DNA Markers for Detection and Genotyping of Xanthomonas euroxanthea
Abstract
1. Introduction
2. Materials and Methods
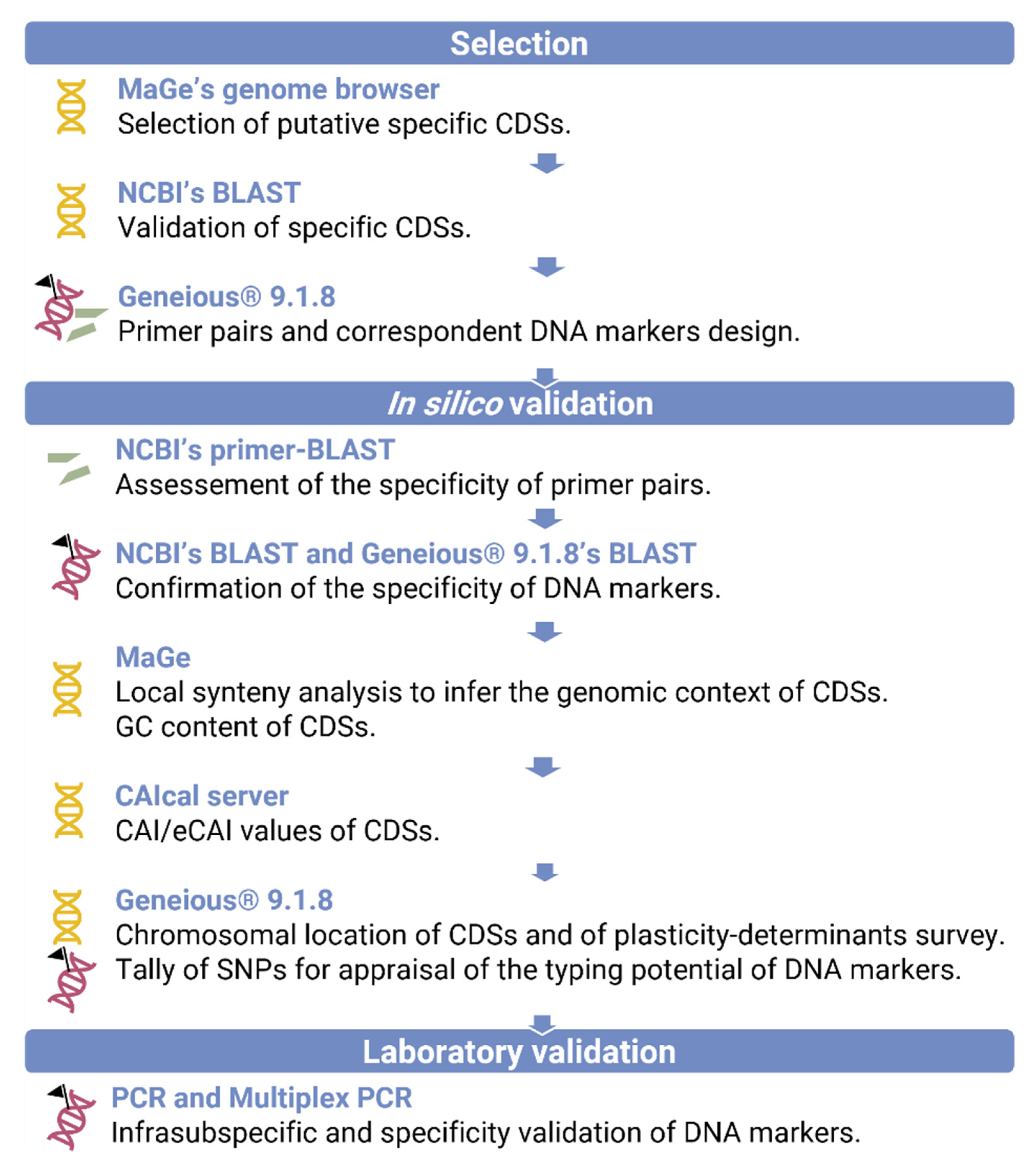
2.1. In Silico Selection and Validation of X. euroxanthea-Specific DNA Markers
2.2. Bacterial Strains, Culture Conditions and DNA Extraction
2.3. Experimental Validation of Putative X. euroxanthea-Specific DNA Markers by Multiplex PCR
2.4. PCR Detection Limit
2.5. Typing Potential of X. euroxanthea-Specific DNA Markers
3. Results
3.1. In Silico Selection of DNA Markers for X. euroxanthea
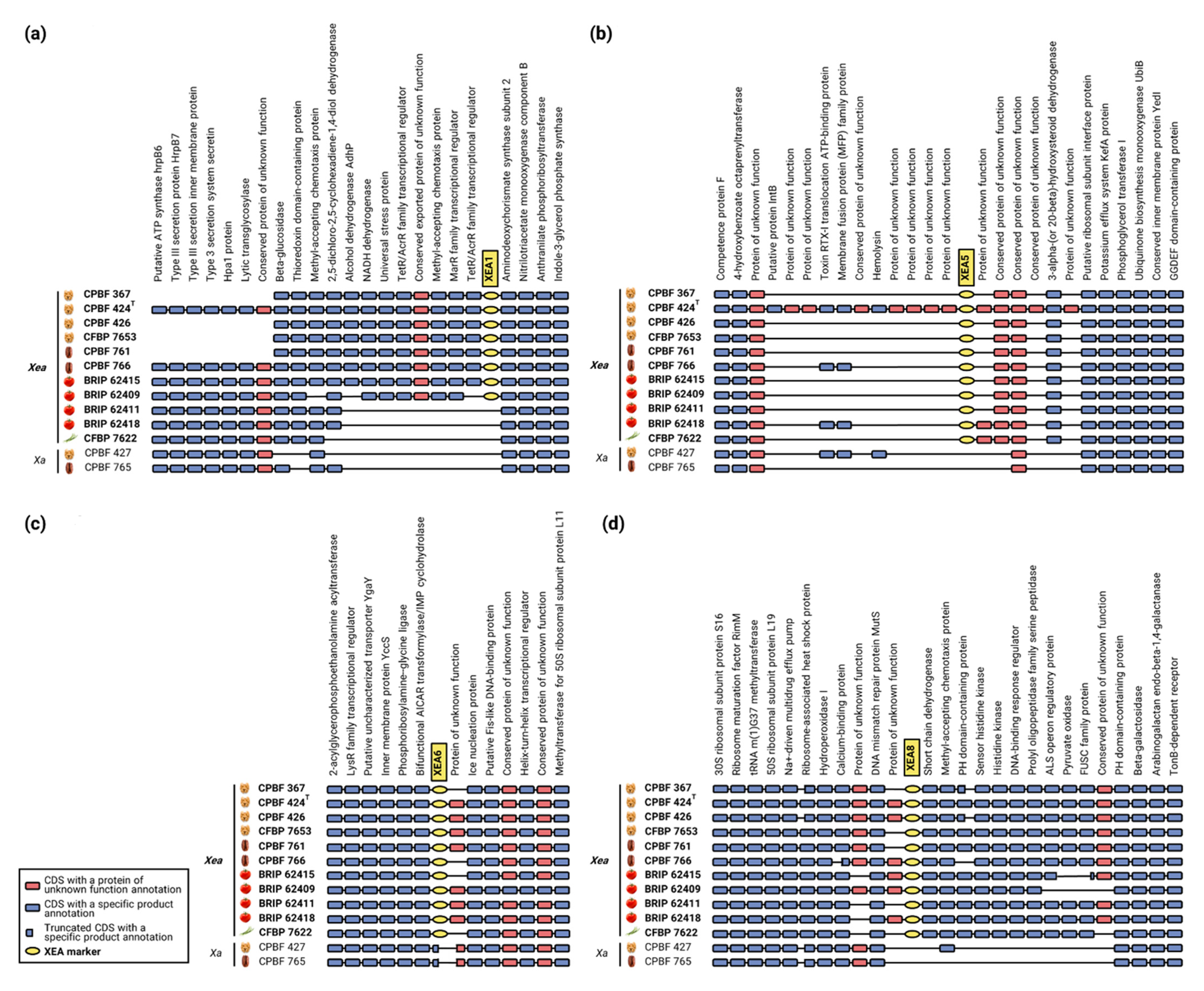
3.2. Genomic Analysis Unearths the Stability of XEA DNA Markers
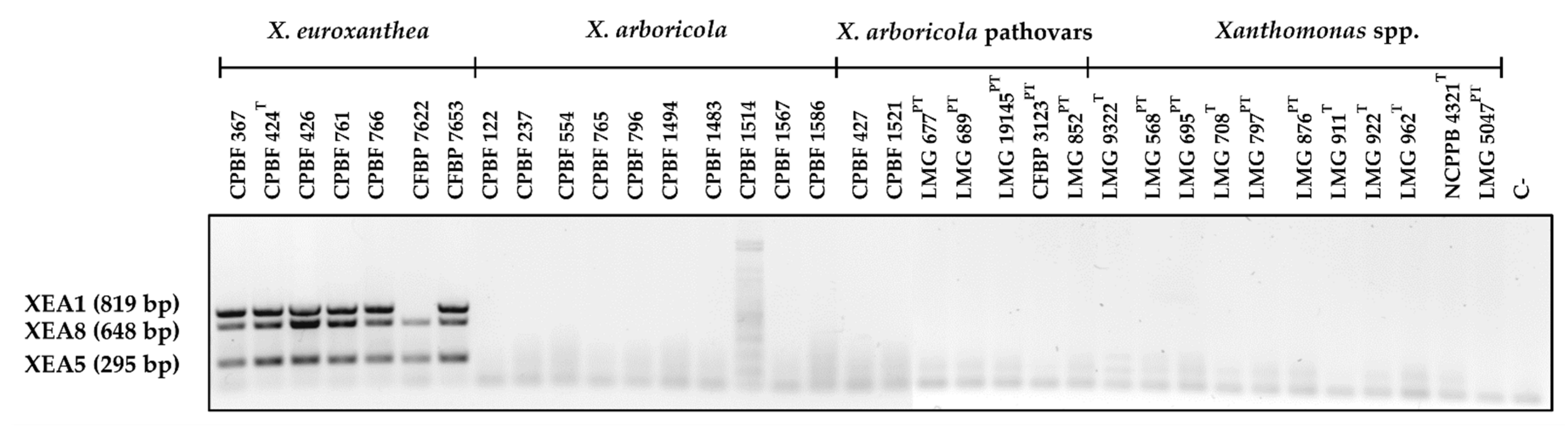
3.3. Multiplex PCR Allows for the Confident Identification of X. euroxanthea Strains
3.4. Detection Limit of Multiplex PCR with XEA DNA Markers
3.5. Typing Potential of Informative XEA DNA Markers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hajri, A.; Meyer, D.; Delort, F.; Guillaumès, J.; Brin, C.; Manceau, C. Identification of a genetic lineage within Xanthomonas arboricola pv. juglandis as the causal agent of vertical oozing canker of Persian (English) walnut in France. Plant Pathol. 2010, 59, 1014–1022. [Google Scholar] [CrossRef]
- Moragrega, C.; Matias, J.; Aletà, N.; Montesinos, E.; Rovira, M. Apical necrosis and premature drop of Persian (English) walnut fruit caused by Xanthomonas arboricola pv. juglandis. Plant Dis. 2011, 95, 1565–1570. [Google Scholar] [CrossRef] [PubMed]
- Moragrega, C.; Özaktan, H. Apical necrosis of persian (english) walnut (Juglans regia): An update. J. Plant Pathol. 2010, 92, S67–S71. [Google Scholar]
- Martins, L.; Fernandes, C.; Blom, J.; Dia, N.C.; Pothier, J.F.; Tavares, F. Xanthomonas euroxanthea sp. nov., a new xanthomonad species including pathogenic and non-pathogenic strains of walnut. Int. J. Syst. Evol. Microbiol. 2020, 70, 9. [Google Scholar] [CrossRef]
- Fernandes, C.; Blom, J.; Pothier, J.F.; Tavares, F. High-Quality Draft Genome Sequence of Xanthomonas sp. Strain CPBF 424, a Walnut-Pathogenic Strain with Atypical Features. Microbiol. Resour. Announc. 2018, 7, e00921-18. [Google Scholar] [CrossRef]
