46].
2.4. Male Infertility
S. haemolyticus causes infection of the male genital system and it could be responsible for male infertility. S. haemolyticus infection decreases sperm motility and viability [47,48]. In addition, contact of S. haemolyticus with the ejaculated spermatozoa can affect the architecture of the sperm plasma membrane and therefore lead to male infertility [47,48]. Moreover, exposure of human spermatozoa to S. haemolyticus leads to an increase in phosphatidylserine externalization, DNA fragmentation, and the percentage of apoptotic as well as necrotic sperm cells [49]. Furthermore, S. haemolyticus infection decreases the percentage of sperm with normal mitochondrial transmembrane potential [49]. Pindar and Viau described a case of S. haemolyticus bacteremia secondary to epididymo-orchitis, and there is no involvement of venous or urinary catheters [50].
2.5. Other Human Diseases
In addition to the aforementioned clinical conditions, S. haemolyticus infection is also recorded in different human infections such as chronic prostatitis [51], coeliac disease [52], community-acquired skin, and soft-tissue infections [53], and continuous ambulatory peritoneal dialysis-associated peritonitis [22]. In addition, S. haemolyticus are among the predominant organisms colonizing the periurethral and urethra in males and females, they regularly account for about 10% of UTIs [54,55]. Furthermore, S. haemolyticus-associated ventricular atrial shunt nephritis was recorded [56]. Moreover, S. haemolyticus causes meningitis in an allogeneic stem cell transplant patient following central catheter-related bacteremia with no previous history of neurosurgical procedures [19]. Furthermore, S. haemolyticus was isolated from a 73-year-old man who presented with liver abscess and silent colon cancer [57].
2.6. Animal Disease
Methicillin-resistant S. haemolyticus (MRSH) was isolated from dogs, but not from cats or horses [14]. MRSH was also isolated from pure-breed kennels and a kennel owner [14]. The previous findings suggest the possibility of bacteria transmission from the animals to the owners and the veterinary personnel. Importantly, the isolates that infect humans and animals are highly resistant to available antibiotics including β-lactams, macrolides, gentamicin, and tetracycline [14]. Moreover, multidrug-resistant S. haemolyticus was found in animal food suggesting that these bacteria could aid in the spread of resistance to antimicrobial agents in the places of food manufacture [58].
3. Antibiotic Resistance in S. heamolyticus
Over recent years, different investigators have described an increasing frequency of multidrug-resistant strains of S. haemolyticus [16,59,60]. S. haemolyticus is notably more resistant to antibiotics than any other CoNS, and the widest spectrum of resistance was observed among strains isolated from the hospital environment [60,61,62]. The presence of resistance genes in S. haemolyticus and its spread in the hospital environment constitutes a potential risk since this bacterium can store the resistance genes and transmit them to other species [63]. Bakthavatchalam et al. used next-generation sequencing technology to characterize the whole genome of multidrug-resistant S. haemolyticus, and they characterized three antibiotic-resistant genes. The first two genes named blaZ and norA are responsible for resistance to β-lactam and quinolone, respectively. The third gene “msr (A)” mediates the cross-resistance to different antimicrobial agents such as macrolides, lincosamide, and streptogramin B [64]. The genome of S. haemolyticus contains large quantities of the mobile genetic elements (insertion sequences, IS) such as IS256 and IS1272 which participate in the bacterial evolution, shaping the population structure through the DNA recombination process [46]. Moreover, these IS play an important role in bacterial adaption to host and hospital environment and genome flexibility [46]. Furthermore, they could transfer the drug resistance to other staphylococcal species, as shown that the sequences of beta-lactamase and qacA genes were identical in both S. aureus and S. haemolyticus indicating interspecies transfer of IS between these species [64,65,66,67]. Similarly, Kim and Jang recognized the integration of S. aureus plasmid, pS0385-1 into the chromosome of S. haemolyticus IPK_TSA25 and this integration confers the resistance to antibiotics mainly tetracyclines [68].
Furthermore, the efflux pump mechanism is documented in S. haemolyticus human clinical isolates, and it is associated with resistance to gentamicin, erythromycin, ciprofloxacin, chloramphenicol, and tetracycline [69]. The multidrug-resistant pump is mediated by several genes such as qacG, qacH, and qacJ genes [69]. Interestingly, the qac genes also confer the resistance of S. haemolyticus to antiseptics, and these resistance genes can be horizontally transferred among bacteria [69]. Table 1 summarizes the resistance of S. haemolyticus to common antibiotics.

Table 1.
Resistance of S. haemolyticus to antibiotics.
3.1. β-Lactam
Some isolates of S. haemolyticus are not susceptible to β-lactams. Barros and colleagues studied the antibiotic profile of 64 clinical isolates of S. haemolyticus. They found that 95% of the isolates were resistant to penicillin and ampicillin and 88% of the isolates were resistant to oxacillin and cefoxitin [80]. Similarly, Manoharan et al. recorded that the susceptibility of 356 clinical isolates of S. haemolyticus to cefoxitin and penicillin was very low, 8.7% and 5.9%, respectively [81]. Likewise, De Vecchi et al. showed that S. haemolyticus, isolated from joint infections, have the highest level of oxacillin resistance (83%), compared to S. aures and other CoNS species isolated from the same patients [82]. MRSH became the reason for a significant limitation in the use of β-lactam antibiotics [62].
3.2. Methicillin
Methicillin-resistant CoNS was first isolated in 1961 in a clinical laboratory in the UK at rates higher than in S. aureus [83]. Nevertheless, CoNS was not thought to be particularly pathogenic to human beings at that time.
Both S. aureus and CoNS harbor the mecA gene. Therefore, they share the resistance mechanism to methicillin which is mediated by modified transpeptidase enzymes (PBP2a) that crosslink the peptidoglycan layers by synthesizing the pentaglycine bridges in peptidoglycan. This modified PBP2 binds the methicillin at a much lower affinity leading to therapeutic failure [70].
The sequences of the mecA gene of S. aureus, S. haemolyticus, and S. epidermidis are similar by 99.95%. Such a degree of similarity supports the hypothesis of the interspecies transfer of the mecA gene. Unfortunately, the investigated CoNS groups are often not specified to the species levels, which hinders the accurate evaluation of S. haemolyticus resistance rates to methicillin [84]. Molecular analysis demonstrated that the S. haemolyticus genome comprises the ccr gene complex that contains a chromosomal recombinase, which enables the combination of the mec cassette with chromosomal DNA, and sometimes also other resistance and virulence genes [85,86]. Both the mec gene and the ccr gene complex form the staphylococcal cassette chromosome mec (SCCmec) cassettes that mediate bacteria pathogenesis [87]. In S. aureus, about 11 types of SCCmec cassettes have been identified. The largest diversity of SCCmec sequences is observed amongst S. epidermidis, S. haemolyticus, and S. hominis strains [55]. SCCmec types III, IV, and V were detected in methicillin-resistant CoNS and some bacteria contain several types [87]. In S. haemolyticus, type V is the most frequently identified SCCmec cassette [55,87]. Importantly, MRSH isolates are widespread in the hospital environment. In South Korea, 51.4% of X-ray cassettes were contaminated with genetic similarities to MRSH strains [88]. In another study, 96% of S. haemolyticus strains were resistant to methicillin among Brazilian isolates [12]. MRSH strains were detected in 67.5% of CoNS isolated from patients with nosocomial bacteremia in ICU in Istanbul [89].
The ability of S. haemolyticus to transfer genes to other species was elucidated at the neonatal ICU in Orebro University Hospital in Sweden in 2008 where a case of SCCmec type V cassette was transferred from MRSH to methicillin-susceptible S. aureus [90]. During 20 years of analysis in Zurich, Switzerland between 1986 and 2005, the detection rate of methicillin resistance CoNS bacteria jumped five folds [91].
Some recent studies showed that the incidence of oxacillin resistance of S. haemolyticus isolates has exceeded 80% [92,93]. Some strains of S. haemolyticus were even resistant to ceftobiprole which is a fifth-generation cephalosporin. These strains had MIC ranges of 1 to 4 µg/mL [94].
