Spontaneous Bacterial Peritonitis: The Incremental Value of a Fast and Direct Bacterial Identification from Ascitic Fluids Inoculated in Blood Culture Bottles by MALDI-TOF MS for a Better Management of Patients
Abstract
:1. Introduction
2. Materials and Methods
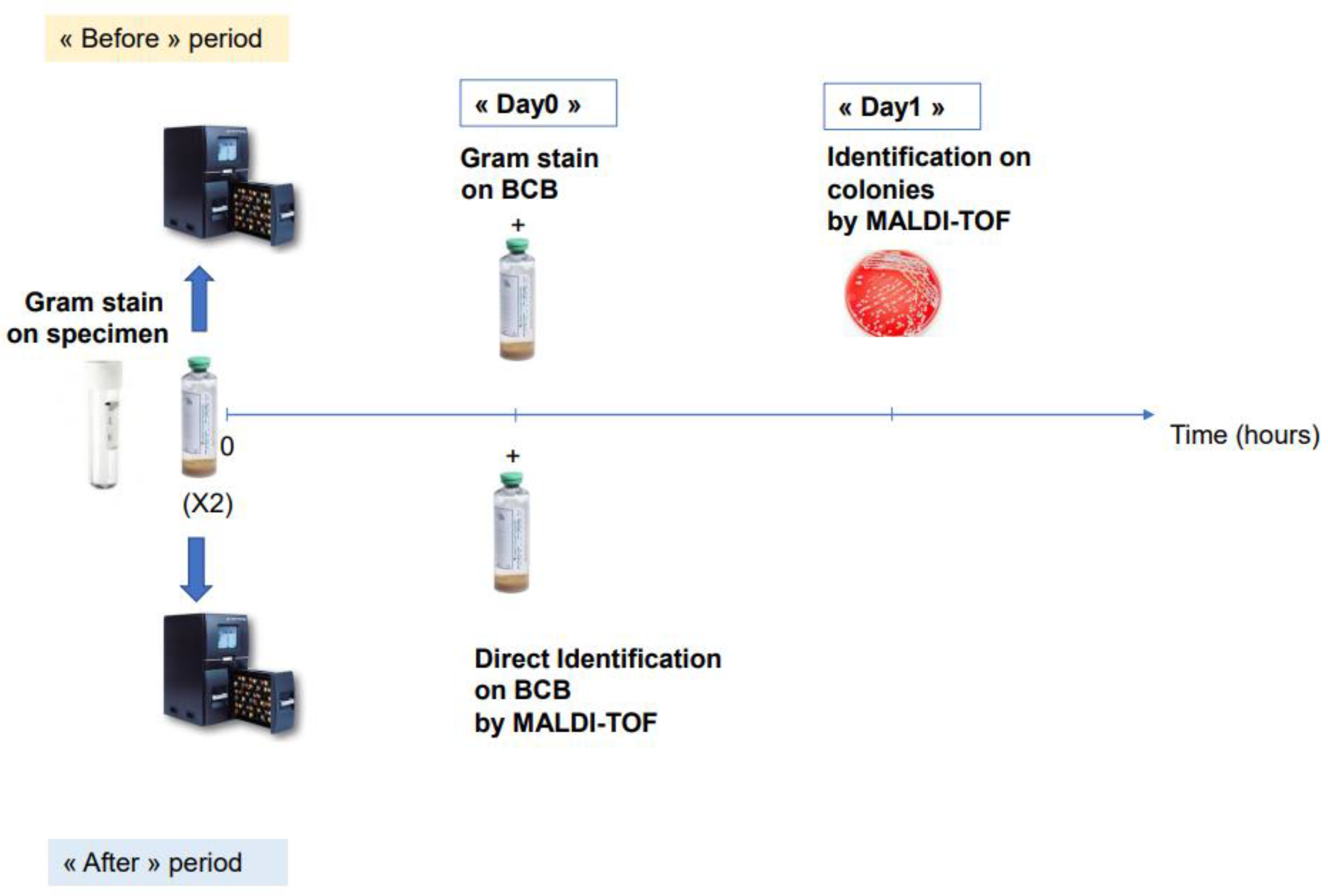
2.1. Study Design and Samples Procedure
2.2. MALDI-TOF MS
2.3. Patients and Data Collection
2.4. Evaluation of Decreasing Antibiotic Optimization Turnaround Time
2.5. Statistical Analyses
3. Results
3.1. Analysis of Direct Bacterial Identification by MALDI-TOF MS for Ascitic Fluid
3.2. Impact of Our Method for the Management of Infected Patients: Before-and-After Case–Control Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rimola, A.; García-Tsao, G.; Navasa, M.; Piddock, L.J.V.; Planas, R.; Bernard, B.; Inadomi, J.M. Diagnosis, Treatment and Prophylaxis of Spontaneous Bacterial Peritonitis: A Consensus Document. J. Hepatol. 2000, 32, 142–153. [Google Scholar] [CrossRef]
- Caly, W.R.; Strauss, E. A Prospective Study of Bacterial Infections in Patients with Cirrhosis. J. Hepatol. 1993, 18, 353–358. [Google Scholar] [CrossRef]
- Fernández, J.; Navasa, M.; Gómez, J.; Colmenero, J.; Vila, J.; Arroyo, V.; Rodés, J. Bacterial Infections in Cirrhosis: Epidemiological Changes with Invasive Procedures and Norfloxacin Prophylaxis: Bacterial Infections in Cirrhosis: Epidemiological Changes With Invasive Procedures and Norfloxacin Prophylaxis. Hepatology 2002, 35, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Wong, F. Sepsis in Cirrhosis: Report on the 7th Meeting of the International Ascites Club. Gut 2005, 54, 718–725. [Google Scholar] [CrossRef] [Green Version]
- European Association For The Study Of The Liver. EASL Clinical Practice Guidelines on the Management of Ascites, Spontaneous Bacterial Peritonitis, and Hepatorenal Syndrome in Cirrhosis. J. Hepatol. 2010, 53, 397–417. [Google Scholar] [CrossRef]
- Garcia-Tsao, G. Current Management of the Complications of Cirrhosis and Portal Hypertension: Variceal Hemorrhage, Ascites, and Spontaneous Bacterial Peritonitis. Dig. Dis. 2016, 34, 382–386. [Google Scholar] [CrossRef]
- Nousbaum, J.-B. Infection du Liquide D’ascite : Diagnostic, Traitement et Prévention. Available online: https://www.fmcgastro.org/wp-content/uploads/file//pdf-2015/099_106_Nousbaum.pdf (accessed on 12 May 2022).
- Simon, L.; Ughetto, E.; Gaudart, A.; Degand, N.; Lotte, R.; Ruimy, R. Direct Identification of 80 Percent of Bacteria from Blood Culture Bottles by Matrix-Assisted Laser Desorption Ionization–Time of Flight Mass Spectrometry Using a 10-Minute Extraction Protocol. J. Clin. Microbiol. 2019, 57, e01278-18. [Google Scholar] [CrossRef] [Green Version]
- La Scola, B.; Raoult, D. Direct Identification of Bacteria in Positive Blood Culture Bottles by Matrix-Assisted Laser Desorption Ionisation Time-of-Flight Mass Spectrometry. PLoS ONE 2009, 4, e8041. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, L.; Sánchez-Juanes, F.; Porras-Guerra, I.; García-García, M.I.; García-Sánchez, J.E.; González-Buitrago, J.M.; Muñoz-Bellido, J.L. Microorganisms Direct Identification from Blood Culture by Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry. Clin. Microbiol. Infect. 2011, 17, 546–551. [Google Scholar] [CrossRef] [Green Version]