- Teixeira, M.; Martins, L.; Fernandes, C.; Chaves, C.; Pinto, J.; Tavares, F.; Fonseca, N.A. Complete Genome Sequences of Walnut-Associated Xanthomonas euroxanthea Strains CPBF 367 and CPBF 426 Obtained by Illumina/Nanopore Hybrid Assembly. Microbiol. Resour. Announc. 2020, 9, e00902-20. [Google Scholar] [CrossRef]
- Martins, L.; Teixeira, M.; Fernandes, C.; Pothier, J.F.; Koebnik, R.; Fonseca, N.A.; Tavares, F. Genomic Features of Xanthomonas arboricola and X. euroxanthea Sharing the Same Plant Host Species Preferences (Walnut, Pecan and Tomato) Seems Unbiased by the Host Species; CIBIO, Universidade do Porto: Porto, Portugal, 2022; manuscript in preparation. [Google Scholar]
- Fernandes, C.; Martins, L.; Teixeira, M.; Blom, J.; Pothier, J.F.; Fonseca, N.A.; Tavares, F. Comparative Genomics of Xanthomonas euroxanthea and Xanthomonas arboricola pv. juglandis Strains Isolated from a Single Walnut Host Tree. Microorganisms 2021, 9, 624. [Google Scholar] [CrossRef]
- Roach, R.; Mann, R.; Gambley, C.G.; Shivas, R.G.; Rodoni, B. Identification of Xanthomonas species associated with bacterial leaf spot of tomato, capsicum and chilli crops in eastern Australia. Eur. J. Plant Pathol. 2018, 150, 595–608. [Google Scholar] [CrossRef]
- Kałużna, M.; Fischer-Le Saux, M.; Pothier, J.F.; Jacques, M.; Obradović, A.; Tavares, F.; Stefani, E. Xanthomonas arboricola pv. juglandis and pv. corylina: Brothers or distant relatives? Genetic clues, epidemiology, and insights for disease management. Mol. Plant Pathol. 2021, 22, 1481–1499. [Google Scholar] [CrossRef]
- Zarei, S.; Taghavi, S.M.; Rahimi, T.; Mafakheri, H.; Potnis, N.; Koebnik, R.; Saux, M.F.-L.; Pothier, J.F.; Palacio-Bielsa, A.; Cubero, J.; et al. Taxonomic Refinement of Xanthomonas arboricola. Phytopathology, 2022; Online ahead of print. [Google Scholar] [CrossRef]
- Fernandes, C.; Albuquerque, P.; Mariz-Ponte, N.; Cruz, L.; Tavares, F. Comprehensive diversity assessment of walnut-associated xanthomonads reveal the occurrence of distinct Xanthomonas arboricola lineages and of a new species (Xanthomonas euroxanthea) within the same tree. Plant Pathol. 2021, 70, 5. [Google Scholar] [CrossRef]