3.3. Glycopeptides
Glycopeptides are given to patients with severe infections caused by multidrug-resistant CoNS. Glycopeptide antibiotics block the late stages of peptidoglycan cross-linking by binding to the dipeptide terminus D-Ala-D-Ala leading to the inhibition of bacterial cell wall synthesis [95]. Vancomycin and teicoplanin are members of available glycopeptides. The bacterial resistance to glycopeptides was developed due to the uncontrolled use [96,97]. Glycopeptide heteroresistance is common in MRSH, but rare in methicillin-susceptible S. haemolyticus [64]. Bakthavatchalam et al. reported the first isolate of methicillin-susceptible S. haemolyticus was in India which is resistant to teicoplanin and with decreased sensitivity to vancomycin [64]. The resistance of S. haemolyticus to glycopeptide in the previous case is due to the alteration of the glycopeptide resistance-associated histidine kinase (GraS) mediated by the insertion of two amino acids (leucine and proline) at adjacent sites inside the GraS target. Furthermore, Billot-Klein et al. showed that the alteration in the cross-bridge between the peptidoglycan layers of S. haemolyticus decreases the efficiency of binding of glycopeptides to the bacterial cell wall leading to antibiotic resistance [71].
3.3.1. Vancomycin
In 2002, in the US, the first vancomycin-resistant S. aureus was reported which contained the vanA gene. Up to the present, the frequency of staphylococci resistance to vancomycin is infrequently reported [98]. The resistance of S. haemolyticus to vancomycin was recorded earlier. Vancomycin-resistant S. haemolyticus isolates were first recorded in a patient with peritonitis, these isolates showed resistance to vancomycin in vivo and in vitro [99]. The exact mechanism of CoNS resistance to vancomycin remains unclearly defined. One of the reported mechanisms for the reduced susceptibility of S. epidermidis and S. haemolyticus to vancomycin is the increase in cell wall thickening [100,101]. Some vancomycin-resistant CoNS had an overproduction of cell wall peptidoglycan material leading to an excess of glycopeptide binding sites [102]. Therefore, it seems that S. aureus and CoNS share the same mechanism of reduced susceptibility to glycopeptides.
One of the disadvantages of vancomycin is its reduced activity on biofilms and its low intracellular penetration power. In contrast, rifampicin is very effective in the eradication of biofilms caused by Staphylococci. However, it should be given with other antibiotics to avoid the rapid development of resistance [11].
3.3.2. Teicoplanin
Teicoplanin is effective in the treatment of S. haemolyticus infections, with rare homogenous resistance reports [103]. However, compared with other CoNS, teicoplanin is less potent in vitro against S. haemolyticus isolates [21]. The first cases of teicoplanin-resistant S. haemolyticus were described in the US and UK in 1986. S. haemolyticus isolated from both reports were also resistant to methicillin but sensitive to vancomycin [103,104]. S. haemolyticus which developed resistance to teicoplanin was also resistant to vancomycin [104]. Several reports showed that the rate of resistance to teicoplanin is higher than vancomycin among S. haemolyticus isolates [21,105,106].
During the period 2000–2003, S. haemolyticus was the second most frequent organism of CoNS isolated from patients with bacteremia (following S. epidermidis). Teicoplanin resistance was detected in 11–29% of these isolates [66]. Bakthavatchalam and colleagues reported that teicoplanin resistance operon (tcaRAB) plays a crucial role in the resistance of S. haemolyticus to teicoplanin [64]. Substitutions in three amino acids in tcaA (I3N, I390N, and L450I), and/or mutations in the transcriptional regulator tcaR (L44V, G52V, and S87P) were associated with the resistance of S. haemolyticus to teicoplanin [64].
3.4. Linezolid
Linezolid is recommended in cases of severe bacterial infections caused by methicillin-resistant staphylococci or vancomycin-resistant bacteria [61,72]. Linezolid inhibits bacterial protein synthesis by interfering with the peptidyl transferase of 23S rRNA in the 50S ribosomal subunit [107]. Linezolid-resistant S. haemolyticus has been isolated from four patients’ pus samples. Two samples were isolated from patients with chronic osteomyelitis, and two isolates were detected in cases of pemphigus vulgaris [74]. Some S. haemolyticus variants show a mucoid appearance. These mucoid colonies were associated with linezolid-resistant and were difficult to treat [108]. Linezolid-resistant S. haemolyticus isolates were responsible for an outbreak in an Italian intensive care unit [42]. The resistant isolates harbored the G2576T mutation that confers the resistance to linezolid which was retained for several passages [42]. S. haemolyticus isolates that carry the G2576T mutation in the 23S rRNA gene can disseminate in the hospital and ICU and are associated with the spread of nosocomial infections [61,109]. Furthermore, Kumari and colleagues described several mutations such as G2576T, G2447U, and C2534U at the domain V of the 23S ribosomal RNA gene and are associated with linezolid resistance [73]. Interestingly, the previous mutations were recorded in multiple, not single, clones of S. haemolyticus and were not associated with inappropriate use of linezolid [73]. Importantly, all linezolid-resistant S. haemolyticus isolates carry the cfr gene which confers methylation of 23S ribosomal RNA at A2503 and exhibits resistance to chloramphenicol, florfenicol, clindamycin, streptogramin A, and linezolid [74,75,110]. The previous findings suggest that the use of linezolid especially in the case of S. haemolyticus should be controlled to preserve the drug clinical utility.
3.5. Lincosamides
Lincosamides such as lincomycin, clindamycin, and Pirlimycin are effective against gram-positive cocci, and they suppress the bacterial protein expression by acting on the 50s ribosome. S. haemolyticus was resistant to high levels of lincomycin through plasmid-mediated inactivation of the lincomycin and clindamycin [111]. The enzyme responsible for the inactivation of lincosamides is the lincosamide O-nucleotidyltransferase that is encoded by lnu (A) and lnu (A′) (formerly lin) genes [76,77]. Novotna et al. identified S. haemolyticus isolates that are resistant to both clindamycin and lincomycin but sensitive to erythromycin [112]. The mechanism of resistance to lincosamides in the previous isolates was not identified, and it was not related to the resistance gene, ribosomal mutation, and/or inactivation resistance [78,112]. Further study showed that the resistance of these isolates to lincosamides was due to efflux of the drug which was mediated by the vga (A)LC resistance gene, that formed the ATP-binding cassette (ABC) family [78]. The mechanism of resistance of S. haemolyticus to lincosamides via efflux mechanism is similar to the resistance of Staphylococci to macrolide-streptogramin B via by Msr (A) gene [78].
3.6. Mupirocin
Mupirocin is an intranasal antibiotic used for the eradication of staphylococci infection. Mupirocin targets isoleucyl-tRNA synthetase which is required for protein synthesis, and resistance to this antibiotic arise from mutation in isoleucyl-tRNA (low level of resistance) or similarity between this target and eukaryotic enzymes (high level of resistance) [79]. The resistance to mupirocin is recorded in S. haemolyticus isolates due to the presence of the mupA gene in mupirocin resistance (MupR) plasmids [13]. Interestingly, an insertion sequence (IS257) is flanking the mupA gene which could aid in the horizontal transfer of S. haemolyticus mupA gene to the environment or other bacteria [13]. Rossi and colleagues reported the transfer of MupR from S. haemolyticus clinical isolates to S. aureus suggesting that S. haemolyticus act as a reservoir for MupR [67]. Not only in humans but high levels of MupR were recorded in MRSH in healthy and diseased dogs [113,114].
4. Virulence Factors of S. haemolyticus
Genome sequencing of common S. haemolyticus strain C10A has illustrated the brief detection of multiple antibiotic resistance and virulence genes [115]. However, many of these virulence factors remained virtually unexplored [116]. Surface substances and cytolysins have crucial effects on the virulence of S. haemolyticus [62]. Bacterial adherence and internalization are mediated by biofilm and fibronectin-binding proteins (FnBP). Following the entry to the host cell, toxins and enzymes are released by S. haemolyticus which mediate tissue damage, activation of proinflammatory cytokines, and apoptosis of host cells. Figure 2 summarizes the virulence factors that are associated with the pathogenesis of S. haemolyticus.
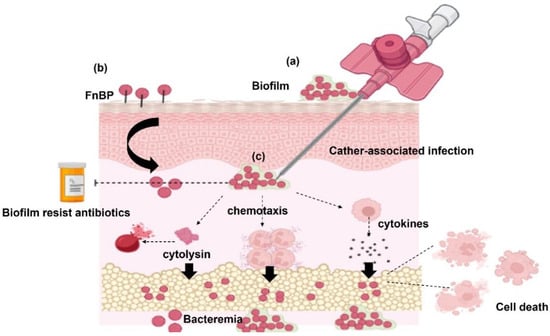
Figure 2.