- Schubert, S.; Weinert, K.; Wagner, C.; Gunzl, B.; Wieser, A.; Maier, T.; Kostrzewa, M. Novel, Improved Sample Preparation for Rapid, Direct Identification from Positive Blood Cultures Using Matrix-Assisted Laser Desorption/Ionization Time-of-Flight (MALDI-TOF) Mass Spectrometry. J. Mol. Diagn. 2011, 13, 701–706. [Google Scholar] [CrossRef]
- Foster, A.G.W. Rapid Identification of Microbes in Positive Blood Cultures by Use of the Vitek MS Matrix-Assisted Laser Desorption Ionization–Time of Flight Mass Spectrometry System. J. Clin. Microbiol. 2013, 51, 3717–3719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanguinetti, M.; Posteraro, B. Mass Spectrometry Applications in Microbiology beyond Microbe Identification: Progress and Potential. Expert Rev. Proteom. 2016, 13, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Angeletti, S. Matrix Assisted Laser Desorption Time of Flight Mass Spectrometry (MALDI-TOF MS) in Clinical Microbiology. J. Microbiol. Methods 2017, 138, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Meex, C.; Neuville, F.; Descy, J.; Huynen, P.; Hayette, M.-P.; De Mol, P.; Melin, P. Direct Identification of Bacteria from BacT/ALERT Anaerobic Positive Blood Cultures by MALDI-TOF MS: MALDI Sepsityper Kit versus an in-House Saponin Method for Bacterial Extraction. J. Med. Microbiol. 2012, 61, 1511–1516. [Google Scholar] [CrossRef]
- Christner, M.; Rohde, H.; Wolters, M.; Sobottka, I.; Wegscheider, K.; Aepfelbacher, M. Rapid Identification of Bacteria from Positive Blood Culture Bottles by Use of Matrix-Assisted Laser Desorption-Ionization Time of Flight Mass Spectrometry Fingerprinting. J. Clin. Microbiol. 2010, 48, 1584–1591. [Google Scholar] [CrossRef] [Green Version]
- Huang, A.M.; Newton, D.; Kunapuli, A.; Gandhi, T.N.; Washer, L.L.; Isip, J.; Collins, C.D.; Nagel, J.L. Impact of Rapid Organism Identification via Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Combined With Antimicrobial Stewardship Team Intervention in Adult Patients With Bacteremia and Candidemia. Clin. Infect. Dis. 2013, 57, 1237–1245. [Google Scholar] [CrossRef]
- Galar, A.; Leiva, J.; Espinosa, M.; Guillén-Grima, F.; Hernáez, S.; Yuste, J.R. Clinical and Economic Evaluation of the Impact of Rapid Microbiological Diagnostic Testing. J. Infect. 2012, 65, 302–309. [Google Scholar] [CrossRef]
- Lallemand, E.; Arvieux, C.; Coiffier, G.; Polard, J.-L.; Albert, J.-D.; Guggenbuhl, P.; Jolivet-Gougeon, A. Use of MALDI-TOF Mass Spectrometry after Liquid Enrichment (BD BactecTM) for Rapid Diagnosis of Bone and Joint Infections. Res. Microbiol. 2017, 168, 122–129. [Google Scholar] [CrossRef]
- Noll, C.; Nasruddin-Yekta, A.; Sternisek, P.; Weig, M.; Groß, U.; Schilling, A.F.; Beil, F.T.; Bader, O. Rapid Direct Detection of Pathogens for Diagnosis of Joint Infections by MALDI-TOF MS after Liquid Enrichment in the BacT/Alert Blood Culture System. PLoS ONE 2020, 15, e0243790. [Google Scholar] [CrossRef]