- Catara, V.; Cubero, J.; Pothier, J.F.; Bosis, E.; Bragard, C.; Ðermić, E.Ð.; Holeva, M.C.; Jacques, M.-A.; Petter, F.; Pruvost, O.; et al. Trends in Molecular Diagnosis and Diversity Studies for Phytosanitary Regulated Xanthomonas. Microorganisms 2021, 9, 862. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.; Albuquerque, P.; Sousa, R.; Cruz, L.; Tavares, F. Multiple DNA Markers for Identification of Xanthomonas arboricola pv. juglandis Isolates and its Direct Detection in Plant Samples. Plant Dis. 2017, 101, 858–865. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Martins, L.; Fernandes, C.; Albuquerque, P.; Tavares, F. Assessment of Xanthomonas arboricola pv. juglandis Bacterial Load in Infected Walnut Fruits by Quantitative PCR. Plant Dis. 2019, 103, 2577–2586. [Google Scholar] [CrossRef] [PubMed]
- Pothier, J.F.; Pagani, M.C.; Pelludat, C.; Ritchie, D.F.; Duffy, B. A duplex-PCR method for species- and pathovar-level identification and detection of the quarantine plant pathogen Xanthomonas arboricola pv. pruni. J. Microbiol. Methods 2011, 86, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Loreti, S.; Pucci, N.; Perez, G.; Catara, V.; Scortichini, M.; Bella, P.; Ferrante, P.; Giovanardi, D.; Stefani, E. Detection and identification of Xanthomonas arboricola pv. pruni from symptomless plant material: Results of an Italian test performance study. EPPO Bull. 2015, 45, 41–51. [Google Scholar] [CrossRef]
- Palacio-Bielsa, A.; Cubero, J.; Cambra, M.A.; Collados, R.; Berruete, I.M.; López, M.M. Development of an efficient real-time quantitative PCR protocol for detection of Xanthomonas arboricola pv. pruni in Prunus species. Appl. Environ. Microbiol. 2011, 77, 89–97. [Google Scholar] [CrossRef]
- Palacio-Bielsa, A.; López-Soriano, P.; Bühlmann, A.; van Doorn, J.; Pham, K.; Cambra, M.A.; Berruete, I.M.; Pothier, J.F.; Duffy, B.; Olmos, A.; et al. Evaluation of a real-time PCR and a loop-mediated isothermal amplification for detection of Xanthomonas arboricola pv. pruni in plant tissue samples. J. Microbiol. Methods 2015, 112, 36–39. [Google Scholar] [CrossRef]
