Clustered Regularly Interspaced Short Palindromic Repeats in Xanthomonas citri—Witnesses to a Global Expansion of a Bacterial Pathogen over Time
Abstract
1. Introduction
2. Materials and Methods
2.1. Genomic Resources
2.2. Prediction of CRISPR Loci in Genome Assemblies
2.3. Prediction of CRISPR Loci in a Sequence Read Archive (SRA)
3. Results
3.1. Curated Database of X. citri Genome Sequences
3.2. Inventory of CRISPR Spacers from Genome Sequences of X. citri pv. citri
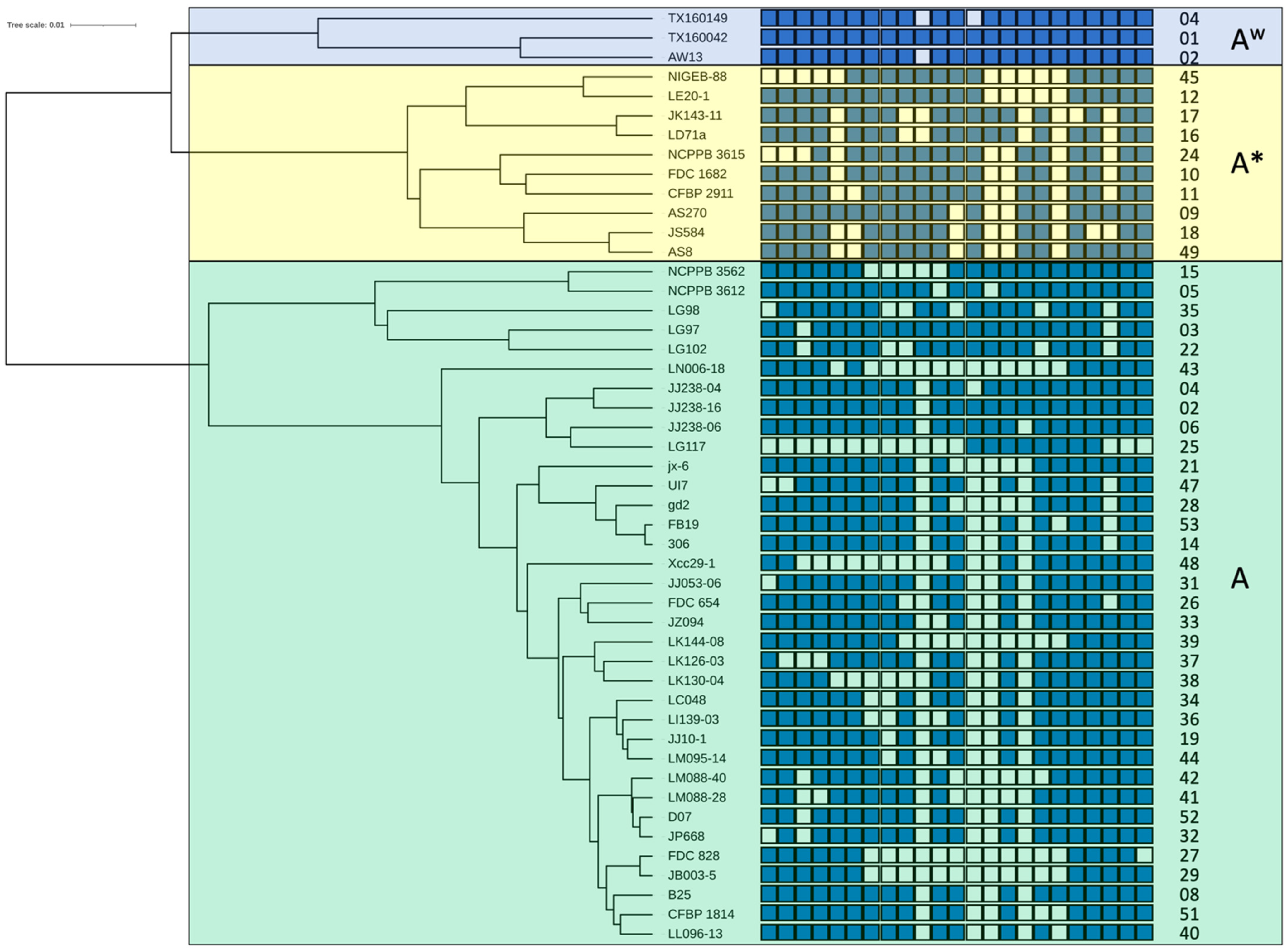
3.3. Spoligotypes of X. citri pv. citri
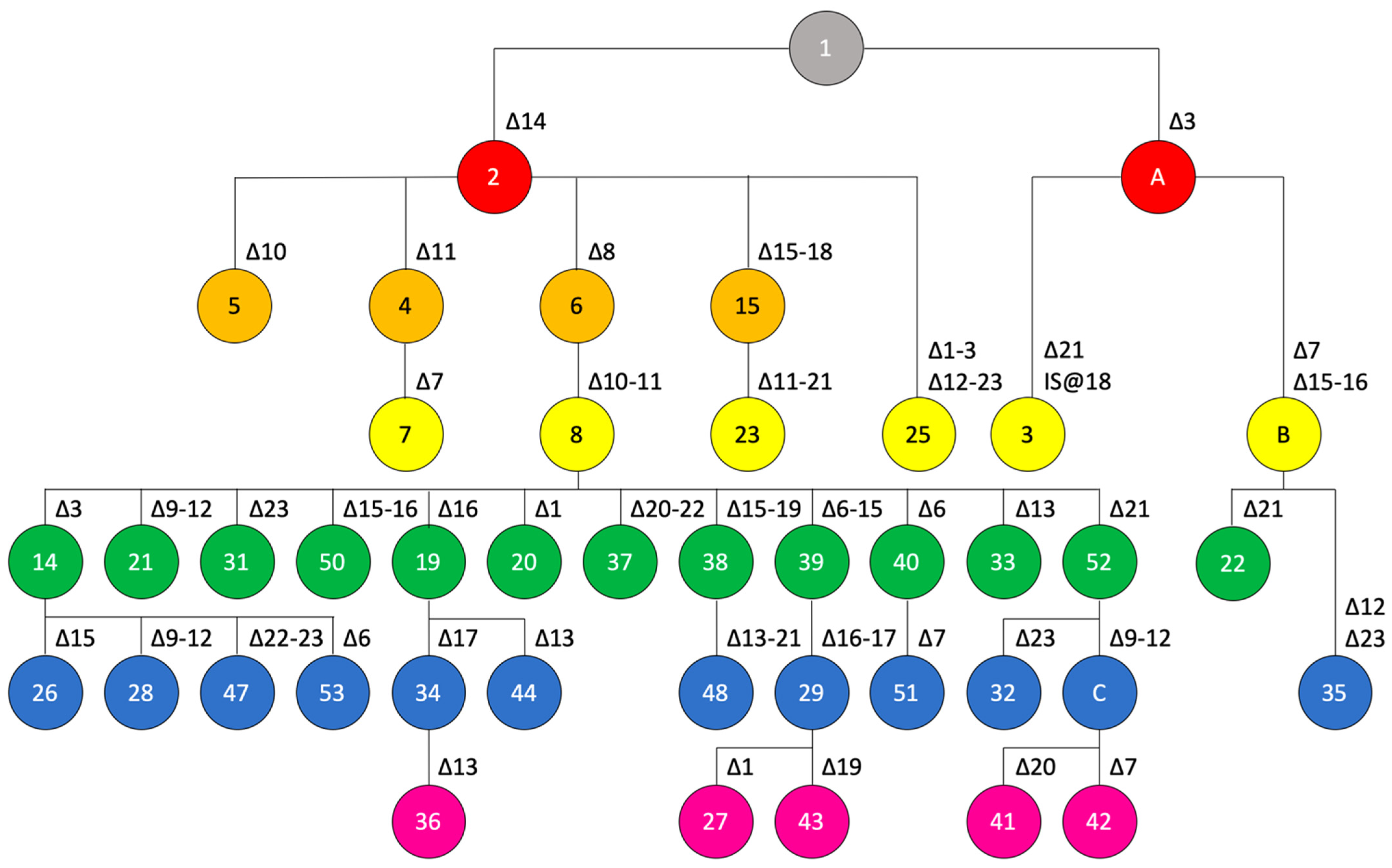
3.4. Genealogy of CRISPR Loci in X. citri pv. citri
3.5. Characterization of the CRISPR Locus from an Ancient X. citri pv. citri
3.6. Analysis of CRISPR Loci in Other Pathovars of X. citri
4. Discussion
4.1. Genomics-Informed Analysis of X. citri pv. citri Doubles the Number of Known Spoligotypes and Allows Reconstructing Their Probable Evolutionary Trajectory/Provides Information about Lineage Descendance of CRISPR Loci
4.2. The Spoligotype Genealogy Framework Contains Information on the Global Spread of the Citrus Canker Pathogen, as Exemplified by the Two Introductions in West Africa
4.3. Metagenomic Data from a Herbarium Sample Enable Reconstruction of the Spoligotype of an Ancient Citrus Canker Pathogen and Its Positioning in the CRISPR-Based Genealogy
4.4. The X. citri pv. citri CRISPR Locus Is Conserved in Some Additional Pathovars of X. citri and Different Pathovars Tend to Share Their Oldest Spacers
4.5. Some Spacers Observed in X. citri Pathovars beyond the Citri Pathovar Originated from Bacteriophages
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Timilsina, S.; Potnis, N.; Newberry, E.A.; Liyanapathiranage, P.; Iruegas-Bocardo, F.; White, F.F.; Goss, E.M.; Jones, J.B. Xanthomonas diversity, virulence and plant-pathogen interactions. Nat. Rev. Microbiol. 2020, 18, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.A.; Terol, J.; Ibanez, V.; López-García, A.; Pérez-Román, E.; Borredá, C.; Domingo, C.; Tadeo, F.R.; Carbonell-Caballero, J.; Alonso, R.; et al. Genomics of the origin and evolution of Citrus. Nature 2018, 554, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.N.; Ali, M.S.; Choi, S.J.; Hyun, J.W.; Baek, K.H. Biocontrol of citrus canker disease caused by Xanthomonas citri subsp. citri using an endophytic Bacillus thuringiensis. Plant Pathol. J. 2019, 35, 486–497. [Google Scholar] [CrossRef]
