Abstract
Elizabethkingia spp. is a ubiquitous pathogenic bacterium that has been identified as the causal agent for a variety of conditions such as meningitis, pneumonia, necrotizing fasciitis, endophthalmitis, and sepsis and is emerging as a global threat including in Southeast Asia. Elizabethkingia infections tend to be associated with high mortality rates (18.2–41%) and are mostly observed in neonates and immunocompromised patients. Difficulties in precisely identifying Elizabethkingia at the species level by traditional methods have hampered our understanding of this genus in human infections. In Southeast Asian countries, hospital outbreaks have usually been ascribed to E. meningoseptica, whereas in Singapore, E. anophelis was reported as the main Elizabethkingia spp. associated with hospital settings. Misidentification of Elizabethkingia spp. could, however, underestimate the number of cases attributed to the bacterium, as precise identification requires tools such as MALDI-TOF MS, and particularly whole-genome sequencing, which are not available in most hospital laboratories. Elizabethkingia spp. has an unusual antibiotic resistance pattern for a Gram-negative bacterium with a limited number of horizontal gene transfers, which suggests an intrinsic origin for its multidrug resistance. Efforts to prevent and further understand Elizabethkingia spp. infections and limit its spread must rise to this new challenge.
1. Introduction
The Gram-negative bacteria of the genus Elizabethkingia have recently emerged as an important pathogen in hospital-acquired infections and are generally associated with high mortality [1]. Recent literature has reported several cases of severe infection in humans owing to this organism, with neonatal meningitis most commonly presented in children [2], accompanied by a range of other clinical manifestations such as septicemia and bacteremia [3,4], osteomyelitis [5], urinary tract infections [6,7], endogenous endophthalmitis [8], endocarditis [9], epididymo-orchitis [10], pulmonary abscess [11], necrotizing fasciitis [12,13], cystic fibrosis [14], hydrocephalus [15], and secondary infections with a high mortality rate, particularly in immunocompromised patients [16]. Elizabethkingia meningoseptica infections have also been associated with COVID-19 patients [17]. Elizabethkingia spp. infects not only immunocompromised patients but also immunocompetent ones [18,19,20].
Historically, the first report of human infection due to Elizabethkingia was that of 19 cases of meningitis in infants in the United States of America [21]. Even in its earliest description, the isolates were demonstrated to be multidrug-resistant. Not long after King’s (1959) report, an outbreak of meningitis infection with E. meningoseptica was reported among neonates in the Congo [22] with varying sensitivities to chloramphenicol, carbomycin, magnamycin, and erythromycin.
Worldwide infections caused by E. meningoseptica were reportedly high amongst immunocompetent neonates as well as hospitalized patients with existing underlying infections, and in a comprehensive review, Dzuiban et al. [2] showed that from 283 cases reported from 28 countries from 1944 to 2017, 76% of them were neonates aged 0–1 month. From the 283 cases that were reviewed, 209 of the patients were diagnosed with meningitis [2]. Infections by this pathogen have been reported in many parts of the world, including in Southeast Asian countries such as Malaysia [2], Singapore [23], Thailand [24], Indonesia [25], and Cambodia [26]. However, until now, there have been no published reports from other Southeast Asian countries such as the Philippines, Brunei, Myanmar, Laos, and Timor-Leste. Although for Vietnam, there have been no published reports of clinical Elizabethkingia spp. infections, the isolation of the pathogen from the environment [27,28] suggests the existence of infections that could have been misidentified and/or have not been published. In Malaysia, there were only 32 cases from four published reports [2], indicating the scarcity of data for Elizabethkingia spp. infections in most countries around the region. The aim of this review is to summarize our current understanding of the characteristics of Elizabethkingia spp., the current epidemiological developments, and clinical manifestations of Elizabethkingia spp. in Southeast Asia.
2. Identification
When first discovered in 1959, the suggested name for the bacterium was Flavobacterium meningosepticum, which was later recommended to be changed to Chryseobacterium meningosepticum (in 1994) [29]. In 2005, it was assigned to the genus Elizabethkingia (named after the first scientist to report its’ discovery, Elizabeth King) under the Flavobacteriaceae family based on 16S rRNA phylogenetic studies [30]. Recently, whole-genome sequence analysis along with optical mapping and MALDI-TOF mass spectrometry led to the revision of the genus Elizabethkingia into eight species, namely E. meningoseptica, E. miricola, E. anophelis, E. bruuniana, E. ursingii, E. occulta [31], E. argenteiflava sp. nov. [32], and the latest E. umeracha [33].
Since correct identification of Elizabethkingia is difficult using traditional microbiological methods and misidentification of E. anophelis with E. meningoseptica has been found to be common (Lau et al., 2016), it is therefore highly likely for this pathogen to be underreported. Correct identification of the organism is crucial for the diagnosis and management strategies, as E. anophelis is a nososcomial pathogen [34]. Hence, differentiation between E. anophelis and E. meningoseptica requires accurate microbial identification, but the phenotypic similarities between E. anophelis and E. meningoseptica present a challenge to accurate identification, particularly for clinically derived isolates; 16S rRNA gene analysis had identified a 98.6% similarity between E. meningoseptica and E. anophelis, which has often led to the misidentification of these bacteria [34].