- Albuquerque, P.; Caridade, C.M.R.; Rodrigues, A.S.; Marcal, A.R.S.; Cruz, J.; Cruz, L.; Santos, C.L.; Mendes, M.V.; Tavares, F. Evolutionary and experimental assessment of novel markers for detection of Xanthomonas euvesicatoria in plant samples. PLoS ONE 2012, 7, e37836. [Google Scholar] [CrossRef]
- Vallenet, D.; Calteau, A.; Dubois, M.; Amours, P.; Bazin, A.; Beuvin, M.; Burlot, L.; Bussell, X.; Fouteau, S.; Gautreau, G.; et al. MicroScope: An integrated platform for the annotation and exploration of microbial gene functions through genomic, pangenomic and metabolic comparative analysis. Nucleic Acids Res. 2020, 48, D579–D589. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647. [Google Scholar] [CrossRef]
- Stothard, P. The sequence manipulation suite: JavaScript programs for analyzing and formatting protein and DNA sequences. Biotechniques 2000, 28, 1102–1104. [Google Scholar] [CrossRef] [PubMed]
- Puigbò, P.; Bravo, I.G.; Garcia-Vallve, S. CAIcal: A combined set of tools to assess codon usage adaptation. Biol. Direct 2008, 3, 38. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Ramasamy, R.P. Current and prospective methods for plant disease detection. Biosensors 2015, 5, 537–561. [Google Scholar] [CrossRef]
- PM 7/064 (2) Xanthomonas arboricola pv. pruni. EPPO Bull. 2021, 51, 240–266. [CrossRef]
- PM 7/022 (1) Xanthomonas arboricola pv. corylina. EPPO Bull. 2004, 34, 179–181. [CrossRef]
- PM 7/023 (2) Xanthomonas axonopodis pv. dieffenbachiae. EPPO Bull. 2009, 39, 393–402. [CrossRef]
- PM 7/044 (1) Xanthomonas axonopodis pv. citri. EPPO Bull. 2005, 35, 289–294. [CrossRef]
- PM 7/065 (1) Xanthomonas fragariae. EPPO Bull. 2006, 36, 135–144. [CrossRef]
- PM 7/080 (1) Xanthomonas oryzae: Diagnostics. EPPO Bull. 2007, 37, 543–553. [CrossRef]
- PM 7/128 (1) Xanthomonas axonopodis pv. allii. EPPO Bull. 2016, 46, 429–443. [CrossRef][Green Version]
- PM 7/110 (1) Xanthomonas spp. (Xanthomonas euvesicatoria, Xanthomonas gardneri, Xanthomonas perforans, Xanthomonas vesicatoria) causing bacterial spot of tomato and sweet pepper. EPPO Bull. 2013, 43, 7–20. [CrossRef]