- Brunings, A.M.; Gabriel, D.W. Xanthomonas citri: Breaking the surface. Mol. Plant Pathol. 2003, 4, 141–157. [Google Scholar] [CrossRef]
- Midha, S.; Patil, P.B. Genomic insights into the evolutionary origin of Xanthomonas axonopodis pv. citri and its ecological relatives. Appl. Environ. Microbiol. 2014, 80, 6266–6279. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bansal, K.; Kumar, S.; Patil, P.B. Phylo-taxonogenomics supports revision of taxonomic status of twenty Xanthomonas pathovars to Xanthomonas citri. Phytopathology 2022, 112, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Horvath, P.; Barrangou, R. CRISPR/cas, the immune system of bacteria and archaea. Science 2010, 327, 167–170. [Google Scholar] [CrossRef]
- Haft, D.H.; Selengut, J.; Mongodin, E.F.; Nelson, K.E. A guild of 45 CRISPR-associated (Cas) protein families and multiple CRISPR/Cas subtypes exist in prokaryotic genomes. PLoS Comput. Biol. 2005, 1, e60. [Google Scholar] [CrossRef]
- Barrangou, R.; Horvath, P. The CRISPR system protects microbes against phages, plasmids. Microbe 2009, 4, 224–230. [Google Scholar] [CrossRef]
- Makarova, K.S.; Wolf, Y.I.; Iranzo, J.; Shmakov, S.A.; Alkhnbashi, O.S.; Brouns, S.J.J.; Charpentier, E.; Cheng, D.; Haft, D.H.; Horvath, P.; et al. Evolutionary classification of CRISPR-Cas systems: A burst of class 2 and derived variants. Nat. Rev. Microbiol. 2020, 18, 67–83. [Google Scholar] [CrossRef]
- Pieretti, I.; Royer, M.; Barbe, V.; Carrere, S.; Koebnik, R.; Couloux, A.; Darrasse, A.; Gouzy, J.; Jacques, M.A.; Lauber, E.; et al. Genomic insights into strategies used by Xanthomonas albilineans with its reduced artillery to spread within sugarcane xylem vessels. BMC Genom. 2012, 13, 658. [Google Scholar] [CrossRef] [PubMed]
- Koebnik, R.; Burokiene, D.; Bragard, C.; Chang, C.; Saux, M.F.; Kölliker, R.; Lang, J.M.; Leach, J.E.; Luna, E.K.; Portier, P.; et al. The complete genome sequence of Xanthomonas theicola, the causal agent of canker on tea plants, reveals novel secretion systems in clade-1 xanthomonads. Phytopathology 2021, 111, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.; Muñoz-Bodnar, A.; Arias Rojas, N.; Poulin, L.; Rodriguez-R, L.M.; Gagnevin, L.; Vernière, C.; Pruvost, O.; Koebnik, R. CRISPR elements provide a new framework for the genealogy of the citrus canker pathogen Xanthomonas citri pv. citri. BMC Genom. 2019, 20, 917. [Google Scholar] [CrossRef]