The four automated bacterial identification systems that are commonly used in diagnostic laboratories are: (1) API/ID32 Phenotyping Kits (bioMérieux, Marcy l’Etoile, France); (2) Phoenix 100 ID/AST Automated Microbiology System (Becton Dickinson Co., Sparks, MD, USA); (3) VITEK 2 Automated Identification System [35]; and (4) MALDI-TOF MS System (bioMérieux, Marcy l’Etoile, France) [36]. At the time of writing this review, the four microbial identification systems that are listed above do not, however, contain all eight species of Elizabethkingia in their reference spectra database. Studies have also shown that misidentification of Elizabethkingia was rife using these automated identification systems, with E. anophelis commonly misidentified as E. meningoseptica [1,35,37]. When the accuracy of the API/ID32, Phoenix 100 ID/AST, Vitek 2, and Vitek MS Elizabethkingia, clinical isolate identifications were compared with 16S rRNA gene sequencing; it was reported that species identification concordance between these identification systems and 16S rRNA gene sequencing was low at only 24.5–26.5% [35]. Nevertheless, MALDI-TOF MS systems with amended databases (labeled as “research-use only” system) either in the Vitek MS Knowledge Base v3.2 and Bruker MALDI Biotyper Library (Bruker Daltonics GmbH, Bremen, Germany) are now able to reliably differentiate E. meningoseptica from E. anophelis, but not the remaining species of the genus Elizabethkingia [35]. In a recent report of 22 clinical and 6 environmental hospital isolates from Queensland, Australia, Burnard et al. (2020) showed that the VITEK MS Knowledge Base v3.2 had a 96.2% accuracy in identifying Elizabethkingia, with a solitary isolate of E. bruuniana being the only species that was misidentified. Whole-genome sequencing confirmed that the majority of the isolates were E. anophelis (n = 22), with the rest being E. miricola (n = 3), E. meningoseptica (n = 2), and E. bruuniana (n = 1) [38].
In the near future, the inclusion of novel Elizabethkingia species spectra into the databases should ensure highly accurate identification using MALDI-TOF MS systems, making it a reliable identification tool in lieu of whole-genome sequencing.
3. Antibiotic Resistance
Elizabethkingia are intrinsically resistant to most β-lactams, β-lactam/lactamase inhibitors, and carbapenems due to the presence of two unique class B metallo-β-lactamases (MBLs), namely blaBlaB and blaGOB, along with a class A extended-spectrum β-lactamase (ESBL), blaCME [39,40,41]. Elizabethkingia are the only known bacteria thus far with multiple chromosomally encoded MBLs [42]. Reports of subclasses of MBL genes such as blaBlaB-1 [40], blaBlaB, and blaGOB in both E. meningoseptica and E. anophelis [41], as well as blaBlaB-16 and blaGOB-19 in E. miricola isolated from a black-spotted frog in China [43], make Elizabethkingia spp. a possible environmental reservoir for β-lactam resistance.
Elizabethkingia isolates are frequently resistant to aminoglycosides, macrolides, tetracycline, and vancomycin but show variable susceptibility to piperacillin, piperacillin-tazobactam, fluoroquinolones, minocycline, tigecycline, and trimethoprim-sulfamethoxazole [3,38,41,44,45,46]; cephalosporins, monobactams, and moderate susceptibilities to piperacillin [47,48,49], ceftazidime, colistin, and meropenem [50]; and levofloxacin [51]. There are currently no established MIC breakpoints for Elizabethkingia, and susceptibilities are largely reported based on Enterobacteriaceae breakpoints of the Clinical and Laboratory Standards Institute (CLSI) M100 guidelines and/or the European Committee on Antimicrobial Susceptibility Testing (EUCAST) pharmacokinetic–pharmacodynamic (PK–PD) “non-species” breakpoints [37,38]. It has been pointed out that susceptibilities, especially for vancomycin and piperacillin-tazobactam as determined by disk diffusion and E-test, are deemed unreliable and inaccurate for Elizabethkingia, and broth microdilution is instead recommended for susceptibility determination [45,52]. Although successful therapy has been attributed to rifampicin, there has been a report of bacterial resistance after three days of starting treatment [53]. A similar case was reported for an E. meningoseptica isolate in the Kuala Lumpur General Hospital, which developed resistance during treatment to cefepime, a cephalosporin antibiotic that is normally highly active against both Gram-positive and Gram-negative organisms [54].