Pathogenesis of S. haemolyticus. (a) S. haemolyticus isolates that form a biofilm adhere to the catheter and internalize with it inside the host. Biofilm-associated S. haemolyticus isolates are resistant to antibiotics. (b) Fibronectin-binding proteins (FnBP) of S. haemolyticus help in bacterial adherence, internalization, and invasion to host cells. (c) S. haemolyticus invades the host cells causing bacteremia through the release of cytolysins, proinflammatory cytokines from the host immune cells, and activation of chemotaxis.
4.1. Biofilm Formation
Biofilm is a polysaccharide layer produced extracellularly and aids bacterial attachment to surfaces and medical devices. S. haemolyticus isolates that form a biofilm participate in catheters and other devices associated with infections [117,118,119,120]. Biofilm-producing S. haemolyticus causes bacteremia, particularly those associated with the use of catheter-associated infections and nosocomial infections [117,118,119].
The biofilm formation by S. haemolyticus is a complex process and is increased in the presence of antimicrobial agents [117]. The impact of antibiotics on the inhibition of biofilm formation is controversial. Pereira-Ribeiro and colleagues reported that the biofilm formation of S. haemolyticus on abiotic surfaces was not inhibited by antibiotics such as linezolid, teicoplanin, vancomycin, tigecycline, rifampicin, etc. [117]. While Szczuka and colleagues showed that tigecycline/rifampicin combination (biofilm inhibitory concentration ranged from 0.062 to 1 µg/mL) was more effective than daptomycin/rifampicin combination (biofilm inhibitory concentration ranged from 0.125 to 2 µg/mL) against ica-independent biofilm, produced by S. haemolyticus [11]. The formation of biofilm and resistance to antibiotics could be the causes of persistent bacterial infections and survival inside hospitals [121].
The formation of biofilm by S. haemolyticus differs from other Staphylococci. The formation of biofilm by S. epidermidis and S. aureus is mediated by the ica operon which codes for the enzymes responsible for the formation of poly-N-acetylglucosamine/polysaccharide intercellular adhesion which participates in the formation of the biofilm matrix [122]. However, the biofilm formation by S. haemolyticus is mainly ica-independent because no icaAD genes were observed in the bacteria [117,118,119]. Moreover, In contrast to S. aureus and S. epidermidis, S. haemolyticus biofilms do not include accumulation-associated protein and biofilm-associated protein genes and are independent of polysaccharide intercellular adhesion (PIA) [118].
Still the process of biofilm formation by S. haemolyticus has not yet been extensively studied. Further studies need to verify the detailed steps of biofilm formation in S. haemolyticus and the factors regulating it.
4.2. S. haemolyticus Surface Proteins Required for Bacteria Adherence
Besides the biofilm formation, S. haemolyticus secretes fibronectin-binding proteins (FnBP) that play an important role in bacterial adherence to the extracellular matrix, bacterial internalization into the host cell, and invasion [15]. The adherence property of clinical S. haemolyticus is different from commensal S. haemolyticus. Commensal S. haemolyticus have high adherence to fibronectin and collagen, while clinical S. haemolyticus have low adherence to fibronectin and collagen [123]. However, using the bacteria surface shaving approach, 65 surface proteins were identified in clinical S. haemolyticus isolates and were associated with adherence to human keratinocytes such as the bacterial Toll/interleukin-1 like (TIRs) domain-containing protein, the bifunctional autolysin Atl, LPXTG, and the transglycosylase SceD [123].
4.3. Toxins and Enzymes
Some S. haemolyticus isolates secrete enterotoxins and/or hemolysins [55,124,125]. Staphylococci enterotoxins act as superantigens that activate the immune cells to produce their cytokines resulting in food poisoning and other diseases such as sepsis and multiorgan dysfunction [126,127]. Several enterotoxin genes were recorded in S. haemolyticus such as sea, seb, sec, seg, and sei and one or more genes could be recorded in the isolates found from blood cultures [125]. S. haemolyticus isolates containing enterotoxin genes are associated with bovine mastitis [128] and peritonitis in continuous ambulatory peritoneal dialysis (CAPD) patients [129]. In addition, enterotoxin-producing S. haemolyticus was isolated from the clinical samples of newborns [130]. Cytotoxins (also known hemolysins) are virulence factors associated with the pathogenesis of S. aureus, but data about the effect of these toxins in CoNS infections is unknown. There are several hemolysins associated with Staphylococci infections including α-hemolysin, β-hemolysin, and δ-hemolysin. α-hemolysin producing S. aureus strains that express a high level of hla gene, causes damage to the skin, neurons, epithelium, endothelial and immune cells, while the strains that are deficient in hla gene are less virulent [131,132]. Interestingly, more than 90% of S. haemolyticus harbor hla gene [125], and these isolates are associated with diabetic ulcers [133]. In addition, β-hemolysin and δ-hemolysin were detected in 81% and 40.5% of the S. haemolyticus isolates, and both toxins were recorded in 30% of the isolates [125]. β-toxin plays a role in the evasion of the pathogen to the immune system and scavenging of nutrients [134], while δ-toxin is encoded by regulatory RNAIII and affects the agr quorum-sensing system [135]. Moreover, Da and colleagues identified phenol-soluble modulins (PSMs) in S. haemolyticus isolates, and these toxins have a broad cytolytic activity [116]. Alpha-type PSM (PSMα) has a potent leucocidin and hemolytic activity, and β-type PSM has anti-gonococcal activity [116]. Furthermore, S. haemolyticus PSMs induce neutrophil chemotaxis resulting in a pronounced pro-inflammatory effect [116]. The previous findings highlight the importance of toxins in the invasion of S. haemolyticus and the development of bacteremia and sepsis.
4.4. Cytotoxicity and Apoptosis of the Host Cells
In vitro studies showed that S. haemolyticus infection could alter the host immune response through its effect on the host cells [15,136]. Krzymińska et al. showed that S. haemolyticus causes injury and loss of mitochondrial membrane potential in macrophages [136]. In addition, S. haemolyticus infection is cytotoxic to the macrophages through induction of caspase-dependent apoptosis [136]. The previous findings show one strategy for S. haemolyticus persistence and dissemination in the host through inhibition and host macrophages. Similarly, Eltwisy et al. showed that S. haemolyticus infection causes damage and apoptosis of primary human skin fibroblast cells, and it induces the release of proinflammatory cytokines from the PBMCs cocultured with the skin fibroblast [15]. Collectively, the previous reports show that S. haemolyticus infection causes damage to the host cells through apoptosis.
5. Conclusions and Future Perspectives
Human skin is colonized by an opportunistic bacterial pathogen S. haemolyticus which carries antibiotic resistance genes. S. haemolyticus, especially the clinical isolates, are mainly multidrug-resistant, and these isolates produce biofilms, toxins, and enzymes leading to infections that are difficult to treat. The increasingly growing spread of multidrug-resistant S. haemolyticus in the hospital environment could have potentially devastating complications. The presence of resistance genes in S. haemolyticus (Table 1) suggests the possibility of resistance gene transfer between S. haemolyticus and other bacteria which explains the widespread resistance to antibiotics and the survival in the hospitals. Still, not all the mechanisms of S. haemolyticus resistance to antibiotics are known, and future studies need to verify other resistance mechanisms. Importantly, the uncontrolled use of antibiotics aids in the spread of resistant S. haemolyticus isolates. Therefore, the use of antibiotics, especially in the case of S. haemolyticus, should be controlled to preserve the drug’s clinical utility.