- Campos, P.E.; Groot Crego, C.; Boyer, K.; Gaudeul, M.; Baider, C.; Richard, D.; Pruvost, O.; Roumagnac, P.; Szurek, B.; Becker, N.; et al. First historical genome of a crop bacterial pathogen from herbarium specimen: Insights into citrus canker emergence. PLoS Pathog. 2021, 17, e1009714. [Google Scholar] [CrossRef]
- Young, J.M.; Dye, D.W.; Bradbury, J.F.; Panagopoulos, C.G.; Robbs, C.F. A proposed nomenclature and classification for plant pathogenic bacteria. N. Z. J. Agric. Res. 1978, 21, 153–177. [Google Scholar] [CrossRef]
- Vauterin, L.; Hoste, B.; Kersters, K.; Swings, J. Reclassification of Xanthomonas. Int. J. Syst. Bacteriol. 1995, 45, 472–489. [Google Scholar] [CrossRef]
- Rademaker, J.L.; Hoste, B.; Louws, F.J.; Kersters, K.; Swings, J.; Vauterin, L.; De Bruijn, F.J. Comparison of AFLP and rep-PCR genomic fingerprinting with DNA-DNA homology studies: Xanthomonas as a model system. Int. J. Syst. Evol. Microbiol. 2000, 50, 665–677. [Google Scholar] [CrossRef]
- Rademaker, J.L.W.; Louws, F.J.; Schultz, M.H.; Rossbach, U.; Vauterin, L.; Swings, J.; De Bruijn, F.J. A comprehensive species to strain taxonomic framework for Xanthomonas. Phytopathology 2005, 95, 1098–1111. [Google Scholar] [CrossRef]
- Constantin, E.C.; Cleenwerck, I.; Maes, M.; Baeyen, S.; Van Malderghem, C.; De Vos, P.; Cottyn, B. Genetic characterization of strains named as Xanthomonas axonopodis pv. dieffenbachiae leads to a taxonomic revision of the X. axonopodis species complex. Plant Pathol. 2016, 65, 792–806. [Google Scholar] [CrossRef]
- Oren, A.; Garrity, G.M. Notification of changes in taxonomic opinion previously published outside the IJSEM. Int. J. Syst. Evol. Microbiol. 2017, 67, 2081–2086. [Google Scholar] [CrossRef]
- Rodriguez-R, L.M.; Konstantinidis, K.T. The enveomics collection: A toolbox for specialized analyses of microbial genomes and metagenomes. PeerJ Prepr. 2016, 4, e1900. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Göker, M. TYGS is an automated high-throughput platform for state-of-the-art genome-based taxonomy. Nat. Commun. 2019, 10, 2182. [Google Scholar] [CrossRef] [PubMed]
