Evaluation of Commercial Concentration Methods for Microscopic Diagnosis of Protozoa and Helminths in Human Stool Samples in a Non-Endemic Area
Abstract
:1. Introduction
2. Materials and Methods
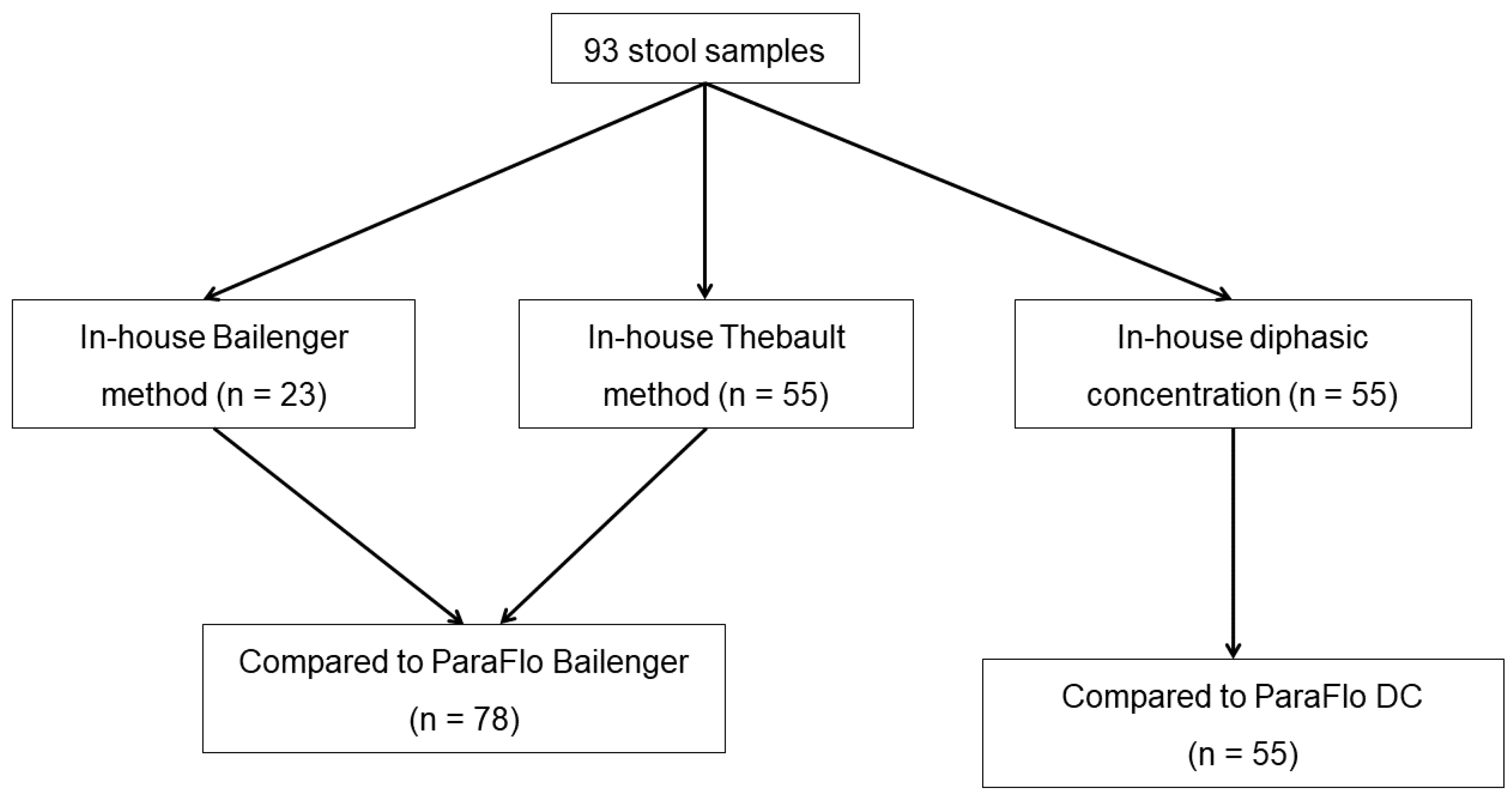
2.1. Clinical Samples
2.2. In-House Concentration Methods
2.3. Commercial Concentration Methods
2.4. Statistical Analysis
3. Results
3.1. Samples and Techniques
3.2. Overall Detection of Parasite Species
3.3. Diagnostic Concordance between In-House and Commercial Assays
3.4. Helminth and Protozoa Detection Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Herricks, J.R.; Hotez, P.J.; Wanga, V.; Coffeng, L.E.; Haagsma, J.A.; Basáñez, M.-G.; Buckle, G.; Budke, C.M.; Carabin, H.; Fèvre, E.M.; et al. The Global Burden of Disease Study 2013: What does it mean for the NTDs? PLoS Negl. Trop. Dis. 2017, 11, e0005424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pullan, R.L.; Smith, J.L.; Jasrasaria, R.; Brooker, S.J. Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasites Vectors 2014, 7, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buonfrate, D.; Bisanzio, D.; Giorli, G.; Odermatt, P.; Fürst, T.; Greenaway, C.; French, M.; Reithinger, R.; Gobbi, F.; Montresor, A.; et al. The global prevalence of Strongyloides stercoralis infection. Pathogens 2020, 9, 468. [Google Scholar] [CrossRef]
- Ayabina, D.; Kura, K.; Toor, J.; Graham, M.; Anderson, R.M.; Hollingsworth, T.D. Maintaining low prevalence of Schistosoma mansoni: Modeling the effect of less frequent treatment. Clin. Infect. Dis. 2021, 72, S140–S145. [Google Scholar] [CrossRef] [PubMed]
- GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 385, 117–171. [Google Scholar] [CrossRef]
- McHardy, I.H.; Wu, M.; Shimizu-Cohen, R.; Couturier, M.R.; Humphries, R.M. Detection of intestinal protozoa in the clinical laboratory. J. Clin. Microbiol. 2014, 52, 712–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailenger, J.; Carcenac, F.; Fourrier, M.F. Comparative value of several classical diphasic methods in parasitic coprology. Ann. Biol. Clin. 1970, 28, 425–430. [Google Scholar]
- Leméteil, D.; Gargala, G.; Razakandrainibe, R.; Ballet, J.J.; Favennec, L.; Costa, D. Comparative evaluation of commercial concentration procedures for human intestinal parasite detection. Lab. Med. 2019, 50, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Barda, B.D.; Rinaldi, L.; Ianniello, D.; Zepherine, H.; Salvo, F.; Sadutshang, T.; Cringoli, G.; Clementi, M.; Albonico, M. Mini-FLOTAC, an innovative direct diagnostic technique for intestinal parasitic infections: Experience from the field. PLoS Negl. Trop. Dis. 2013, 7, e2344. [Google Scholar] [CrossRef] [Green Version]