- Pothier, J.F.; Vorhölter, F.J.; Blom, J.; Goesmann, A.; Pühler, A.; Smits, T.H.M.; Duffy, B. The ubiquitous plasmid pXap41 in the invasive phytopathogen Xanthomonas arboricola pv. pruni: Complete sequence and comparative genomic analysis. FEMS Microbiol. Lett. 2011, 323, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Fujikawa, T.; Takikawa, Y. Detection and identification of Xanthomonas campestris pv. campestris and pv. raphani by multiplex polymerase chain reaction using specific primers. Appl. Microbiol. Biotechnol. 2021, 105, 1991–2002. [Google Scholar] [CrossRef]
- Almeida, A.; Albuquerque, P.; Araujo, R.; Ribeiro, N.; Tavares, F. Detection and discrimination of common bovine mastitis-causing streptococci. Vet. Microbiol. 2013, 164, 370–377. [Google Scholar] [CrossRef]
- Puigbò, P.; Bravo, I.G.; Garcia-Vallvé, S. E-CAI: A novel server to estimate an expected value of Codon Adaptation Index (eCAI). BMC Bioinform. 2008, 9, 65. [Google Scholar] [CrossRef]
- Segerman, B. The genetic integrity of bacterial species: The core genome and the accessory genome, two different stories. Front. Cell. Infect. Microbiol. 2012, 2, 116. [Google Scholar] [CrossRef]
- Fonseca, N.P.; Felestrino, É.B.; Caneschi, W.L.; Sanchez, A.B.; Cordeiro, I.F.; Lemes, C.G.C.; Assis, R.A.B.; Carvalho, F.M.S.; Ferro, J.A.; Varani, A.M.; et al. Detection and identification of Xanthomonas pathotypes associated with citrus diseases using comparative genomics and multiplex PCR. PeerJ 2019, 2019, e7676. [Google Scholar] [CrossRef]
- Bangratz, M.; Wonni, I.; Kini, K.; Sondo, M.; Brugidou, C.; Béna, G.; Gnacko, F.; Barro, M.; Koebnik, R.; Silué, D.; et al. Design of a new multiplex PCR assay for rice pathogenic bacteria detection and its application to infer disease incidence and detect co-infection in rice fields in Burkina Faso. PLoS ONE 2020, 15, e0232115. [Google Scholar] [CrossRef]
- Brown, B.P.; Wernegreen, J.J. Genomic erosion and extensive horizontal gene transfer in gut-associated Acetobacteraceae. BMC Genom. 2019, 20, 472. [Google Scholar] [CrossRef]
- Armbruster, C.R.; Marshall, C.W.; Garber, A.I.; Melvin, J.A.; Zemke, A.C.; Moore, J.; Zamora, P.F.; Li, K.; Fritz, I.L.; Manko, C.D.; et al. Adaptation and genomic erosion in fragmented Pseudomonas aeruginosa populations in the sinuses of people with cystic fibrosis. Cell Rep. 2021, 37, 109829. [Google Scholar] [CrossRef] [PubMed]