Author Contributions
Study design: H.O.E., H.O.T., M.H.R.H., I.M.S. and M.A.E.-M. Literature search and data collection: H.O.E., H.O.T., M.H.R.H., I.M.S. and M.A.E.-M. Manuscript drafting: H.O.E., M.H.R.H. and I.M.S. Manuscript editing and revision: I.M.S. and M.A.E.-M. Supervision: I.M.S. and M.A.E.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The Figure 1 and Figure 2 present in this study were designed using free software www.BioRender.com.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| Aap | accumulation-associated protein |
| Ala | alanine amino acid |
| Bhp | biofilm-associated protein |
| BSIs | Bloodstream infections |
| CC1 | clonal complex 1 |
| CAPD | continuous ambulatory peritoneal dialysis |
| CoNS | Coagulase-negative Staph |
| CRS | catheter-related sepsis |
| eDNA | extracellular DNA |
| FnBP | fibronectin-binding proteins |
| GraS | glycopeptide resistance-associated histidine kinase |
| ica | intercellular adhesion |
| ICU | intensive care unit; IS: insertion sequence |
| MIC | minimum inhibitory concentration |
| MRSH | methicillin-resistant S. haemolyticus |
| PBP | penicillin-binding protein |
| PIA | polysaccharide intercellular adhesion |
| PSMs | phenol-soluble modulins |
| tcaRAB | teicoplanin resistance operon |
| tcaR | transcriptional regulator |
| TIRs | Toll/interleukin-1 like |
| SCCmec | Staphylococcal cassette chromosome mec |
| ST25 | sequence type 25 |
| S. | Staphylococcus. |
| UTIs | urinary tract infections |
References
- Eng, R.H.; Wang, C.; Person, A.; Kiehn, T.E.; Armstrong, D. Species identification of coagulase-negative staphylococcal isolates from blood cultures. J. Clin. Microbiol. 1982, 15, 439–442. [Google Scholar] [CrossRef] [PubMed]
- John, J.F., Jr.; Gramling, P.K.; O’Dell, N.M. Species identification of coagulase-negative staphylococci from urinary tract isolates. J. Clin. Microbiol. 1978, 8, 435–437. [Google Scholar] [CrossRef] [PubMed]
- Liekweg, W.G., Jr.; Greenfield, L.J. Vascular prosthetic infections: Collected experience and results of treatment. Surgery 1977, 81, 335–342. [Google Scholar] [PubMed]
- Rupp, M.E.; Archer, G.L. Coagulase-negative staphylococci: Pathogens associated with medical progress. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 1994, 19, 231–243, quiz 244–235. [Google Scholar] [CrossRef]
- Kloos, W.E.; Schleifer, K.H. Simplified scheme for routine identification of human Staphylococcus species. J. Clin. Microbiol. 1975, 1, 82–88. [Google Scholar] [CrossRef]
- Schaberg, D.R.; Culver, D.H.; Gaynes, R.P. Major trends in the microbial etiology of nosocomial infection. Am. J. Med. 1991, 91, 72S–75S. [Google Scholar] [CrossRef]
- Konstantinidis, K.T.; Ramette, A.; Tiedje, J.M. The bacterial species definition in the genomic era. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2006, 361, 1929–1940. [Google Scholar] [CrossRef]
- Lamers, R.P.; Muthukrishnan, G.; Castoe, T.A.; Tafur, S.; Cole, A.M.; Parkinson, C.L. Phylogenetic relationships among Staphylococcus species and refinement of cluster groups based on multilocus data. BMC Evol. Biol. 2012, 12, 171. [Google Scholar] [CrossRef]
- Ben Saida, N.; Marzouk, M.; Ferjeni, A.; Boukadida, J. A three-year surveillance of nosocomial infections by methicillin-resistant Staphylococcus haemolyticus in newborns reveals the disinfectant as a possible reservoir. Pathol. Biol. 2009, 57, 35. [Google Scholar] [CrossRef]
- Renaud, F.; Etienne, J.; Bertrand, A.; Brun, Y.; Greenland, T.B.; Freney, J.; Fleurette, J. Molecular epidemiology of Staphylococcus haemolyticus strains isolated in an Albanian hospital. J. Clin. Microbiol. 1991, 29, 1493–1497. [Google Scholar] [CrossRef]
- Szczuka, E.; Grabska, K.; Kaznowski, A. In Vitro Activity of Rifampicin Combined with Daptomycin or Tigecycline on Staphylococcus haemolyticus Biofilms. Curr. Microbiol. 2015, 71, 184–189. [Google Scholar] [CrossRef]
- Schuenck, R.P.; Pereira, E.M.; Iorio, N.; Santos, K. Multiplex PCR assay to identify methicillin-resistant Staphylococcus haemolyticus. FEMS Immunol. Med. Microbiol. 2008, 52, 431–435. [Google Scholar] [CrossRef] [PubMed]
- do Ferreira, N.; Schuenck, R.P.; dos Santos, K.; do de Bastos, M.; Giambiagi-deMarval, M. Diversity of plasmids and transmission of high-levelmupirocin mupA resistance gene in Staphylococcus haemolyticus. FEMS Immunol. Med. Microbiol. 2011, 61, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Ruzauskas, M.; Siugzdiniene, R.; Klimiene, I.; Virgailis, M.; Mockeliunas, R.; Vaskeviciute, L.; Zienius, D. Prevalence of methicillin-resistant Staphylococcus haemolyticus in companion animals: A cross-sectional study. Ann. Clin. Microbiol. Antimicrob. 2014, 13, 56. [Google Scholar] [CrossRef] [PubMed]
- Eltwisy, H.O.; Abdel-Fattah, M.; Elsisi, A.M.; Omar, M.M.; Abdelmoteleb, A.; El-Mokhtar, M.A. Pathogenesis of Staphylococcus haemolyticus on primary human skin fibroblast cells. Virulence 2020, 11, 1142–1157. [Google Scholar] [CrossRef]
- Cavanagh, J.P.; Hjerde, E.; Holden, M.T.G.; Kahlke, T.; Klingenberg, C.; Flaegstad, T.; Parkhill, J.; Bentley, S.D.; Sollid, J.U.E. Whole-genome sequencing reveals clonal expansion of multiresistant Staphylococcus haemolyticus in European hospitals. J. Antimicrob. Chemoth. 2014, 69, 2920–2927. [Google Scholar] [CrossRef]
- Hosseinkhani, F.; Jabalameli, F.; Farahani, N.N.; Taherikalani, M.; van Leeuwen, W.B.; Emaneini, M. Variable number of tandem repeat profiles and antimicrobial resistance patterns of Staphylococcus haemolyticus strains isolated from blood cultures in children. Infection 2016, 38, 19–21. [Google Scholar] [CrossRef]
- Ahmed, N.H.; Baruah, F.K.; Grover, R.K. Staphylococcal Blood Stream Infections in Cancer Patients. Ann. Med. Health Sci. Res. 2015, 5, 226–227. [Google Scholar] [CrossRef]
- Bryce, A.N.; Doocey, R.; Handy, R. Staphylococcus haemolyticus meningitis and bacteremia in an allogenic stem cell transplant patient. IDCases 2021, 26, e01259. [Google Scholar] [CrossRef]
- Viale, P.; Stefani, S. Vascular catheter-associated infections: A microbiological and therapeutic update. J. Chemother. 2006, 18, 235–249. [Google Scholar] [CrossRef]
- Sloos, J.H.; Dijkshoorn, L.; van Boven, C.P. Septicaemias caused by a strain of Staphylococcus haemolyticus exhibiting intermediate susceptibility to teicoplanin in multiple intensive care unit patients. J. Antimicrob. Chemother. 2000, 45, 410–411. [Google Scholar] [CrossRef] [PubMed]
- Spare, M.K.; Tebbs, S.E.; Lang, S.; Lambert, P.A.; Worthington, T.; Lipkin, G.W.; Elliott, T.S.J. Genotypic and phenotypic properties of coagulase-negative staphylococci causing dialysis catheter-related sepsis. J. Hosp. Infect. 2003, 54, 272–278. [Google Scholar] [CrossRef]
- Sader, H.S.; Jones, R.N.; Gales, A.C.; Silva, J.B.; Pignatari, A.C.; America, S. SENTRY antimicrobial surveillance program report: Latin american and brazilian results for 1997 through 2001. Braz. J. Infect. Dis. 2004, 8, 25–79. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Satti, L.; Zaman, G.; Gardezi, A.; Sabir, N.; Khadim, M. Catheter related recurrent blood stream infection caused by linezolid-resistant, methicillin resistant Staphylococcus haemolyticus; an emerging super bug. JPMA J. Pak. Med. Assoc. 2019, 69, 261–263. [Google Scholar]