Using disk diffusion, Lau, Chow [1] reported 21 Elizabethkingia isolates from Hong Kong as susceptible to vancomycin. However, studies using broth microdilution tests on isolates from Taiwan [45,55] and Australia [38] indicated that the isolates are likely non-susceptible based on the high MICs obtained (that ranged between 8 and 256 µg/mL). Similar ranges of vancomycin MICs were obtained by Han et al. [37], who investigated Elizabethkingia isolates from South Korea using the agar dilution method and concluded that all isolates were non-susceptible based on the interpretive criteria used for Staphylococcus spp. The vancomycin resistance gene, vanW, was reported in the majority of Elizabethkingia genomes, although the exact function of vanW is currently unknown [38,46]. However, mutations in vanW have been identified in microorganisms with VanB-type glycopeptide resistance [46,56]. In view of these facts and despite some anecdotal reports of success in using intravenous vancomycin alone to treat Elizabethkingia infections [57,58], it was recommended that even if intravenous vancomycin is the favored therapy for Elizabethkingia meningitis, ciprofloxacin, linezolid, or rifampicin should also be included until future clinical studies could be carried out to conclusively determine the clinical efficacy of these vancomycin-combination regiments for treatment [52].
One of the earliest reports of the whole-genome sequences of Elizabethkingia spp. strains from Southeast Asia was from Singapore, whereby sputum isolates obtained from three patients (NUHP1, NUHP2, and NUHP3) and four from the hospital’s sink (NUH1, NUH4, NUH6, and NUH11) at the National University Hospital, Singapore, were compared against five previously sequenced E. anophelis strains Ag1 (PRJNA80705) and R26 (PRJNA178189), E. meningoseptica ATCC 12535 (NITE) (PRJNA199489), E. meningoseptica ATCC 12535 (OSU) (PRJNA198814), and E. meningoseptica 502 (PRJNA176121). This led to the discovery of 16 antibiotic resistance genes from the core genomes and 19 antibiotic resistance genes from the accessory genomes of Elizabethkingia spp., and this included genes that confer resistance to aminoglycosides, β-lactams, fluoroquinolones, glycopeptides, macrolide-lincosamide-streptogramins, tetracyclines, trimethoprim, and rifampicin [40]. A later study on two African isolates, E27017 and E18064, that compared their genomes with the genomes of 18 strains belonging to the genus Elizabethkingia from many different regions, including Malaysian and Singaporean genomic sequences that were available at that time, identified that all Elizabethkingia genomes contained at least 17 antimicrobial resistance genes (Supplementary Table S1) [41].
A whole-genome sequencing study on three isolates of E. meningoseptica collected from an outbreak from three separate patients living in different counties in the Midwest regions of Michigan led to the identification of 22 resistance genes and 18 multidrug resistance efflux pump-encoded genes (Supplementary Table S1) in all samples [59]. While Elizabethkingia spp. genomes shared many antibiotic-resistance genes with each other, minor differences have been reported [3,60]. Hence, genomic investigations of Elizabethkingia spp. offers invaluable novel information on the species, but unfortunately, there have not been any reports of the whole-genome sequence of Elizabethkingia spp. isolates from Southeast Asia besides those from Singapore.
4. Virulence Factors
The mechanisms of pathogenesis of Elizabethkingia spp. are still being studied [59]. When the virulence factor database (VFDB, http://www.mgc.ac.cn/VFs/, accessed on 12 December 2021) was used to predict their presence from the genome sequences of various Elizabethkingia spp., this led to the prediction of a total of 270 putative virulence factor genes. More than fourteen virulence factor classes for Elizabethkingia spp. were identified (see Supplementary Table S2 for the complete list) with the following defined virulence-associated functions: adherence, antimicrobial activity, biofilm, cellular metabolism, effector delivery system, exoenzyme, exotoxin, immune modulation, invasion, motility, nutritional/metabolic factor, post-translational modification, regulation, stress survival, and others. Different species of Elizabethkingia shared the same virulence factors (Figure 1).
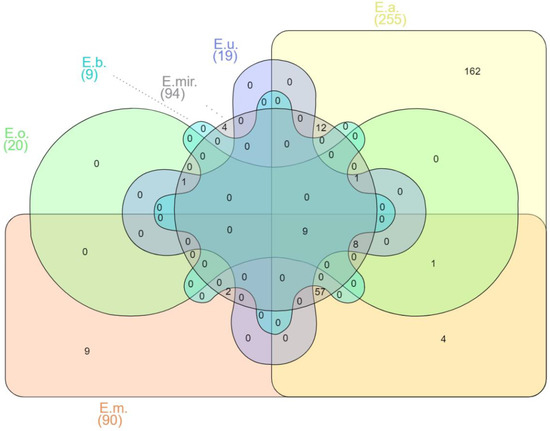
Figure 1.
Venn diagram of shared virulence factor genes of Elizabethkingia spp. E.m.—E. meningoseptica; E.a.—E. anophelis; E.mir.—E. miricola; E.o.—E. occulta; E.u.—E. ursingii; E.b.—E. bruuniana. Edwards mode was used to process virulence factor gene outputs for Venn diagram visualization with InteractiVenn [74].
Among the 270 predicted genes for virulence factors, 162 have been reported as unique in E. anophelis (Supplementary Table S2). E. meningoseptica carried six unique genes involved in adherence that encode curli nucleator protein (csgB), curli assembly proteins (curEm1, curEm2, curEm3, curEm4), a curli production assembly protein (csgG), and two genes involved in immune modulation encoding a capsular polysaccharide synthesis enzyme (cap8O), a gene encoding Rab2-interacting conserved protein A (ricA) and a putative carbonic anhydrase-encoded gene (mig-5) (Supplementary Table S2). Four of the E. miricola unique virulence genes were predicted to be involved with urease accessory protein (ureE), urease alpha subunit (ureA), twitching motility protein (pilG), and sphingomyelinase-c (smcL) (Supplementary Table S2).