| Xanthomonas Species and Pathovars | Strains | GenBank, NCBI Accession/WGS Prefix |
|---|---|---|
| X. euroxanthea | CPBF 367 | LR861803.1 |
| X. euroxanthea | CPBF 424T | LR994544.1 |
| X. euroxanthea | CPBF 426 | LR861805.1 |
| X. euroxanthea | CPBF 761 | HG999363.1 |
| X. euroxanthea | CPBF 766 | HG999364.1 |
| X. euroxanthea | CFBP 7622 | MIGF01.1 |
| X. euroxanthea | CFBP 7653 | MIGK01.1 |
| X. euroxanthea | BRIP 62409 | QEZJ01.1 |
| X. euroxanthea | BRIP 624011 | QEZI01.1 |
| X. euroxanthea | BRIP 62415 | QEZH01.1 |
| X. euroxanthea | BRIP 62418 | QEZG01.1 |
| X. arboricola | CPBF 1494 | HG999362.1 |
| X. arboricola | CPBF 765 | HG999365.1 |
| X. arboricola pv. juglandis | CPBF 427 | LR861807.1 |
| X. campestris pv. campestris | LMG 568PT | NC_003902 |
| X. campestris pv. campestris | 8004 | NC_007086 |
| X. campestris pv. campestris | B100 | NC_010688 |
| X. citri pv. citri | 306 | NC_003919 |
| X. citri pv. bilvae | NCPPB 3213PT | CDHI01 |
| X. phaseoli pv. phaseoli | CFBP 412 | NZ_CP020964.2 |
| X. citri subsp. aurantifolli | ICPB 10535 | ACPY01 |
| X. vasicola pv. musacearum | BCC282 | RRCQ01 |
| X. vasicola pv. musacearum | NCPPB 4381 | ACHT01 |
| X. oryzae pv. oryzae | PX099A | NC_010717.1 |
| X. oryzae pv. oryzae | MAFF 311018 | NC_007705.1 |
| X. oryzae pv. oryzae | KACC 10331 | NC_006834.1 |
| X. oryzae pv. oryzicola | BLS256 | NZ_AAQN |
| X. translucens pv. translucens | 569 | VIWM01.1 |
| X. translucens pv. translucens | LMG 876PT | NZ_CAPJ.1 |
| X. sacchari | NCPPB 4393 | AGDB01.1 |
| X. hortorum pv. gardneri | ICMP 7383 | CP018731.1 |
| X. hortorum pv. gardneri | LMG 962T | NZ_AEQX.1 |
| X. vesicatoria | LMG 911T | NZ_AEQV |
| X. euvesicatoria pv. perforans | 91-118 | NZ_AEQW |
| X. albilineans | GPE PC73 | NC_013722 |
| Xanthomonas Species and Pathovars | Strains 1 | Geographic Origin | Year of Isolation |
|---|---|---|---|
| X. euroxanthea | CPBF 367 | Portugal (Loures) | 2016 |
| X. euroxanthea | CPBF 424T | Portugal (Loures) | 2016 |
| X. euroxanthea | CPBF 426 | Portugal (Loures) | 2016 |
| X. euroxanthea | CPBF 761 | Portugal (Alcobaça) | 2016 |