- Panda, S.; Kar, S.; Sharma, S.; Singh, D.V. Multidrug-resistant Staphylococcus haemolyticus isolates from infected eyes and healthy conjunctivae in India. J. Glob. Antimicrob. Resist. 2016, 6, 154–159. [Google Scholar] [CrossRef]
- Malathi, J.; Sowmiya, M.; Margarita, S.; Madhavan, H.N.; Therese, K.L. Application of PCR based-RFLP for species identification of ocular isolates of methicillin resistant staphylococci (MRS). Indian J. Med. Res. 2009, 130, 78–84. [Google Scholar]
- Makki, A.R.; Sharma, S.; Duggirala, A.; Prashanth, K.; Garg, P.; Das, T. Phenotypic and genotypic characterization of coagulase negative staphylococci (CoNS) other than Staphylococcus epidermidis isolated from ocular infections. Investig. Ophthalmol. Vis. Sci. 2011, 52, 9018–9022. [Google Scholar] [CrossRef]
- Wong, M.; Baumrind, B.R.; Frank, J.H.; Halpern, R.L. Postoperative Endophthalmitis Caused by Staphylococcus haemolyticus following Femtosecond Cataract Surgery. Case Rep. Ophthalmol. 2015, 6, 435–438. [Google Scholar] [CrossRef]
- Panda, S.; Jena, S.; Sharma, S.; Dhawan, B.; Nath, G.; Singh, D.V. Identification of Novel Sequence Types among Staphylococcus haemolyticus Isolated from Variety of Infections in India. PLoS ONE 2016, 11, e0166193. [Google Scholar] [CrossRef]
- Couto, N.; Chlebowicz, M.A.; Raangs, E.C.; Friedrich, A.W.; Rossen, J.W. Complete Genome Sequences of Two Methicillin-Resistant Staphylococcus haemolyticus Isolates of Multilocus Sequence Type 25, First Detected by Shotgun Metagenomics. Genome Announc. 2018, 6, e00036-18. [Google Scholar] [CrossRef]
- Spencer, S.R.; Dealler, S.F.; Hassett, P.D.; Todd, N.J.; Hawkey, P.M.; Noble, B.A. Bacterial contamination of intraocular lenses: The source of the bacteria. Eye 1989, 3, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Kodjikian, L.; Burillon, C.; Roques, C.; Pellon, G.; Freney, J.; Renaud, F.N. Bacterial adherence of Staphylococcus epidermidis to intraocular lenses: A bioluminescence and scanning electron microscopy study. Invest. Ophthalmol. Vis. Sci. 2003, 44, 4388–4394. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Singh, D. Biofilm Formation by ica-Negative Ocular Isolates of Staphylococcus haemolyticus. Front. Microbiol. 2018, 9, 2687. [Google Scholar] [CrossRef] [PubMed]
- Brook, I. Bacteriology of neonatal omphalitis. J. Infect. 1982, 5, 127–131. [Google Scholar] [CrossRef]
- Bjorkqvist, M.; Liljedahl, M.; Zimmermann, J.; Schollin, J.; Soderquist, B. Colonization pattern of coagulase-negative staphylococci in preterm neonates and the relation to bacteremia. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2010, 29, 1085–1093. [Google Scholar] [CrossRef]
- Cave, R.; Misra, R.; Chen, J.; Wang, S.; Mkrtchyan, H.V. Whole genome sequencing revealed new molecular characteristics in multidrug resistant staphylococci recovered from high frequency touched surfaces in London. Sci. Rep. 2019, 9, 9637. [Google Scholar] [CrossRef]
- Mehri, H.; Jahanbakhsh, R.; Shakeri, F.; Ardebili, A.; Behnampour, N.; Khodabakhshi, B.; Ghaemi, E.A. Investigation of Glycopeptide Susceptibility of Coagulase-Negative Staphylococci (CoNS) From a Tertiary Care Hospital in Gorgan, Northern Iran. Arch. Pediatr. Infect. 2017, 5, e37264. [Google Scholar] [CrossRef]
- Soeorg, H.; Huik, K.; Parm, Ü.; Ilmoja, M.-L.; Metelskaja, N.; Metsvaht, T.; Lutsar, I. Genetic Relatedness of Coagulase-negative Staphylococci From Gastrointestinal Tract and Blood of Preterm Neonates With Late-onset Sepsis. Pediatric Infect. Dis. J. 2013, 32, 389–393. [Google Scholar] [CrossRef]
- Perdreau-Remington, F.; Stefanik, D.; Peters, G.; Ruckdeschel, G.; Haas, F.; Wenzel, R.; Pulverer, G. Methicillin-resistant Staphylococcus haemolyticus on the hands of health care workers: A route of transmission or a source? J. Hosp. Infect. 1995, 31, 195–203. [Google Scholar] [CrossRef]
- Klingenberg, C.; Rønnestad, A.; Anderson, A.S.; Abrahamsen, T.G.; Zorman, J.; Villaruz, A.; Flaegstad, T.; Otto, M.; Sollid, J.E. Persistent strains of coagulase-negative staphylococci in a neonatal intensive care unit: Virulence factors and invasiveness. Clin. Microbiol. Infect. 2007, 13, 1100–1111. [Google Scholar] [CrossRef]
- Daniel, B.; Saleem, M.; Naseer, G.; Fida, A. Significance of Staphylococcus Haemolyticus in Hospital Acquired Infections. J. Pioneer Med. Sci. 2014, 4, 119–125. [Google Scholar]
- Mazzariol, A.; Lo Cascio, G.; Kocsis, E.; Maccacaro, L.; Fontana, R.; Cornaglia, G. Outbreak of linezolid-resistant Staphylococcus haemolyticus in an Italian intensive care unit. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2012, 31, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, J.P.; Wolden, R.; Heise, P.; Esaiassen, E.; Klingenberg, C.; Aarag Fredheim, E.G. Antimicrobial susceptibility and body site distribution of community isolates of coagulase-negative staphylococci. APMIS Acta Pathol. Microbiol. Immunol. Scand. 2016, 124, 973–978. [Google Scholar] [CrossRef]
- Degener, J.E.; Heck, M.E.; van Leeuwen, W.J.; Heemskerk, C.; Crielaard, A.; Joosten, P.; Caesar, P. Nosocomial infection by Staphylococcus haemolyticus and typing methods for epidemiological study. J. Clin. Microbiol. 1994, 32, 2260–2265. [Google Scholar] [CrossRef] [PubMed]
- Dziri, R.; Klibi, N.; Lozano, C.; Ben Said, L.; Bellaaj, R.; Tenorio, C.; Boudabous, A.; Ben Slama, K.; Torres, C. High prevalence of Staphylococcus haemolyticus and Staphylococcus saprophyticus in environmental samples of a Tunisian hospital. Diagn. Microbiol. Infect. Dis. 2016, 85, 136–140. [Google Scholar] [CrossRef]
- Bouchami, O.; de Lencastre, H.; Miragaia, M. Impact of Insertion Sequences and Recombination on the Population Structure of Staphylococcus haemolyticus. PLoS ONE 2016, 11, e0156653. [Google Scholar] [CrossRef]
- Fraczek, M.; Piasecka, M.; Gaczarzewicz, D.; Szumala-Kakol, A.; Kazienko, A.; Lenart, S.; Laszczynska, M.; Kurpisz, M. Membrane stability and mitochondrial activity of human-ejaculated spermatozoa during in vitro experimental infection with Escherichia coli, Staphylococcus haemolyticus and Bacteroides ureolyticus. Andrologia 2012, 44, 315–329. [Google Scholar] [CrossRef]
- Fraczek, M.; Wiland, E.; Piasecka, M.; Boksa, M.; Gaczarzewicz, D.; Szumala-Kakol, A.; Kolanowski, T.; Beutin, L.; Kurpisz, M. Fertilizing potential of ejaculated human spermatozoa during in vitro semen bacterial infection. Fertil. Steril. 2014, 102, 711–719.E1. [Google Scholar] [CrossRef]
- Fraczek, M.; Hryhorowicz, M.; Gaczarzewicz, D.; Szumala-Kakol, A.; Kolanowski, T.J.; Beutin, L.; Kurpisz, M. Can apoptosis and necrosis coexist in ejaculated human spermatozoa during in vitro semen bacterial infection? J. Assist. Reprod. Genet. 2015, 32, 771–779.e711. [Google Scholar] [CrossRef]
- Pindar, C.; Viau, R.A. Staphylococcus haemolyticus epididymo-orchitis and bacteraemia: A case report. JMM Case Rep. 2018, 5, e005157. [Google Scholar] [CrossRef]
- Mazzoli, S. Biofilms in chronic bacterial prostatitis (NIH-II) and in prostatic calcifications. FEMS Immunol. Med. Microbiol. 2010, 59, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, E.; Ribes-Koninckx, C.; Calabuig, M.; Sanz, Y. Intestinal Staphylococcus spp. and virulent features associated with coeliac disease. J. Clin. Pathol. 2012, 65, 830. [Google Scholar] [CrossRef] [PubMed]