Identification of 6880 gene families in E. anophelis highlighted the genomic heterogeneity of Elizabethkingia species [41]. Genes homologous to heme iron acquisition, oxidative stress resistance proteins, and hemolysins were reported in earlier studies [34,61,62]. Extensive variations of capsular polysaccharide synthesis genes in E. anopheles were first reported by Breurec, Criscuolo [41], with variable cps clusters observed amongst the different lineages suggesting virulence heterogeneity among Elizabethkingia strains [41]. Identification of the capsule biosynthesis gene, capD [59], and the adeG gene for the AdeFGH efflux pump [20] in all Elizabethkingia species (Supplementary Table S2) leads to possible biofilm formation [44,63], which empowers the bacteria with the ability to persist on various surfaces [59,64]. Thirty clinical isolates from Malaysia, which comprised E. anophelis, E. meningoseptica, and E. miricola, were recently shown to produce biofilms on polystyrene microtiter plates [65].
Nine virulence factor genes were shared between six of the Elizabethkingia spp., including the E. argenteiflava-encoded adeFGH efflux pump, isocitrate lyase (icl), catalase/(hydro)peroxidase (katA), 60K heat shock protein (htpB), phospholipase C (plc), phosphopyruvate hydratase (eno), translation elongation factor (tuf), catalase/peroxidase HPI (katG), and aspartate 1-decarboxylase precursor (panD), which is involved with adherence, biofilm formation, cellular metabolism, exotoxin production, and stress survival (Supplementary Table S2). Isocitrate lyase (icl) plays an important role in the glyoxylate cycle [66], and its presence in Elizabethkingia spp. can predict its essential role in stationary-phase survival. An early report had shown that the presence of icl in Mycobacterium tuberculosis promoted the tenacity of infection by helping the pathogen to survive inside macrophages [67].
However, the specific role of bacterial enzymes in pathogenesis varies with infection. The presence of phospholipases C (plc) in all Elizabethkingia spp. [46] suggest its crucial role in downregulating host immunity [68]. In L. monocytogenes, plc aided bacterial escape toward the cytosol and cell-to-cell propagation, whereas, in C. perfringens, it helped bacteria induce endothelial damage and platelet aggregation, and in P. aeruginosa, it led to the triggering of signaling pathways that lead to inflammation [69].
The catalase-peroxidase genes, katA and katG (encoding hydroperoxidase I), are crucial against oxidative stress [70]. An earlier report showed that strains with katA were resistant to dodecyl sulfate, proteinase K, pepsin, trypsin, chymotrypsin, and the neutrophil protease cathepsin G, and they could survive for a long period once released from lysed cells [71]. Presence of katA [40,41,44,46,60,61,72,73] and katG [44,46,72,73] could also support Elizabethkingia species’ resistance to aminoglycosides.
5. Sources of Isolation and Transmission
The genera Elizabethkingia are aerobic, non-fermenting, non-motile, catalase-positive, oxidase-positive, indole-positive, and Gram-negative bacilli widely distributed in soil, mosquitoes, plants, fresh and marine fish [30,75], food products [76], hospital settings [77], stagnant water, inland wetlands, and rivers [33]. Due to their biofilm-forming ability [63], they have been isolated from sinks and taps where they colonize the most, leading to nosocomial and community infections [78] (Table 1).

Table 1.
Various sources of isolation of Elizabethkingia spp. in Southeast Asia.
Vector-borne transmission of the bacterial pathogen via mosquito bites has been suggested ever since the discovery of E. anophelis in the midgut of the Anopheles gambiae mosquito [79,80] and, more recently, in the salivary glands and saliva of Aedes albopictus [81]. The microbiome of Anopheles mosquitoes has evidently revealed the strong symbiotic nature of E. meningoseptica [82], which has been isolated from various independent sources, including Anopheles stephensi, the vector for the malarial parasite Plasmodium vivax [78,83,84], semi-field Anopheles gambiae females [82,84,85,86], field sampled mosquitoes in Cameroon [87,88], and laboratory-reared mosquitoes where Anopheles were the predominant species [87,89]. Another comparative study on bacterial microbiota isolated from the midgut of various Anopheles spp., which were obtained in the same region of Mae Sot District and Sop Moei District in Thailand, reported on the findings of Elizabethkingia spp. from Anopheles minimus, Anopheles dirus, Anopheles maculatus, Anopheles sawadwongporni, and Anopheles dravidicus mosquitoes [90]. However, sequences associated with the genus Elizabethkingia could not be definitively assigned to either E. anophelis or E. meningoseptica as the V3–V4 region of the 16S rRNA gene used for microbiome profiling could not differentiate between the two species [90]. Despite these multiple discoveries of Elizabethkingia spp. in the midgut and salivary glands of various mosquito species, there is currently a lack of strong direct evidence that supports Elizabethkingia infection, particularly E. anophelis, as a mosquito-borne disease [45], although this should not be ruled out with our current level of knowledge. A comparative genomics study of three cases of E. anophelis also provided evidence of vertical transmission from mother to her baby [62].