- Allam, A.F.; Farag, H.F.; Lotfy, W.; Fawzy, H.H.; Elhadad, H.; Shehab, A.Y. Comparison among FLOTAC, Kato-Katz and formalin ether concentration techniques for diagnosis of intestinal parasitic infections in school children in an Egyptian rural setting. Parasitology 2021, 148, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Coulibaly, J.T.; Ouattara, M.; Becker, S.L.; Lo, N.C.; Keiser, J.; N’Goran, E.K.; Ianniello, D.; Rinaldi, L.; Cringoli, G.; Utzinger, J. Comparison of sensitivity and faecal egg counts of mini-FLOTAC using fixed stool samples and Kato-Katz technique for the diagnosis of Schistosoma mansoni and soil-transmitted helminths. Acta Trop. 2016, 164, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Dirani, G.; Zannoli, S.; Paesini, E.; Farabegoli, P.; Dalmo, B.; Vocale, C.; Liguori, G.; Varani, S.; Sambri, V. EasyscreenTM enteric protozoa assay for the detection of intestinal parasites: A retrospective bi-center study. J. Parasitol. 2019, 105, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Frickmann, H.; Hoffmann, T.; Köller, T.; Hahn, A.; Podbielski, A.; Landt, O.; Loderstädt, U.; Tannich, E. Comparison of five commercial real-time PCRs for in-vitro diagnosis of Entamoeba histolytica, Giardia duodenalis, Cryptosporidium spp., Cyclospora cayetanensis, and Dientamoeba fragilis in human stool samples. Travel Med. Infect. Dis. 2021, 41, 102042. [Google Scholar] [CrossRef] [PubMed]
- Argy, N.; Nourrisson, C.; Aboubacar, A.; Poirier, P.; Valot, S.; Laude, A.; Desoubeaux, G.; Pomares, C.; Machouart, M.; Govic, Y.L.; et al. Selecting a multiplex PCR panel for accurate molecular diagnosis of intestinal protists: A comparative study of Allplex® (Seegene®), G-DiaParaTrio (Diagenode®), and RIDA®GENE (R-Biopharm®) assays and microscopic examination. Parasite 2022, 29, 5. [Google Scholar] [CrossRef] [PubMed]
- Autier, B.; Belaz, S.; Razakandrainibe, R.; Gangneux, J.-P.; Robert-Gangneux, F. Comparison of three commercial multiplex PCR Assays for the diagnosis of intestinal protozoa. Parasite 2018, 25, 48. [Google Scholar] [CrossRef] [PubMed]
- Momčilović, S.; Cantacessi, C.; Arsić-Arsenijević, V.; Otranto, D.; Tasić-Otašević, S. Rapid diagnosis of parasitic diseases: Current scenario and future needs. Clin. Microbiol. Infect. 2019, 25, 290–309. [Google Scholar] [CrossRef] [Green Version]
- Autier, B.; Gangneux, J.-P.; Robert-Gangneux, F. Evaluation of the AllplexTM Gastrointestinal Panel-Parasite Assay for protozoa detection in stool samples: A retrospective and prospective study. Microorganisms 2020, 8, 569. [Google Scholar] [CrossRef]
- Weinreich, F.; Hahn, A.; Eberhardt, K.A.; Kann, S.; Köller, T.; Warnke, P.; Dupke, S.; Dekker, D.; May, J.; Frickmann, H.; et al. Multicentric evaluation of SeeGene Allplex Real-Time PCR assays targeting 28 bacterial, microsporidal and parasitic nucleic acid sequences in human stool samples. Diagnostics 2022, 12, 1007. [Google Scholar] [CrossRef]