- Shittu, A.; Lin, J.; Morrison, D.; Kolawole, D. Isolation and molecular characterization of multiresistant Staphylococcus sciuri and Staphylococcus haemolyticus associated with skin and soft-tissue infections. J. Med. Microbiol. 2004, 53, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Gunn, B.A.; Davis, C.E. Staphylococcus haemolyticus urinary tract infection in a male patient. J. Clin. Microbiol. 1988, 26, 1055–1057. [Google Scholar] [CrossRef]
- Takeuchi, F.; Watanabe, S.; Baba, T.; Yuzawa, H.; Ito, T.; Morimoto, Y.; Kuroda, M.; Cui, L.; Takahashi, M.; Ankai, A.; et al. Whole-genome sequencing of staphylococcus haemolyticus uncovers the extreme plasticity of its genome and the evolution of human-colonizing staphylococcal species. J. Bacteriol. 2005, 187, 7292–7308. [Google Scholar] [CrossRef]
- Suen, K.; Mashhadian, A.; Figarsky, I.; Payumo, J.; Liu, A. A rare and important case of Staphylococcus haemolyticus-associated ventricular atrial shunt nephritis. Clin. Case Rep. 2017, 5, 2012–2016. [Google Scholar] [CrossRef]
- Gamberini, S.; Anania, G.; Incasa, E.; Zangirolami, A.; Tampieri, M.; Boari, B.; Benea, G.; Manfredini, R. Staphylococcus hemolyticus liver abscess as an uncommon presentation of silent colonic cancer: A case report. J. Am. Geriatr. Soc. 2006, 54, 1619–1620. [Google Scholar] [CrossRef]
- Bhargava, K.; Zhang, Y. Multidrug-resistant coagulase-negative Staphylococci in food animals. J. Appl. Microbiol. 2012, 113, 1027–1036. [Google Scholar] [CrossRef]
- Hope, R.; Livermore, D.M.; Brick, G.; Lillie, M.; Reynolds, R.; Su, B.W.P.R. Non-susceptibility trends among staphylococci from bacteraemias in the UK and Ireland, 2001–2006. J. Antimicrob. Chemoth. 2008, 62, Ii65–Ii74. [Google Scholar] [CrossRef]
- Bouchami, O.; Achour, W.; Mekni, M.A.; Rolo, J.; Ben Hassen, A. Antibiotic resistance and molecular characterization of clinical isolates of methicillin-resistant coagulase-negative staphylococci isolated from bacteremic patients in oncohematology. Folia Microbiol. 2011, 56, 122–130. [Google Scholar] [CrossRef]
- Rodriguez-Aranda, A.; Daskalaki, M.; Villar, J.; Sanz, F.; Otero, J.R.; Chaves, F. Nosocomial spread of linezolid-resistant Staphylococcus haemolyticus infections in an intensive care unit. Diagn. Microbiol. Infect. Dis. 2009, 63, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Czekaj, T.; Ciszewski, M.; Szewczyk, E.M. Staphylococcus haemolyticus—An emerging threat in the twilight of the antibiotics age. Microbiology 2015, 161, 2061–2068. [Google Scholar] [CrossRef] [PubMed]
- Hosseinkhani, F.; Emaneini, M.; van Leeuwen, W. High-Quality Genome Sequence of the Highly Resistant Bacterium Staphylococcus haemolyticus, Isolated from a Neonatal Bloodstream Infection. Genome Announc. 2017, 5, e00683-17. [Google Scholar] [CrossRef] [PubMed]
- Bakthavatchalam, Y.D.; Sudarsanam, T.D.; Babu, P.; Munuswamy, E.; Muthuirulandi Sethuvel, D.P.; Devanga Ragupathi, N.K.; Veeraraghavan, B. Methicillin-Susceptible Teicoplanin-Resistant Staphylococcus haemolyticus Isolate from a Bloodstream Infection with Novel Mutations in the tcaRAB Teicoplanin Resistance Operon. Jpn J. Infect. Dis. 2017, 70, 458–460. [Google Scholar] [CrossRef] [PubMed]
- Anthonisen, I.L.; Sunde, M.; Steinum, T.M.; Sidhu, M.S.; Sørum, H. Organization of the antiseptic resistance gene qacA and Tn552-related beta-lactamase genes in multidrug- resistant Staphylococcus haemolyticus strains of animal and human origins. Antimicrob. Agents Chemother. 2002, 46, 3606–3612. [Google Scholar] [CrossRef]
- Falcone, M.; Giannella, M.; Raponi, G.; Mancini, C.; Venditti, M. Teicoplanin use and emergence of Staphylococcus haemolyticus: Is there a link? Clin. Microbiol. Infect. 2006, 12, 96–97. [Google Scholar] [CrossRef]
- Rossi, C.C.; Ferreira, N.C.; Coelho, M.L.; Schuenck, R.P.; Bastos Mdo, C.; Giambiagi-deMarval, M. Transfer of mupirocin resistance from Staphylococcus haemolyticus clinical strains to Staphylococcus aureus through conjugative and mobilizable plasmids. FEMS Microbiol. Lett. 2016, 363, fnw121. [Google Scholar] [CrossRef]
- Kim, H.J.; Jang, S. Draft genome sequence of multidrug-resistant Staphylococcus haemolyticus IPK_TSA25 harbouring a Staphylococcus aureus plasmid, pS0385-1. J. Glob. Antimicrob. Resist. 2017, 11, 8–9. [Google Scholar] [CrossRef]
- Correa, J.E.; Paulis, D.A.; Predari, S.; Sordelli, D.O.; Jeric, P.E. First report of qacG, qacH and qacJ genes in Staphylococcus haemolyticus human clinical isolates. J. Antimicrob. Chemoth. 2008, 62, 956–960. [Google Scholar] [CrossRef]
- Bochniarz, M.; Wawron, W.; Szczubial, M. Resistance to methicillin of coagulase-negative staphylococci (CNS) isolated from bovine mastitis. Pol. J. Vet. Sci. 2013, 16, 687–692. [Google Scholar] [CrossRef][Green Version]
- Billot-Klein, D.; Gutmann, L.; Bryant, D.; Bell, D.; Van Heijenoort, J.; Grewal, J.; Shlaes, D.M. Peptidoglycan synthesis and structure in Staphylococcus haemolyticus expressing increasing levels of resistance to glycopeptide antibiotics. J. Bacteriol. 1996, 178, 4696–4703. [Google Scholar] [CrossRef] [PubMed]
- Quiles-Melero, I.; Gómez-Gil, R.; Romero-Gómez, M.P.; Sánchez-Díaz, A.M.; Pablos, M.d.; García-Rodriguez, J.; Gutiérrez, A.; Mingorance, J. Mechanisms of Linezolid Resistance among Staphylococci in a Tertiary Hospital. J. Clin. Microbiol. 2013, 51, 998–1001. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Rawre, J.; Trikha, A.; Sreenivas, V.; Sood, S.; Kapil, A.; Dhawan, B. Linezolid-resistant Staphylococcus haemolyticus: Emergence of G2447U & C2534U mutations at the domain V of 23S ribosomal RNA gene in a tertiary care hospital in India. Indian J. Med. Res. 2019, 149, 795–798. [Google Scholar] [CrossRef] [PubMed]
- Brijwal, M.; Dhawan, B.; Rawre, J.; Sebastian, S.; Kapil, A. Clonal dissemination of linezolid-resistant Staphylococcus haemolyticus harbouring a G2576T mutation and the cfr gene in an Indian hospital. J. Med. Microbiol. 2016, 65, 698–700. [Google Scholar] [CrossRef]
- Long, K.S.; Vester, B. Resistance to linezolid caused by modifications at its binding site on the ribosome. Antimicrob. Agents Chemother. 2012, 56, 603–612. [Google Scholar] [CrossRef]
- Brisson-Noel, A.; Delrieu, P.; Samain, D.; Courvalin, P. Inactivation of lincosaminide antibiotics in Staphylococcus. Identification of lincosaminide O-nucleotidyltransferases and comparison of the corresponding resistance genes. J. Biol. Chem. 1988, 263, 15880–15887. [Google Scholar] [CrossRef]
- Brisson-Noël, A.; Courvalin, P. Nucleotide sequence of gene linA encoding resistance to lincosamides in Staphylococcus haemolyticus. Gene 1986, 43, 247–253. [Google Scholar] [CrossRef]
- Novotna, G.; Janata, J. A New Evolutionary Variant of the Streptogramin A Resistance Protein, Vga(A)LC, from Staphylococcus haemolyticus with Shifted Substrate Specificity towards Lincosamides. Antimicrob. Agents Chemother. 2006, 50, 4070–4076. [Google Scholar] [CrossRef]
- Thomas, C.M.; Hothersall, J.; Willis, C.L.; Simpson, T.J. Resistance to and synthesis of the antibiotic mupirocin. Nat. Rev. Microbiol. 2010, 8, 281–289. [Google Scholar] [CrossRef]