- Klein, S.; Zimmermann, S.; Köhler, C.; Mischnik, A.; Alle, W.; Bode, K.A. Integration of Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry in Blood Culture Diagnostics: A Fast and Effective Approach. J. Med. Microbiol. 2012, 61, 323–331. [Google Scholar] [CrossRef] [Green Version]
- Tandon, P.; Garcia-Tsao, G. Bacterial Infections, Sepsis, and Multiorgan Failure in Cirrhosis. Semin. Liver Dis. 2008, 28, 26–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biggins, S.W.; Angeli, P.; Garcia-Tsao, G.; Ginès, P.; Ling, S.C.; Nadim, M.K.; Wong, F.; Kim, W.R. Diagnosis, Evaluation, and Management of Ascites, Spontaneous Bacterial Peritonitis and Hepatorenal Syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2021, 74, 1014–1048. [Google Scholar] [CrossRef] [PubMed]
- Shelat, V.G.; Wang, Q.; Chia, C.L.; Wang, Z.; Low, J.K.; Woon, W.W. Patients with Culture Negative Pyogenic Liver Abscess Have the Same Outcomes Compared to Those with Klebsiella Pneumoniae Pyogenic Liver Abscess. Hepatobiliary Pancreat. Dis. Int. 2016, 15, 504–511. [Google Scholar] [CrossRef]
- Fernández, J.; Acevedo, J.; Castro, M.; Garcia, O.; Rodríguez de Lope, C.; Roca, D.; Pavesi, M.; Sola, E.; Moreira, L.; Silva, A.; et al. Prevalence and Risk Factors of Infections by Multiresistant Bacteria in Cirrhosis: A Prospective Study. Hepatology 2012, 55, 1551–1561. [Google Scholar] [CrossRef]
| Groups | Total No. | No. Concordant | % Concordant |
|---|---|---|---|
| Gram-positive bacteria | 98 | 71 | 73% |
| Gram-negative bacteria | 58 | 50 | 86% |
| Total | 156 | 121 | 78% |
| Staphylococcus aureus | 9 | 9 | 100% |
| Coagulase-negative Staphylococci | 37 | 26 | 70% |
| Total | 46 | 35 | 76% |
| Streptococci | 13 | 5 | 39% |
| Enterococci | 25 | 21 | 84% |
| Other Gram-positive cocci 1 | 4 | 2 | 50% |
| Total | 42 | 28 | 67% |
| Enterobacteriaceae | 44 | 40 | 91% |
| Pseudomonas aeruginosa | 9 | 5 | 56% |
| Bacteroides fragilis | 3 | 3 | 100% |
| Total | 56 | 48 | 86% |
| Aerobic Gram-positive bacilli 2 | 8 | 7 | 88% |
| Anaerobic Gram-positive bacilli 3 | 2 | 1 | 50% |
| Total | 10 | 8 | 80% |
| Moraxella osloensis | 2 | 2 | 100% |
| Total | 2 | 2 | 100% |
| Before Period | After Period | Statistical Analysis (+) | |
|---|---|---|---|
| Number of patients | 41 | 41 | |
| Sex ratio (male/female) | 33/8 | 28/13 | p = 0.2 (ns) |
| Age | 65 +/− 11 | 62 +/− 11 | p = 0.32 (ns) |
| Monomicrobial samples | 37/41 | 37/3 | p > 0.99 (ns) |
| Polymicrobial samples | 4/41 | 4/41 | p > 0.99 (ns) |
| Mean time for bacterial growth in BCBs | 26.4 | 25.6 | p = 0.76 (ns) |
| Enterobacteriacae | 17/45 | 17/45 | p > 0.99 (ns) |
| Enterococcus sp./Streptococcus sp. | 18/45 | 14/45 | p = 0.37 (ns) |
| Staphylococcus spp. | 5/45 | 7/44 | p = 0.53 (ns) |
| Non-fermenting Bacilli | 3/45 | 3/45 | p > 0.99 (ns) |
| Anaerobic bacteria | 1/45 | 2/45 | p = 0.55 (ns) |
| Other bacteria | 1/45 | 2/45 | p = 0.55 (ns) |