- Autier, B.; Gangneux, J.-P.; Robert-Gangneux, F. Evaluation of the AllplexTM GI-Helminth(I) Assay, the first marketed multiplex PCR for helminth diagnosis. Parasite 2021, 28, 33. [Google Scholar] [CrossRef]
- Hartuis, S.; Lavergne, R.-A.; Nourrisson, C.; Verweij, J.; Desoubeaux, G.; Lussac-Sorton, F.; Lemoine, J.-P.; Cateau, E.; Jeddi, F.; Poirier, P.; et al. The Novodiag® Stool Parasites Assay, an innovative high-plex technique for fast detection of protozoa, helminths and microsporidia in stool samples: A retrospective and prospective study. Parasite 2022, 29, 27. [Google Scholar] [CrossRef]
- Hoffmann, T.; Hahn, A.; Verweij, J.J.; Leboulle, G.; Landt, O.; Strube, C.; Kann, S.; Dekker, D.; May, J.; Frickmann, H.; et al. Differing effects of standard and harsh nucleic acid extraction procedures on diagnostic helminth real-time PCRs applied to human stool samples. Pathogens 2021, 10, 188. [Google Scholar] [CrossRef] [PubMed]
- Autier, B.; Boukthir, S.; Degeilh, B.; Belaz, S.; Dupuis, A.; Chevrier, S.; Gangneux, J.-P.; Robert-Gangneux, F. Clinical value of serology for the diagnosis of strongyloidiasis in travelers and migrants: A 4-year retrospective study using the Bordier IVD® Strongyloides ratti ELISA Assay. Parasite 2021, 28, 79. [Google Scholar] [CrossRef] [PubMed]
- Buonfrate, D.; Sequi, M.; Mejia, R.; Cimino, R.O.; Krolewiecki, A.J.; Albonico, M.; Degani, M.; Tais, S.; Angheben, A.; Requena-Mendez, A.; et al. Accuracy of five serologic tests for the follow up of Strongyloides stercoralis infection. PLoS Negl. Trop. Dis. 2015, 9, e0003491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peiffer-Smadja, N.; Dellière, S.; Rodriguez, C.; Birgand, G.; Lescure, F.-X.; Fourati, S.; Ruppé, E. Machine learning in the clinical microbiology laboratory: Has the time come for routine practice? Clin. Microbiol. Infect. 2020, 26, 1300–1309. [Google Scholar] [CrossRef]
| Parasite Species | Number of Positive Samples Detected With | |||
|---|---|---|---|---|
| In-House Bailenger or Thebault | ParaFlo® Bailenger | In-House DC | ParaFlo® DC | |
| Entamoeba coli | 19 | 13 | 4 | 6 |
| Endolimax nana | 19 | 12 | 4 | 1 |
| Entamoeba histolytica/dispar | 4 | 0 | 0 | 1 |
| Entamoeba hartmanni | 4 | 1 | 1 | 0 |
| Blastocystis hominis | 2 | 0 | 0 | 0 |
| Cryptosporidium sp. | 1 | 1 | 0 | 0 |
| Sarcocystis hominis | 0 | 1 | 0 | 0 |
| Giardia intestinalis | 12 | 7 | 2 | 1 |
| Chilomastix mesnilii | 2 | 2 | 0 | 0 |
| Schistosoma mansoni | 5 | 6 | 12 | 13 |
| Hymenolepis nana | 2 | 3 | 3 | 4 |
| Enterobius vermicularis | 0 | 0 | 1 | 0 |
| Ancylostomatidae | 1 | 1 | 0 | 0 |