- Couvin, D.; Bernheim, A.; Toffano-Nioche, C.; Touchon, M.; Michalik, J.; Néron, B.; Rocha, E.P.C.; Vergnaud, G.; Gautheret, D.; Pourcel, C. CRISPRCasFinder, an update of CRISRFinder, includes a portable version, enhanced performance and integrates search for Cas proteins. Nucleic Acids Res. 2018, 46, W246–W251. [Google Scholar] [CrossRef] [PubMed]
- Richard, D.; Rieux, A.; Lefeuvre, P.; Hamza, A.; Lobin, K.K.; Naiken, M.; Stravens, R.; Boyer, C.; Boyer, K.; Javegny, S.; et al. Draft genome sequences of 284 Xanthomonas citri pv. citri strains causing Asiatic citrus canker. Microbiol. Resour. Announc. 2021, 10, e01024-20. [Google Scholar] [CrossRef]
- Hu, F.P.; Young, J.M.; Stead, D.E.; Goto, M. Transfer of Pseudomonas cissicola (Takimoto 1939) Burkholder 1948 to the Genus Xanthomonas. Int. J. Syst. Evol. Microbiol. 1997, 47, 228–230. [Google Scholar] [CrossRef][Green Version]
- Rudra, B.; Gupta, R.S. Phylogenomic and comparative genomic analyses of species of the family Pseudomonadaceae: Proposals for the genera Halopseudomonas gen. nov. and Atopomonas gen. nov., merger of the genus Oblitimonas with the genus Thiopseudomonas, and transfer of some misclassified species of the genus Pseudomonas into other genera. Int. J. Syst. Evol. Microbiol. 2021, 71, 9. [Google Scholar] [CrossRef]
- Richard, D.; Pruvost, O.; Balloux, F.; Boyer, C.; Rieux, A.; Lefeuvre, P. Time-calibrated genomic evolution of a monomorphic bacterium during its establishment as an endemic crop pathogen. Mol. Ecol. 2021, 30, 1823–1835. [Google Scholar] [CrossRef]
- Kajava, A.V.; Lindow, S.E. A model of the three-dimensional structure of ice nucleation proteins. J. Mol. Biol. 1993, 232, 709–717. [Google Scholar] [CrossRef]
- Midha, S.; Bansal, K.; Kumar, S.; Girija, A.M.; Mishra, D.; Brahma, K.; Laha, G.S.; Sundaram, R.M.; Sonti, R.V.; Patil, P.B. Population genomic insights into variation and evolution of Xanthomonas oryzae pv. oryzae. Sci. Rep. 2017, 7, 40694. [Google Scholar] [CrossRef]
- Gétaz, M.; Krijger, M.; Rezzonico, F.; Smits, T.H.M.; van der Wolf, J.M.; Pothier, J.F. Genome-based population structure analysis of the strawberry plant pathogen Xanthomonas fragariae reveals two distinct groups that evolved independently before its species description. Microb. Genom. 2018, 4, e000189. [Google Scholar] [CrossRef]
- Johnson, S.C. Hierarchical clustering schemes. Psychometrika 1967, 32, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v4: Recent updates and new developments. Nucleic Acids Res. 2019, 47, W256–W259. [Google Scholar] [CrossRef] [PubMed]