| Total % of change in antibiotic treatment | 15/41 (37%) | 15/41 (37%) | p = 0.99 (ns) |
| % change in antibiotic treatment at first result at Day 0 on BCBs (Gram before vs identification after) | 6/41 (15%) | 15/41 (37%) | p = 0.02 (*) |
| Mean time to first change in antibiotic (hours) | 41.3 | 24.3 | p < 0.0001 (****) |
| Patient | Gender | Age | Clinical Features | First Antibiotic | Leukocyte Count (Cells/mm3) | GRAM on Positive BCBs (D0) | Identification (Day 0) | Time to First Change in Antibiotic (Hours) (T0) | Antibiotic Optimization | Identification (Day 1) | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | Male | 58 | SBP in a patient with ethylic liver cirrhosis | Tazocillin | 11,900 | Gram-positive rod | L. monocytogenes | 20 | Amoxicillin | L. monocytogenes | Favorable evolution after 10 days of amoxicillin |
| Patient 2 | Female | 75 | SBP in a patient with HCV-related liver cirrhosis | Amoxicillin and clavulanic acid | 400 | Gram-negative rod | E. cloacae | 24 | Cefepime and metronidazole | E. cloacae and P. aeruginosa | Not favorable after 5 days of cefepime and metronidazole switched for imipenem. |
| Patient 3 | Male | 83 | SBP | Cefotaxime | 315 | Gram-positive cocci in chain | E. faecalis | 18.5 | Amoxicillin | E. faecalis | Favorable evolution after 10 days of amoxicillin |
| Patient 4 | Male | 77 | SBP | Cefotaxime | 5940 | Gram-positive cocci in chain | E. faecalis | 20 | Amoxicillin | E. faecalis | Favorable evolution after 10 days of amoxicillin |
| Patient 5 | Female | 67 | SBP in a patient with endometrial cancer | Tazocillin | 2700 | Gram-negative bacilli | E. cloacae | 18.5 | Cefepime | E. cloacae | Favorable evolution after 7 days of cefepime |
| Patient 6 | Male | 67 | SBP in a patient with ethylic liver cirrhosis | Tazocillin | 270 | Gram-negative bacilli | E. coli | 24 | Cefotaxime | E. coli | Favorable evolution after 4 days of cefotaxime followed by a oral amoxicillin and clavulanic acid for a total of 7 days |
| Patient 7 | Female | 85 | SBP in a patient with secondary liver involvement by lymphoma | No antibiotic | 250 | Gram-positive cocci in clusters | S. aureus | 22 | Introduction of cefazolin | S. aureus | Not favorable. Death 10 days after antibiotic initiation. |
| Patient 8 | Male | 54 | SBP in a patient with ethylic liver cirrhosis and bleedings of varices. Past medical history of SBP caused by S. pneumoniae | Cefotaxime | 22,140 | Gram-negative bacilli | B. fragilis | 69 | Addition of metronidazole | B. fragilis | Not favorable. Recurrence of SBP at 5 days of antibiotic initiation and switched for imipenem |
| Patient 9 | Male | 71 | SBP and sepsis in a patient with ethylic liver cirrhosis. | Imipenem | 1200 | Gram-positive cocci in chains and Gram-negative bacilli | E. coli and E. faecium | 21.5 | Addition of Daptomycin | E. coli and E. faecium | Not favorable. Patient died 7 days after appropriate antibiotic treatment |
| Patient 10 | Male | 67 | SBP in a patient hospitalized for drainage of refractory ascites caused by K. pneumoniae producing ESBL. | No antibiotic | 315 | Gram-negative bacilli | K. pneumoniae | 16 | Initiation of imipenem | K. pneumoniae | Not favorable. Death 19 days after antibiotic initiation. |