- Barros, E.M.; Ceotto, H.; Bastos, M.C.F.; dos Santos, K.R.N.; Giambiagi-deMarval, M. Staphylococcus haemolyticus as an Important Hospital Pathogen and Carrier of Methicillin Resistance Genes. J. Clin. Microbiol. 2012, 50, 166–168. [Google Scholar] [CrossRef]
- Manoharan, M.; Sistla, S.; Ray, P. Prevalence and Molecular Determinants of Antimicrobial Resistance in Clinical Isolates of Staphylococcus haemolyticus from India. Microb. Drug Resist. 2021, 27, 501–508. [Google Scholar] [CrossRef] [PubMed]
- De Vecchi, E.; George, D.A.; Romanò, C.L.; Pregliasco, F.E.; Mattina, R.; Drago, L. Antibiotic sensitivities of coagulase-negative staphylococci and Staphylococcus aureus in hip and knee periprosthetic joint infections: Does this differ if patients meet the International Consensus Meeting Criteria? Infect. Drug Resist. 2018, 11, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Stewart, G.T. Changes in Sensitivity of Staphylococci to Methicillin. Br. Med. J. 1961, 1, 863. [Google Scholar] [CrossRef]
- John, J.F.; Harvin, A.M. History and evolution of antibiotic resistance in coagulase-negative staphylococci: Susceptibility profiles of new anti-staphylococcal agents. Ther. Clin. Risk Manag. 2007, 3, 1143–1152. [Google Scholar] [PubMed]
- Hanssen, A.M.; Ericson Sollid, J.U. SCCmec in staphylococci: Genes on the move. FEMS Immunol. Med. Microbiol. 2006, 46, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Katayama, Y.; Asada, K.; Mori, N.; Tsutsumimoto, K.; Tiensasitorn, C.; Hiramatsu, K. Structural comparison of three types of staphylococcal cassette chromosome mec integrated in the chromosome in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2001, 45, 1323–1336. [Google Scholar] [CrossRef]
- Zong, Z.; Peng, C.; Lü, X. Diversity of SCCmec elements in methicillin-resistant coagulase-negative staphylococci clinical isolates. PLoS ONE 2011, 6, e20191. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, H.S.; Park, J.Y.; Koo, H.S.; Choi, C.S.; Song, W.; Cho, H.C.; Lee, K.M. Contamination of X-ray cassettes with methicillin-resistant Staphylococcus aureus and methicillin-resistant Staphylococcus haemolyticus in a radiology department. Ann. Lab. Med. 2012, 32, 206–209. [Google Scholar] [CrossRef]
- Koksal, F.; Yasar, H.; Samasti, M. Antibiotic resistance patterns of coagulase-negative staphylococcus strains isolated from blood cultures of septicemic patients in Turkey. Microbiol. Res. 2009, 164, 404–410. [Google Scholar] [CrossRef]
- Berglund, C.; Söderquist, B. The origin of a methicillin-resistant Staphylococcus aureus isolate at a neonatal ward in Sweden—possible horizontal transfer of a staphylococcal cassette chromosome mec between methicillin-resistant Staphylococcus haemolyticus and Staphylococcus aureus. Clin. Microbiol. Infect. 2008, 14, 1048–1056. [Google Scholar] [CrossRef]
- Guggenheim, M.; Zbinden, R.; Handschin, A.E.; Burns, G.-A. Changes in bacterial isolates from burn wounds and their antibiograms: A 20-year study (1986–2005). Burns 2009, 35, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Kresken, M.; Becker, K.; Seifert, H.; Leitner, E.; Korber-Irrgang, B.; von Eiff, C.; Loschmann, P.A.; Study, G. Resistance trends and in vitro activity of tigecycline and 17 other antimicrobial agents against Gram-positive and Gram-negative organisms, including multidrug-resistant pathogens, in Germany. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2011, 30, 1095–1103. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sader, H.S.; Jones, R.N. Antimicrobial activity of daptomycin in comparison to glycopeptides and other antimicrobials when tested against numerous species of coagulase-negative Staphylococcus. Diagn. Microbiol. Infect. Dis. 2012, 73, 212–214. [Google Scholar] [CrossRef] [PubMed]
- von Eiff, C.; Friedrich, A.W.; Becker, K.; Peters, G. Comparative in vitro activity of ceftobiprole against staphylococci displaying normal and small-colony variant phenotypes. Antimicrob. Agents Chemother. 2005, 49, 4372–4374. [Google Scholar] [CrossRef]
- James, R.C.; Pierce, J.G.; Okano, A.; Xie, J.; Boger, D.L. Redesign of glycopeptide antibiotics: Back to the future. ACS Chem. Biol. 2012, 7, 797–804. [Google Scholar] [CrossRef]
- Tacconelli, E.; Tumbarello, M.; de Gaetano Donati, K.; Bettio, M.; Spanu, T.; Leone, F.; Sechi, L.A.; Zanetti, S.; Fadda, G.; Cauda, R. Glycopeptide Resistance among Coagulase-Negative Staphylococci that Cause Bacteremia: Epidemiological and Clinical Findings from a Case-Control Study. Clin. Infect. Dis. 2001, 33, 1628–1635. [Google Scholar] [CrossRef]
- Aubert, G.; Passot, S.; Lucht, F.; Dorche, G. Selection of vancomycin- and teicoplanin-resistant Staphylococcus haemolyticus during teicoplanin treatment of S. epidermidis infection. J. Antimicrob. Chemother. 1990, 25, 491–493. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Staphylococcus aureus resistant to vancomycin--United States, 2002. MMWR. Morb. Mortal. Wkly. Rep. 2002, 51, 565–567. [Google Scholar]
- Schwalbe, R.S.; Stapleton, J.T.; Gilligan, P.H. Emergence of vancomycin resistance in coagulase-negative staphylococci. N. Engl. J. Med. 1987, 316, 927–931. [Google Scholar] [CrossRef]
- Sanyal, D.; Greenwood, D. An electronmicroscope study of glycopeptide antibiotic-resistant strains of Staphylococcus epidermidis. J. Med. Microbiol. 1993, 39, 204–210. [Google Scholar] [CrossRef]
- Giovanetti, E.; Biavasco, F.; Pugnaloni, A.; Lupidi, R.; Biagini, G.; Varaldo, P.E. An electron microscopic study of clinical and laboratory-derived strains of teicoplanin-resistant Staphylococcus haemolyticus. Microb. Drug Resist. 1996, 2, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Biavasco, F.; Vignaroli, C.; Varaldo, P.E. Glycopeptide resistance in coagulase-negative staphylococci. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2000, 19, 403–417. [Google Scholar] [CrossRef]
- Cunningham, R.; Gurnell, M.; Bayston, R.; Cockayne, A.; Shelton, A. Teicoplanin resistance in Staphylococcus haemolyticus, developing during treatment. J. Antimicrob. Chemother. 1997, 39, 438–439. [Google Scholar] [CrossRef] [PubMed]
- Schwalbe, R.S.; Ritz, W.J.; Verma, P.R.; Barranco, E.A.; Gilligan, P.H. Selection for vancomycin resistance in clinical isolates of Staphylococcus haemolyticus. J. Infect. Dis. 1990, 161, 45–51. [Google Scholar] [CrossRef]
- Tsakris, A.; Papadimitriou, E.; Douboyas, J.; Antoniadis, A. Emergence of teicoplanin-resistant Staphylococcus haemolyticus clinical isolates in Greece. J. Antimicrob. Chemother. 2000, 46, 1040–1041. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Blans, M.; Troelstra, A. Glycopeptide resistance in Staphylococcus haemolyticus during treatment with teicoplanin. Infect. Control Hosp. Epidemiol. 2001, 22, 263–264. [Google Scholar] [CrossRef][Green Version]
- Rajan, V.; Kumar, V.G.; Gopal, S. A cfr-positive clinical staphylococcal isolate from India with multiple mechanisms of linezolid-resistance. Indian J. Med. Res. 2014, 139, 463–467. [Google Scholar]
- Matlani, M.; Shende, T.; Bhandari, V.; Dawar, R.; Sardana, R.; Gaind, R. Linezolid-resistant mucoid Staphylococcus haemolyticus from a tertiary-care centre in Delhi. New Microbes New Infect. 2016, 11, 57–58. [Google Scholar] [CrossRef][Green Version]