- Leduc, A.; Traoré, Y.N.; Boyer, K.; Magne, M.; Grygiel, P.; Juhasz, C.C.; Boyer, C.; Guerin, F.; Wonni, I.; Ouedraogo, L.; et al. Bridgehead invasion of a monomorphic plant pathogenic bacterium: Xanthomonas citri pv. citri, an emerging citrus pathogen in Mali and Burkina Faso. Environ. Microbiol. 2015, 17, 4429–4442. [Google Scholar] [CrossRef]
- Bansal, K.; Midha, S.; Kumar, S.; Patil, P.B. Ecological and evolutionary insights into Xanthomonas citri pathovar diversity. Appl. Environ. Microbiol. 2017, 83, e02993-16. [Google Scholar] [CrossRef] [PubMed]
- Patané, J.S.L.; Martins, J., Jr.; Rangel, L.T.; Belasque, J.; Digiampietri, L.A.; Facincani, A.P.; Ferreira, R.M.; Jaciani, F.J.; Zhang, Y.; Varani, A.M.; et al. Origin and diversification of Xanthomonas citri subsp. citri pathotypes revealed by inclusive phylogenomic, dating, and biogeographic analyses. BMC Genom. 2019, 20, 700. [Google Scholar] [CrossRef]
- Srinivasan, M.C.; Patel, M.K. Two new phytopathogenic bacteria on verbenacious hosts. Curr. Sci. 1957, 26, 90–91. [Google Scholar]


| Correct Taxonomic Status | GenBank Annotation | Subgroup a | Complete Genome Sequences | Draft Genome Sequences | CRISPR Subtype b |
|---|---|---|---|---|---|
| X. citri pv. anacardii | X. citri pv. anacardii | 9.6 | 1 | 3 | w/o |
| X. citri pv. aurantifolii | X. citri pv. aurantifolii | 9.6 | 4 | 6 | w/o |
| X. citri pv. azadirachtae | X. campestris pv. azadirachtae | 9.5 | 0 | 1 | w/o |
| X. citri pv. bauhiniae | X. axonopodis pv. bauhiniae | 9.5 | 0 | 1 | w/o |
| X. citri pv. bilvae | X. citri pv. bilvae | 9.5 | 0 | 1 | w/o |
| X. citri pv. cajani | X. axonopodis pv. cajani | 9.5 | 0 | 1 | IC |
| X. citri pv. centellae | X. campestris pv. centellae | 9.5 | 0 | 1 | w/o |
| X. citri pv. citri | X. axonopodis | 9.5 | 1 | 0 | IC |
| X. citri | 9.5 | 1 | 0 | ||
| X. citri pv. citri | 9.5 | 45 | 68 | ||
| X. citri pv. citri c | 9.5 | 0 | 251 | ||
| X. citri pv. clitoriae | X. axonopodis pv. clitoriae | 9.5 | 0 | 1 | IC |
| X. citri pv. dieffenbachiae | 9.6 | w/o | |||
| X. citri pv. durantae | X. campestris pv. durantae | 9.5 | 0 | 1 | IC |
| X. citri pv. eucalyptorum | X. axonopodis pv. eucalyptorum | 9.6 | 0 | 1 | w/o |
| X. citri pv. fuscans | X. citri pv. fuscans | 9.6 | 10 | 25 | w/o |
| X. citri pv. phaseoli var. fuscans | 9.6 | 13 | 0 | ||