| Trichuris trichiura | 2 | 2 | 1 | 1 |
| Taenia sp. | 1 | 1 | 0 | 0 |
| Total | 74 | 50 | 28 | 27 |
| No. of Samples With | ParaFlo® Bailenger vs. In-House Bailenger N = 23 | ParaFlo® Bailenger vs. Thebault Method N = 55 | ParaFlo®DC vs. In-House DC N = 55 |
|---|---|---|---|
| Concordant results | 16 (70) | 38 (69) | 41 (75) |
| Concordant negative results, n (%) | 10 (43.5) | 18 (33) | 29 (52.7) |
| Concordant positive results, n (%) | 6 (26) | 20 (36) | 12 (21.8) |
| Partially positive results of ParaFlo® compared to in-house method, n (%) | 0 | 8 (15) | 2 (3.5) |
| Partially positive results of in-house method compared to ParaFlo®, n (%) | 0 | 2 (3) | 1 (2) |
| False negative result of ParaFlo® method, n (%) | 6 (26) | 6 * (11) | 6 (11) |
| False negative result of in-house method, n (%) | 1 (4.5) | 2 * (3) | 5 (9) |
| Comparison of Techniques | Overall Concordance % (n/N) | No. of Samples with False Negative Results Using: | p-Value 1 | |
|---|---|---|---|---|
| The Commercial Technique | The In-House Technique | |||
| Detection of protozoa | ||||
| ParaFlo® Bailenger vs. in-house Bailenger | 74% (17/23) | 5 | 1 | 0.114 ns |
| ParaFlo® Bailenger vs. Thebault method | 75% (41/55) | 13 | 1 | <0.001 |
| ParaFlo® DC vs. in-house DC | 85% (47/55) | 5 | 3 | 0.65 ns |
| Detection of helminths | ||||
| ParaFlo® Bailenger vs. in-house Bailenger | 96% (22/23) | 1 | 0 | 1 ns |
| ParaFlo® Bailenger vs. Thebault method | 93% (51/55) | 1 | 3 | 0.586 ns |
| ParaFlo® DC vs. in-house DC | 87% (48/55) | 3 | 4 | 1 ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Autier, B.; Gangneux, J.-P.; Robert-Gangneux, F. Evaluation of Commercial Concentration Methods for Microscopic Diagnosis of Protozoa and Helminths in Human Stool Samples in a Non-Endemic Area. Microorganisms 2022, 10, 1237. https://doi.org/10.3390/microorganisms10061237
Autier B, Gangneux J-P, Robert-Gangneux F. Evaluation of Commercial Concentration Methods for Microscopic Diagnosis of Protozoa and Helminths in Human Stool Samples in a Non-Endemic Area. Microorganisms. 2022; 10(6):1237. https://doi.org/10.3390/microorganisms10061237
Chicago/Turabian StyleAutier, Brice, Jean-Pierre Gangneux, and Florence Robert-Gangneux. 2022. "Evaluation of Commercial Concentration Methods for Microscopic Diagnosis of Protozoa and Helminths in Human Stool Samples in a Non-Endemic Area" Microorganisms 10, no. 6: 1237. https://doi.org/10.3390/microorganisms10061237
APA StyleAutier, B., Gangneux, J.-P., & Robert-Gangneux, F. (2022). Evaluation of Commercial Concentration Methods for Microscopic Diagnosis of Protozoa and Helminths in Human Stool Samples in a Non-Endemic Area. Microorganisms, 10(6), 1237. https://doi.org/10.3390/microorganisms10061237