- de Almeida, L.M.; Lincopan, N.; de Araújo, M.R.; Mamizuka, E.M. Clonal dissemination of linezolid-resistant Staphylococcus haemolyticus exhibiting the G2576T mutation in the 23S rRNA gene in a tertiary care hospital in Brazil. Antimicrob. Agents Chemother. 2012, 56, 2792–2793. [Google Scholar] [CrossRef]
- Kehrenberg, C.; Schwarz, S.; Jacobsen, L.; Hansen, L.H.; Vester, B. A new mechanism for chloramphenicol, florfenicol and clindamycin resistance: Methylation of 23S ribosomal RNA at A2503. Mol. Microbiol. 2005, 57, 1064–1073. [Google Scholar] [CrossRef]
- Leclercq, R.; Carlier, C.; Duval, J.; Courvalin, P. Plasmid-mediated resistance to lincomycin by inactivation in Staphylococcus haemolyticus. Antimicrob. Agents Chemother. 1985, 28, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Novotna, G.; Adamkova, V.; Janata, J.; Melter, O.; Spizek, J. Prevalence of resistance mechanisms against macrolides and lincosamides in methicillin-resistant coagulase-negative staphylococci in the Czech Republic and occurrence of an undefined mechanism of resistance to lincosamides. Antimicrob. Agents Chemother. 2005, 49, 3586–3589. [Google Scholar] [CrossRef] [PubMed]
- Bean, D.C.; Wigmore, S.M.; Wareham, D.W. Draft Genome Sequence of a Canine Isolate of Methicillin-Resistant Staphylococcus haemolyticus. Genome Announc. 2017, 5, e00146-17. [Google Scholar] [CrossRef] [PubMed]
- Kizerwetter-Świda, M.; Chrobak-Chmiel, D.; Rzewuska, M. High-level mupirocin resistance in methicillin-resistant staphylococci isolated from dogs and cats. BMC Vet. Res. 2019, 15, 238. [Google Scholar] [CrossRef]
- Chan, K.-G.; Ng, K.; Chong, T.; Pang, Y.; Kamarulzaman, A.; Yin, W.-F.; Tee, K. Antibiotic Resistant and Virulence Determinants of Staphylococcus haemolyticus C10A as Revealed by Whole Genome Sequencing. J. Genom. 2015, 3, 72–74. [Google Scholar] [CrossRef]
- Da, F.; Joo, H.-S.; Cheung, G.Y.C.; Villaruz, A.E.; Rohde, H.; Luo, X.; Otto, M. Phenol-Soluble Modulin Toxins of Staphylococcus haemolyticus. Front. Cell. Infect. Microbiol. 2017, 7, 206. [Google Scholar] [CrossRef]
- Pereira-Ribeiro, P.M.; Sued-Karam, B.R.; Faria, Y.V.; Nogueira, B.A.; Colodette, S.S.; Fracalanzza, S.E.; Duarte, J.L.; Junior, R.H.; Mattos-Guaraldi, A.L. Influence of antibiotics on biofilm formation by different clones of nosocomial Staphylococcus haemolyticus. Future Microbiol. 2019, 14, 789–799. [Google Scholar] [CrossRef]
- Fredheim, E.; Klingenberg, C.; Rohde, H.; Frankenberger, S.; Gaustad, P.; Flægstad, T.; Sollid, J. Biofilm Formation by Staphylococcus haemolyticus. J. Clin. Microbiol. 2009, 47, 1172–1180. [Google Scholar] [CrossRef]
- Silva, P.V.; Cruz, R.S.; Keim, L.S.; Paula, G.R.; Carvalho, B.T.; Coelho, L.R.; Carvalho, M.C.; Rosa, J.M.; Figueiredo, A.M.; Teixeira, L.A. The antimicrobial susceptibility, biofilm formation and genotypic profiles of Staphylococcus haemolyticus from bloodstream infections. Mem. Inst. Oswaldo Cruz 2013, 108, 812–813. [Google Scholar] [CrossRef]
- Flahaut, S.; Vinogradov, E.; Kelley, K.A.; Brennan, S.; Hiramatsu, K.; Lee, J.C. Structural and biological characterization of a capsular polysaccharide produced by Staphylococcus haemolyticus. J. Bacteriol. 2008, 190, 1649–1657. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- de Araujo, G.L.; Coelho, L.R.; de Carvalho, C.B.; Maciel, R.M.; Coronado, A.Z.; Rozenbaum, R.; Ferreira-Carvalho, B.T.; Figueiredo, A.M.; Teixeira, L.A. Commensal isolates of methicillin-resistant Staphylococcus epidermidis are also well equipped to produce biofilm on polystyrene surfaces. J. Antimicrob. Chemother. 2006, 57, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Wolden, R.; Pain, M.; Karlsson, R.; Karlsson, A.; Aarag Fredheim, E.G.; Cavanagh, J.P. Identification of surface proteins in a clinical Staphylococcus haemolyticus isolate by bacterial surface shaving. BMC Microbiol. 2020, 20, 80. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.; Gomez-Lucia, E.; Piriz, S.; Goyache, J.; Orden, J.A.; Vadillo, S. Enterotoxin production by staphylococci isolated from healthy goats. Appl. Environ. Microbiol. 1990, 56, 1323–1326. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, L.; Brito, C.I.; de Oliveira, A.; Martins, P.Y.; Pereira, V.C.; da Cunha Mde, L. Staphylococcus epidermidis and Staphylococcus haemolyticus: Molecular Detection of Cytotoxin and Enterotoxin Genes. Toxins 2015, 7, 3688–3699. [Google Scholar] [CrossRef]
- da Cunha Mde, L.; Calsolari, R.A.; Júnior, J.P. Detection of enterotoxin and toxic shock syndrome toxin 1 genes in Staphylococcus, with emphasis on coagulase-negative staphylococci. Microbiol. Immunol. 2007, 51, 381–390. [Google Scholar] [CrossRef]
- Taylor, A.L.; Llewelyn, M.J. Superantigen-induced proliferation of human CD4+CD25- T cells is followed by a switch to a functional regulatory phenotype. J. Immunol. 2010, 185, 6591–6598. [Google Scholar] [CrossRef]
- de Freitas Guimarães, F.; Nóbrega, D.B.; Richini-Pereira, V.B.; Marson, P.M.; de Figueiredo Pantoja, J.C.; Langoni, H. Enterotoxin genes in coagulase-negative and coagulase-positive staphylococci isolated from bovine milk. J. Dairy Sci. 2013, 96, 2866–2872. [Google Scholar] [CrossRef]
- Barretti, P.; Montelli, A.C.; Batalha, J.E.; Caramori, J.C.; Cunha Mde, L. The role of virulence factors in the outcome of staphylococcal peritonitis in CAPD patients. BMC Infect. Dis. 2009, 9, 212. [Google Scholar] [CrossRef]
- Vasconcelos, N.G.; Pereira, V.C.; Araújo Júnior, J.P.; da Cunha Mde, L. Molecular detection of enterotoxins E, G, H and I in Staphylococcus aureus and coagulase-negative staphylococci isolated from clinical samples of newborns in Brazil. J. Appl. Microbiol. 2011, 111, 749–762. [Google Scholar] [CrossRef]
- Breuer, K.; Wittmann, M.; Kempe, K.; Kapp, A.; Mai, U.; Dittrich-Breiholz, O.; Kracht, M.; Mrabet-Dahbi, S.; Werfel, T. Alpha-toxin is produced by skin colonizing Staphylococcus aureus and induces a T helper type 1 response in atopic dermatitis. Clin. Exp. Allergy 2005, 35, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Berube, B.J.; Bubeck Wardenburg, J. Staphylococcus aureus α-toxin: Nearly a century of intrigue. Toxins 2013, 5, 1140–1166. [Google Scholar] [CrossRef] [PubMed]
- Moraveji, Z.; Tabatabaei, M.; Shirzad Aski, H.; Khoshbakht, R. Characterization of hemolysins of Staphylococcus strains isolated from human and bovine, southern Iran. Iran. J. Vet. Res. 2014, 15, 326–330. [Google Scholar] [PubMed]
- Huseby, M.; Shi, K.; Brown, C.K.; Digre, J.; Mengistu, F.; Seo, K.S.; Bohach, G.A.; Schlievert, P.M.; Ohlendorf, D.H.; Earhart, C.A. Structure and biological activities of beta toxin from Staphylococcus aureus. J. Bacteriol. 2007, 189, 8719–8726. [Google Scholar] [CrossRef]
- Novick, R.P. Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol. Microbiol. 2003, 48, 1429–1449. [Google Scholar] [CrossRef]
- Krzyminska, S.; Szczuka, E.; Kaznowski, A. Staphylococcus haemolyticus strains target mitochondria and induce caspase-dependent apoptosis of macrophages. Antonie Van Leeuwenhoek 2012, 102, 611–620. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).