| X. citri pv. glycines | X. citri pv. glycines | 9.5 | 12 | 6 | w/o |
| X. citri pv. khayae | X. axonopodis pv. khayae | 9.5 | 0 | 1 | IC |
| X. citri pv. leeana | X. campestris pv. leeana | 9.5 | 0 | 1 | w/o |
| X. citri pv. malvacearum | X. citri pv. malvacearum | 9.5 | 8 | 4 | w/o |
| X. citri pv. mangiferaeindicae | X. citri pv. mangiferaeindicae | 9.5 | 3 | 4 | w/o |
| X. citri pv. martyniicola | X. axonopodis pv. martyniicola | 9.5 | 0 | 1 | w/o |
| X. citri pv. melhusii | X. axonopodis pv. melhusii | 9.5 | 0 | 1 | IC |
| X. citri pv. merremiae | X. campestris pv. merremiae | 9.6 | 0 | 2 | w/o |
| X. citri pv. punicae | X. citri pv. punicae | 9.5 | 10 | 2 | IC |
| X. citri pv. sesbaniae | X. citri pv. sesbaniae | 9.6 | 0 | 1 | w/o |
| X. citri pv. thespesiae | X. campestris pv. thespesiae | 9.5 | 0 | 1 | w/o |
| X. citri pv. thirumalacharii | X. citri pv. thirumalacharii | 9.6 | 0 | 1 | w/o |
| X. citri pv. trichodesmae | X. campestris pv. trichodesmae | 9.6 | 0 | 2 | w/o |
| X. citri pv. vignicola | X. citri pv. vignicola | 9.6 | 3 | 0 | w/o |
| X. citri pv. viticola | X. citri pv. viticola | 9.5 | 0 | 2 | w/o |
| X. citri pv. vitiscarnosae | X. campestris pv. vitiscarnosae | 9.5 | 0 | 1 | w/o |
| X. citri pv. vitistrifoliae | X. campestris pv. vitistrifoliae | 9.5 | 0 | 1 | w/o |
| X. citri pv. vitiswoodrowii | X. campestris pv. vitiswoodrowii | 9.5 | 0 | 1 | w/o |
| X. citri [pv. cissicola?] | Pseudomonas cissicola | 9.5 | 0 | 2 | w/o |
| X. citri not pv. citri | X. citri | 9.5 | 0 | 5 | w/o |
| X. citri not pv. citri | X. citri | 9.6 | 4 | 1 | w/o |
| Total | 115 | 401 |
| Strain | Spacer ID | DR Size (bp) | Spacer Size (bp) | Homolog a |
|---|---|---|---|---|
| Canonical sequence | 31 | 34–37 | CRISPR array | |
| LG98 | Xanci2264 | 31 | 28 | |
| LI070-01 | Xanci2535 Xanci2536 | 27 | 28–29 | Hypothetical protein in Staphylococcus aureus [8 × 10−23] |
| LK142-04 | Xanci3512 | 27 | 43 | Hypothetical protein in Escherichia coli [1 × 10−13] |
| LM057-04 | Xanci4667 | 29 | 60 | |
| LM057-15 | Xanci4706 | 28 | 60 | |
| LM088-25 | Xanci4801 | 27 | 29 | Hypothetical protein in Staphylococcus aureus [2 × 10−11] |
| LM095-04 | Xanci4987 Xanci4988 | 29 | 19–22 | |
| LP029-13 | Xanci5825 | 28 | 62 |
| Spacer | Variant Sequence | Canonical Sequence | Strains |
|---|---|---|---|
| Xcc_03a | AAGAAGACCAGTCTGCGGCGTCGCGGCATCCTTGGG | AAGAAGACCAGTCTGCGGCGTCGCGGCATCCTGGGG | JJ009-1 |