Zainuri et al. (2013) reported on the isolation of E. meningoseptica from American bullfrogs (Lithobates catesbeianus or Rana catesbeiana) suffering from red leg syndrome and cataract in Sabah, Malaysia [106]. Isolation of E. meningoseptica from bullfrogs was also described in an earlier study, in which the isolates obtained were found to be resistant to multiple antibiotics [107]. E. miricola, which had been implicated in acute infections in humans, caused a disease outbreak associated involving the internal organs of different anuran species, including northern leopard frogs (Lithobates pipiens), Chapa bug-eyed frogs (Theloderma bicolor), and Vietnamese warty toads (Bombina microdeladigitora) captured in Vietnam. The presence of β-lactamases and putative virulence genes in the E. miricola isolates were detected in silico [27].
E. miricola was also reportedly isolated from Tra catfish (Pangasius hypophthalmus) fillets in the industrial processing lines in Vietnam [109]. Tra catfish is a type of freshwater fish, which is one of the major fish species in the Mekong River, and its processed fillets are exported to more than 80 different countries worldwide [28]. Other scientists have also reported the isolation of E. meningoseptica from retail sausages in Kampar, Malaysia, although the identification was performed by traditional biochemical methods and identified as Chryseobacterium meningosepticum [76].
Furthermore, 454 pyrosequencing of the 16S rRNA gene from the bacterial community of the root of the gnetalean gymnosperm Gnetum gnemon and nearby bulk soils of a tropical forest arboretum at the Forest Research Institute of Malaysia (FRIM) at Kepong, near Kuala Lumpur, identified the mutualistic presence of E. meningoseptica and E. miricola [110]. Elizabethkingia spp. was surprisingly found in relative abundance (4.9%) on the leaves of Gnetum gnemon in comparison with rhizoplane (1.4%) [111]. These reports indicate the ubiquity of Elizabethkingia spp. in the environment and, thus, the difficulty in tracing an outbreak should one occur in the community and outside of hospital settings.
6. Malaysia Reports
Most reported cases of Elizabethkingia spp. infections in Malaysia occurred as isolated cases rather than outbreaks, and the most dominant strain is E. meningoseptica (Table 2). These early identifications of E. meningoseptica were made before laboratories could reliably distinguish between the different Elizabethkingia spp. Although there are currently no published reports on E. anophelis infections in Malaysia, whole-genome sequencing and assembly of a clinical isolate of E. anophelis B2D had been submitted to NCBI (Accession: PRJNA248328) by the University of Malaya but as E. meningoseptica. This led a group of scientists from the University of Malaya to revive thirty archived lyophilized isolates collected from their University Hospital that were initially identified as Flavobacterium meningosepticum [65]. Re-identification using 16S rRNA sequencing revealed that 24 were actually E. anophelis, whereas the remaining six were E. miricola [65]. None of the isolates were identified as E. meningoseptica, underlining the very high possibility of misidentification of these pathogens from earlier publications, particularly those that relied on traditional biochemical tests for their identification. Hence, the cases that had been previously reported as infections due to E. meningoseptica and reviewed below should be taken with caution.

Table 2.
Elizabethkingia spp. isolated from various Southeastern Asian countries based on the published reports until March 2022.
The first case of E. meningoseptica (then reported as Flavobacterium meningosepticum) infection that was recorded in Malaysia involved three neonates with meningitis, where the infection was rapidly controlled by the use of rifamycin [97]. Another study reported six epidemiologically distinct isolates of E. meningoseptica (then reported as Flavobacterium meningosepticum) collected over a two-year period from neonates with meningitis in Kuala Lumpur [98]. From 1972 to 1981, the University of Malaya Medical Centre (UMMC) reported seven confirmed cases of Flavobacterium meningosepticum isolates in infants from the cerebrospinal fluid, three from the blood, and one from the peritoneal fluid [91]. Out of the seven infants, two of them died before receiving intraventricular chemotherapy, and the rest were treated with rifamycin SV, erythromycin, and novobiocin. Two of the surviving patients developed post-infection hydrocephalus, mental retardation, and spasticity (Thong et al., 1981). According to a prospective study carried out over a 12-month period (July 1986 and June 1987) among neonates positive for septicemia at the Special Care Nursery in Hospital Kuala Lumpur (HKL), 6 out of 155 were reported to be positive with E. meningoseptica infection and were treated with rifampicin, erythromycin, and novobiocin [112].