| X. euroxanthea | CPBF 766 | Portugal (Alcobaça) | 2016 |
| X. euroxanthea | CFBP 7622 | USA | 1985 |
| X. euroxanthea | CFBP 7653 | France | 2008 |
| X. arboricola | CPBF 122 | Portugal (Ponte da Barca) | 2015 |
| X. arboricola | CPBF 237 | Portugal (Ponte de Lima) | 2015 |
| X. arboricola | CPBF 554 | Portugal (Carrazeda de Ansiães) | 2016 |
| X. arboricola | CPBF 765 | Portugal (Alcobaça) | 2016 |
| X. arboricola | CPBF 796 | Portugal (Alcobaça) | 2016 |
| X. arboricola | CPBF 1494 | Portugal (Alcobaça) | 2014 |
| X. arboricola | CPBF 1483 | Portugal (Alcobaça) | 2014 |
| X. arboricola | CPBF 1514 | Portugal (Estremoz) | 2014 |
| X. arboricola | CPBF 1567 | Portugal (Bombarral) | 2015 |
| X. arboricola | CPBF 1586 | Portugal (Loures) | 2015 |
| X. arboricola pv. juglandis | CPBF 427 | Portugal (Loures) | 2016 |
| X. arboricola pv. juglandis | CPBF 1521 | Portugal (Loures) | 2014 |
| X. arboricola pv. celebensis | LMG 677PT | New Zealand | 1960 |
| X. arboricola pv. corylina | LMG 689PT | USA | 1939 |
| X. arboricola pv. fragariae | LMG 19145PT | Italy | 1993 |
| X. arboricola pv. populi | CFBP 3123PT | Netherlands | 1979 |
| X. arboricola pv. pruni | LMG 852PT | New Zealand | 1953 |
| X. citri pv. citri | LMG 9322T | USA | 1989 |
| X. campestris pv. campestris | LMG 568PT | United Kingdom | 1957 |
| X. axonopodis pv. dieffenbachiae | LMG 695PT | Brazil | 1965 |
| X. fragariae | LMG 708T | USA | 1960 |
| X. oryzae pv. oryzicola | LMG 797PT | Malaysia | 1964 |
| X. translucens pv. translucens | LMG 876PT | USA | 1933 |
| X. vesicatoria | LMG 911T | New Zealand | 1955 |
| X. euvesicatoria pv. euvesicatoria | LMG 922 | USA | 1939 |
| X. hortorum pv. gardneri | LMG 962T | Yugoslavia | 1953 |
| X.euvesicatoria pv. perforans | NCPPB 4321T | USA | 1933 |
| X.oryzae pv. oryzae | LMG 5047PT | India | 1965 |
| DNA Markers | CDS (MaGe) 1 | Locus Tag (NCBI) | Gene Annotation (MaGe) | Primers | Sequences (5′→3′) | Length (bp) | Best BLASTn Hit with Non-X. euroxanthea (E Value/Query Coverage) |
|---|---|---|---|---|---|---|---|
| XEA1 | XE424_v1_a0582 | XTG_000508 | Conserved protein of unknown function | XEA1F | CTGCCGAGCGTGAAATCCAG | 819 | - |