| Xcc_03b | AAGAAGACCAGTCTGCGGCGTCGCGGCATCTTGGGG | AAGAAGACCAGTCTGCGGCGTCGCGGCATCCTGGGG | LL098-02 |
| Xcc_03c | AAGAAGACCAGTCTGCGGCGTCGCGGCATCCTGGGGG | AAGAAGACCAGTCTGCGGCGTCGCGGCATCCTGGGG | LK136-05 |
| Xcc_13a | GCCATCATGCTTTGAATGCGCCTACCCACGGCGAA | GCCATCATGCTTTGAATGCGCTTACCCACGGCGAA | UI6, UI7 |
| Xcc_18a | GTGCCACCGACAGCGACGCACGTGGACCTGCATGTT | GTGCCACCGACAGCGACGCACGTGGACCTGCAGATC | LG97 |
| Xcc_19a | TCGAGCGCATCGATGACGGTCACCCATCCCC_AATG | TCGAGCGCATCGATGACGGTCACCCATCCCCCAATG | LK169-03 |
| Xcc_19b | GTGCCACCGATGACGGTCACCCATCCCCCAAT_ | TCGAGCGCATCGATGACGGTCACCCATCCCCCAATG | jx4 |
| Strains | Sequence | Remark |
|---|---|---|
| All | GTCGCGCCCTCACGGGCGCGTGGATTGAAAC | Canonical sequence |
| Most | GGCGCGCCCTCACGGGCGCGTGGATTGAAAC | DR1, degenerate variant of terminal repeat |
| Most | TTCGCGCCCTCATGGGCGCGTGGATTGAAAC | DR2, degenerate variant of the penultimate repeat |
| FDC 828 | TTCGCGCCCTCACGGGCGCGTGGATTGAAAC | Hybrid of DR1 and DR2 |
| LK130-09 | TTCGCGCCCTCATGGGCGCGTGGATTGAA_C | |
| LK169-03 | GTCGCGCCCTCACGGGCGCGTGGATTGAAAAC | |
| LM089-02, LMG 9322, LN003-10, MN10, MN11, MN12 | GTCGCGCCCTCACGGGCGCGTGGATTGGAAC | |
| NCPPB 3610 | GTCGCGCCCTCCCGGGCGCGTGGATTGAAAC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellanger, N.; Dereeper, A.; Koebnik, R. Clustered Regularly Interspaced Short Palindromic Repeats in Xanthomonas citri—Witnesses to a Global Expansion of a Bacterial Pathogen over Time. Microorganisms 2022, 10, 1715. https://doi.org/10.3390/microorganisms10091715
Bellanger N, Dereeper A, Koebnik R. Clustered Regularly Interspaced Short Palindromic Repeats in Xanthomonas citri—Witnesses to a Global Expansion of a Bacterial Pathogen over Time. Microorganisms. 2022; 10(9):1715. https://doi.org/10.3390/microorganisms10091715
Chicago/Turabian StyleBellanger, Ninon, Alexis Dereeper, and Ralf Koebnik. 2022. "Clustered Regularly Interspaced Short Palindromic Repeats in Xanthomonas citri—Witnesses to a Global Expansion of a Bacterial Pathogen over Time" Microorganisms 10, no. 9: 1715. https://doi.org/10.3390/microorganisms10091715
APA StyleBellanger, N., Dereeper, A., & Koebnik, R. (2022). Clustered Regularly Interspaced Short Palindromic Repeats in Xanthomonas citri—Witnesses to a Global Expansion of a Bacterial Pathogen over Time. Microorganisms, 10(9), 1715. https://doi.org/10.3390/microorganisms10091715