Another case study of resistant E. meningoseptica was confirmed at HKL and was successfully treated with the fourth-generation cephalosporin, cefepime [54]. Ali and Reddy (2007) reported an unusual finding of E. meningoseptica isolate in a bilateral simultaneous hypopyon corneal ulcer in a contact lens wearer caused by polymicrobial infection [101]. In addition, a study conducted at the adult ICU in Hospital Universiti Sains Malaysia (HUSM) involving 1869 organisms isolated in the period between 2005 and 2007 reported that 1% of the isolates comprised of E. meningoseptica [113]. In another study conducted at HKL, it was reported that out of five positive samples for microorganisms on CSF culture and sensitivity, one sample was positive with E. meningoseptica [99]. Septicemia due to E. meningoseptica in Malaysia was reported in a hemorrhagic stroke patient who developed septic shock during prolonged neuro-intensive care management at HUSM [100].
Findings from a study conducted at a 562-bed tertiary hospital in Selangor, Malaysia [102], showed that 4 out of 358 samples collected and analyzed were E. meningoseptica. Most of the isolates were from the surgical wards. Another study [15] reported on the isolation of E. meningoseptica from the blood and cerebrospinal fluid (CSF) of a premature infant of a dichorionic diamniotic (DCDA) twin with neonatal meningitis. The infant required non-invasive continuous pressure ventilation and, after 10 days, was noted to be febrile, less active, and developed seizures. The patient was successfully treated with intravenous vancomycin and ciprofloxacin for 6 weeks, and oral rifampicin was given for a total of 8 days according to the susceptibility testing of the organism. However, the patient’s recovery was complicated with hydrocephalus.
A prospective cohort study was conducted on gastrointestinal tuberculosis-suspected patients at the Queen Elizabeth Adult Hospital in Kota Kinabalu, Sabah, Malaysia [114]. Interestingly, blood cultures revealed the presence of E. meningoseptica in one of the cases, which was classified as a “non-tuberculosis case” using standard case definitions.
7. Singapore Reports
Although E. anophelis had been reported as the dominant species of Elizabethkingia in Singapore [92], it is not short of the presence of E. meningoseptica. Between April and June 2011, the National University Hospital (NUH) Singapore isolated three imipinem-resistant E. meningoseptica from rectal swabs of patients [105]. Another study by NUH reported an increasing prevalence of E. meningoseptica in ICUs after an environmental sampling control. About 44% (35/79) of the collected tap water samples were positive with E. meningoseptica [104].
During a three-week period in 2012, an investigation of an outbreak by the hospital infection control team at NUH showed that three cardiothoracic ICU patients and two surgical ICU patients were initially diagnosed with E. meningoseptica infection as identified by MALDI-TOF MS [115]. All patients were treated with intravenous piperacillin and tazobactam, cotrimoxazole, or levofloxacin, either alone or in combination; however, three of the patients succumbed to their infections due to septicemia. Three subsequent samples collected from the cardiothoracic ICU patients (NUHP1, NUHP2, and NUHP3) and four samples collected from the sink area (NUH1, NUH4, NUH6, and NUH11) were sent for whole-genome sequencing. Findings showed that the isolates obtained were more closely related to E. anophelis strains that were isolated from the midgut of the Anopheles gambiae malaria mosquito vector than to E. meningoseptica [40,115], thus making this the first case of E. anophelis outbreak in an ICU in Singapore.
Another case involved a 75-year-old patient with numerous comorbidities diagnosed with E. meningoseptica in all her four blood cultures after spending 2 months in the hospital for treatment of other conditions [8]. Three days after the diagnosis and starting treatment with intravenous cotrimoxazole, levofloxacin, and minocycline, she started developing redness and pain in her left eye with blurred vision. An intravitreal tap for vitreous culture was taken, and again, her results showed positive with E. meningoseptica. The patient was injected with intravitreal vancomycin and amikacin and had started on hourly fortified gentamicin and cefazolin eyedrops. The patient’s vitreous culture results showed that E. meningoseptica was susceptible to several antibiotics, including ciprofloxacin, levofloxacin, cotrimoxazole, and minocycline, but nevertheless resistant to ceftazidime, gentamicin, and amikacin. Thus, the intravitreal administration was switched to ciprofloxacin (100 µg/0.05 mL) and repeated five times. The patient’s anterior chamber fibrin clot progressively resolved and the inflammatory material in the vitreous cavity became organized, whereas vision was not recovered [8]. Another study also reported infections with carbapenem-resistant E. meningoseptica after 30 days of hospitalization at Tan Tock Seng Hospital (TTSH), Singapore [116].
Three patients aged 2.8 months, 4.9 months, and 4.8 years were diagnosed with E. meningoseptica using MALDI-TOF MS (VITEK MS) within 13 days in the ICU at Kandang Kerbau Women’s and Children’s Hospital (KKH), Singapore in 2017 [93]. Further investigation was conducted, and 27 environmental samples were collected from the three patients’ rooms or cubicles. Of 27 samples collected from tap water outlets and sinks, 10 samples showed positive with E. anophelis, and 1 sample was positive with E. meningoseptica. E. meningoseptica was isolated from a water tap not associated with any of the cases, and E. anophelis was isolated from an aerator [93].