| XEA1R | CCTTCAGTTGCACCGAACGC | ||||||
| XEA2 | XE424_v1_a2605 | XTG_002379 | Conserved protein of unknown function | XEA2F | AGTCCACCAATGCCATCGCC | 425 | - |
| XEA2R | AGTCCACCAATGCCATCGCC | ||||||
| XEA3 | XE424_v1_a2606 | XTG_002380 | Conserved protein of unknown function | XEA3F | CGGATCGGACAATGACGCTG | 612 | - |
| XEA3R | GCTCTACATCGCCGCTGGAG | ||||||
| XEA4 | XE424_v1_a1415 | XTG_001287 | TetR/AcrR family transcriptional regulator | XEA4F | GACGCATCCGCCCACGACC | 341 | Dickeya zeae A586-S18-A17 (2 × 10−50/95%) |
| XEA4R | TAGGCGGCAGACCCCTTCC | ||||||
| XEA5 | XE424_v1_a0462 | XTG_000401 | MarR family transcriptional regulator | XEA5F | AACGACGCTGACCTGGACC | 295 | Sphingomonas sp. AP4-R1 (5 × 10−13/73%) |
| XEA5R | CGACACCGCACGACCCCG | ||||||
| XEA6 | XE424_v1_a0617 | XTG_000542 | Conserved protein of unknown function | XEA6F | GCGGCTGCAGCGTCGTTG | 237 | Xanthomonas sp. GW (2 × 10−4/19%) |
| XEA6R | TCACCTGATGATCGAAGCCTGG | ||||||
| XEA7 | XE424_v1_a1414 | n/a | Protein of unknown function | XEA7F | GGACGCGCCATGATCTGCC | 212 | - |
| XEA7R | GGTGTCCGAGGMTCAGGTGC | ||||||
| XEA8 | 2 | 2 | 2 | XEA8F | ATCGCCTCTGGATGACGGC | 648 | Dickeya zeae A586-S18-A17 (1 × 10−95/73%) |
| XEA8R | GGTGATGTCGGCAAGCTCG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, K.G.; Martins, L.; Teixeira, M.; Pothier, J.F.; Tavares, F. DNA Markers for Detection and Genotyping of Xanthomonas euroxanthea. Microorganisms 2022, 10, 1078. https://doi.org/10.3390/microorganisms10061078
Silva KG, Martins L, Teixeira M, Pothier JF, Tavares F. DNA Markers for Detection and Genotyping of Xanthomonas euroxanthea. Microorganisms. 2022; 10(6):1078. https://doi.org/10.3390/microorganisms10061078
Chicago/Turabian StyleSilva, Kayla Gisela, Leonor Martins, Miguel Teixeira, Joël F. Pothier, and Fernando Tavares. 2022. "DNA Markers for Detection and Genotyping of Xanthomonas euroxanthea" Microorganisms 10, no. 6: 1078. https://doi.org/10.3390/microorganisms10061078
APA StyleSilva, K. G., Martins, L., Teixeira, M., Pothier, J. F., & Tavares, F. (2022). DNA Markers for Detection and Genotyping of Xanthomonas euroxanthea. Microorganisms, 10(6), 1078. https://doi.org/10.3390/microorganisms10061078