| Patient 11 | Male | 52 | SBP in a patient hospitalized for hepatocellular carcinoma | Tazocillin | 250 | Gram-positive bacilli | L. monocytogenes | 17.5 | Amoxicillin | L. monocytogenes | Favorable after 7 days of amoxicillin |
| Patient 12 | Male | 60 | SBP in a patient with peritoneal carcinomatosis and treated by cefoxitine for a PICC line infection | Cefoxitine | 110 | Gram-positive cocci in chain | E. faecalis | 17 | Amoxicillin | E. faecalis | Favorable after 7 days of amoxicillin |
| Patient 13 | Male | 57 | SBP in a patient with acute liver failure complicating a primary sclerosing cholangitis | No antibiotic | 210 | Gram-positive cocci in chain | S. anginosus | 20 | Amoxicillin | S. anginosus | Favorable after 7 days of amoxicillin |
| Patient 14 | Male | 54 | SBP in a patient with ethylic liver cirrhosis | No antibiotic | 550 | Gram-positive cocci in chain | S. gallolyticus | 39 | Tazocillin | S. gallolyticus | Favorable after 24 h of tazocillin followed by a total of 10 days of oral amoxicillin |
| Patient 15 | Male | 60 | SBP in a patient with ethylic liver cirrhosis | No antibiotic | 300 | Gram-positive cocci in clusters | S. aureus | 17 | Amoxicillin and clavulanic acid | S. aureus | Favorable after 10 days of amoxicillin and clavulanic acid |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lotte, R.; Courdurié, A.; Gaudart, A.; Emery, A.; Chevalier, A.; Tran, A.; Payen, M.; Ruimy, R. Spontaneous Bacterial Peritonitis: The Incremental Value of a Fast and Direct Bacterial Identification from Ascitic Fluids Inoculated in Blood Culture Bottles by MALDI-TOF MS for a Better Management of Patients. Microorganisms 2022, 10, 1188. https://doi.org/10.3390/microorganisms10061188
Lotte R, Courdurié A, Gaudart A, Emery A, Chevalier A, Tran A, Payen M, Ruimy R. Spontaneous Bacterial Peritonitis: The Incremental Value of a Fast and Direct Bacterial Identification from Ascitic Fluids Inoculated in Blood Culture Bottles by MALDI-TOF MS for a Better Management of Patients. Microorganisms. 2022; 10(6):1188. https://doi.org/10.3390/microorganisms10061188
Chicago/Turabian StyleLotte, Romain, Audrey Courdurié, Alice Gaudart, Audrey Emery, Alicia Chevalier, Albert Tran, Mathilde Payen, and Raymond Ruimy. 2022. "Spontaneous Bacterial Peritonitis: The Incremental Value of a Fast and Direct Bacterial Identification from Ascitic Fluids Inoculated in Blood Culture Bottles by MALDI-TOF MS for a Better Management of Patients" Microorganisms 10, no. 6: 1188. https://doi.org/10.3390/microorganisms10061188
APA StyleLotte, R., Courdurié, A., Gaudart, A., Emery, A., Chevalier, A., Tran, A., Payen, M., & Ruimy, R. (2022). Spontaneous Bacterial Peritonitis: The Incremental Value of a Fast and Direct Bacterial Identification from Ascitic Fluids Inoculated in Blood Culture Bottles by MALDI-TOF MS for a Better Management of Patients. Microorganisms, 10(6), 1188. https://doi.org/10.3390/microorganisms10061188