In another retrospective study from 2009 to May 2017 conducted by Chew et al. (2018), seventy-nine blood isolates analyzed with Bruker MALDI Biotyper (bioMérieux) resulted in the identification of either E. meningoseptica (96.2%) or E. miricola (3.8%). Further 16S rRNA gene sequencing using universal primers was performed, and 77 samples showed closer nucleotide identity to E. anophelis. One sample each had a closer nucleotide identity to E. anophelis subsp. Endophytica and E. meningoseptica [92]. Due to the high resemblance between Elizabethkingia species, many isolates were initially misidentified as E. meningoseptica (Yung et al., 2018). Among the 77 isolates collected from hospital waste matters, the blaSHV-producing E. meningoseptica strain was identified among the most predominant taxa (1.4%), showing resistance to extended-spectrum cephalosporins and carbapenems [103].
An eight-year retrospective descriptive study (2010–2017) conducted in a tertiary pediatric hospital in Singapore reported 13 cases of patients with E. meningoseptica infection from the blood and 4 from CSF samples [23]. A 15.4% (2/13) mortality was reported among patients with E. meningoseptica bacteremia. However, a high (75%) number of morbidities consist of patients presented with meningitis. Most patients had developed post-infectious conditions such as hydrocephalus, quadriplegic cerebral palsy with severe disability, and reduced academic performance, whereas another had moderate global developmental delay.
A hemodialysis patient was confirmed positive for E. meningoseptica infection in a retrospective study conducted between January 2011 and June 2012 at the Singapore General Hospital (SGH). The patient was among 118 adult hemodialysis patients confirmed with vascular-access-associated bloodstream infection (VAABSI) [94].
Another case involved a preterm infant born with marked generalized abdominal distension and respiratory distress in the third trimester (33rd week), who was transferred to the neonatal intensive care unit for further care, and three weeks later, the patient was diagnosed with sepsis and subsequently, cloxacillin and amikacin were initiated. Blood samples were collected, and the patient was confirmed to be positive for E. meningoseptica infection. Antibiotic therapy was changed to rifampicin and piperacillin/tazobactam for a period of 4 weeks until full recovery [95].
Combined antibiotic therapy is reported to be the choice of treatment for E. meningoseptica patients in Singapore. The most commonly used combination is piperacillin/tazobactam with trimethoprim/sulfamethoxazole, followed by piperacillin/tazobactam with fluroquinolone [23,95]. Other antimicrobial agents used include minocycline [8,23], clindamycin, rifampicin, ciprofloxacin, cotrimoxazole, and levofloxacin [8].
Singapore and Wisconsin outbreak isolates have type I cps cluster [44]. Additionally, these outbreak strains carry a disrupted DNA repair mutY gene caused by the insertion of an integrative and conjugative element (ICEEa1). Genetic and morphological changes could have substantially contributed to the evolutionary dynamics of the outbreak strains that could have increased their concomitant adaptability eventuating in a hypermutator phenotype [44]. These “outbreak” strain features have not been studied in other Southeast Asian strains due to the lack of WGS investigation. It would not be surprising to find new Southeast Asian or geo-specific lineages, as was recently revealed by a distinct Taiwan strain [117].
8. Thailand Reports
In comparison with Malaysia and Singapore, there are not as many reports on Elizabethkingia infections from Thailand. Nevertheless, a retrospective study conducted by researchers from the University of Bangkok and Chulalongkorn University among eight hospitalized patients at King Chulalongkorn Memorial Hospital (KCMH) led to the first reported cases of E. meningoseptica infection in Thailand [24]. All isolates had shown resistance to cephalosporins, carbapenems, aminoglycosides, vancomycin, and colistin. Patients were treated with combined therapy of ciprofloxacin and cotrimoxazole, followed by levofloxacin, rifampicin, vancomycin, and imipenem. Despite the treatment, the mortality rate was high at 50% [24]. A subsequent report of MALDI-TOF analysis of 54 clinical isolates obtained from Siriraj Hospital, Mahidol University, showed that three of them were E. meningoseptica [36].
9. Indonesia Reports
Reports of Elizabethkingia spp. infections in Indonesia only appeared in the past couple of years. In Malang, Indonesia, a three-month-old infant presented to the Emergency Department of a tertiary hospital with a history of a 15-day fever associated with lethargy [13]. The patient was diagnosed with necrotizing fasciitis with cerebral salt wasting and disseminated intravascular coagulation. The patient had undergone fasciotomy and distal phalanges amputation. A postoperative blood sample revealed the presence of E. meningoseptica using the VITEK 2 system (bioMérieux). The isolate was further tested with various antimicrobials and showed susceptibility to cefepime, tigecycline, trimethoprim-sulfamethoxazole, intermediate susceptibility to combined antibiotics piperacillin-tazobactam, and resistance to ampicillin, ampicillin-sulbactam, cefazolin, ceftazidime, aztreonam, meropenem, amikacin, and gentamicin. The patient was put on three weeks cefepime (50 mg/kg per body weight/day) followed by mechanical ventilation, fluid and electrolyte therapy, intravenous hypertonic saline infusion, intravenous inotropic therapy, thrombocyte concentrate and fresh frozen plasma transfusion, and oral fluid cortisone therapy. After eight weeks of treatment, the patient showed clinical improvement.
In Jakarta, a group of researchers from the Eijkman Institute reported the presence of E. meningoseptica in one non-dengue febrile patient [118]. A retrospective cohort study conducted at the Pediatric Intensive Care Unit at Sanglah Hospital in Denpasar, Bali, Indonesia, from January 2015 to April 2017 led to the isolation of E. meningoseptica from one of their patients’ blood samples diagnosed with septicemia [25]. In a cross-sectional study conducted between April and August of 2015 in the neonatology ward of Haji Adam Malik Hospital in Medan, North Sumatra, 3 out of 43 neonates were found positive for E. meningoseptica from their blood samples, although the method of identification was not stated [96].
10. Cambodia Reports
Reports of Elizabethkingia spp. infections in Cambodia are rare. Reed et al. (2020) reported a case in which a 7-day-old female patient with presumed late-onset neonatal sepsis was transferred to the pediatric ICU at Angkor Hospital for Children, Siem Reap, Cambodia [26]. The patient had experienced symptoms of meningitis, including fever and seizures. Intravenous meropenem (40 mg/kg three times a day) was initiated, and after three days of hospitalization, blood culture isolated was identified as E. anophelis through MALDI-TOF MS using the bioMérieux VITEK MS. Later, the patient’s antibiotic therapy was replaced with intravenous ciprofloxacin (10 mg/kg, 2 per day) and vancomycin (15 mg/kg, 1 per day), and the patient was discharged after 28 days. A month later, the patient was hospitalized, and clinical features showed raised intracranial pressure, including neurologic deficits. The patient was later diagnosed with hydrocephalus. This had led the researchers to the retrieval of the seven isolates stored at −80 °C from January 2012 to October 2018 that had been previously identified as C. meningosepticum, C. miricola, or Elizabethkingia spp. Isolates were subcultured and analyzed using VITEK MS MALDI-TOF mass spectrometry; six of them were re-identified as E. anophelis and one isolate as E. meningoseptica. It is worth noting that four out of seven patients had died due to ventilator-associated pneumonia (VAP) and sepsis [26].
11. Conclusions
Our review of case reports involving Elizabethkingia spp. infections in Southeast Asia showed the dearth of knowledge in the majority of countries within the region. Of the eleven Southeast Asian nations, only Singapore had the most data and published reports, while we were unable to find any clinical case reports from the Philippines, Brunei, Vietnam, Laos, Myanmar, and Timor-Leste. Genomic data are also available mainly from Singapore, with currently only a single isolate of E. anophelis from Malaysia in GenBank (accession no. JNCG01000000) that was (erroneously) deposited in 2014 as E. meningoseptica. The paucity of our knowledge is an important challenge when dealing with Elizabethkingia infections and requires urgent attention from researchers and medical and health officials. Elizabethkingia spp. is still an understudied pathogen with an intrinsic multidrug resistance phenotype that has been reported in several countries around the world, causing opportunistic infections with high mortality rates. The apparent ubiquity of the pathogen, being found in diverse environments and animal hosts, and the presence of multiple antimicrobial resistance genes in its genome is a cause of serious concern as it could serve as a natural reservoir of antimicrobial resistance genes for horizontal transmission to other pathogenic microorganisms. Since currently, only whole-genome sequencing (and perhaps, MALDI-TOF MS in the near future) is able to accurately identify Elizabethkingia to the species level, cheaper but equally specific and sensitive identification or diagnostic methods need to be developed, as genome sequencing and MALDI-TOF MS are not available to most hospital diagnostic laboratories in poorer Southeast Asian countries. Nevertheless, a deeper understanding of the pathogen along with more precise diagnostic procedures, better accessibility of treatment options either with antibiotics or other alternatives such as bacteriophage therapy, and improvements in the prevention of transmission and infection will eventually enable better control of this extremely pathogenic and highly resistant bacteria.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms10050882/s1, Table S1: Genes encoding enzymes/proteins and efflux pumps involved in antibiotic resistance of the Elizabethkingia spp.; Table S2: Potential virulence-associated features among Elizabethkingia spp. as predicted using the Virulence Factor Database (VFDB) [39,40,41,43,44,46,59,60,61,62,72,73,77,119,120,121,122,123,124,125,126,127,128,129,130,131].
Author Contributions
Conceptualization, A.Z. and C.C.Y.; writing—original draft preparation, A.Z.; writing—review and editing, A.Z., J.T. and C.C.Y.; visualization, A.Z.; supervision, C.C.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lau, S.K.; Chow, W.-N.; Foo, C.-H.; Curreem, S.O.; Lo, G.C.-S.; Teng, J.L.; Chen, J.H.; Ng, R.H.; Wu, A.K.; Cheung, I.Y.; et al. Elizabethkingia anophelis bacteremia is associated with clinically significant infections and high mortality. Sci. Rep. 2016, 6, 26045. [Google Scholar] [CrossRef] [PubMed]
- Dziuban, E.J.; Franks, J.L.; So, M.; Peacock, G.; Blaney, D.D. Elizabethkingia in children: A comprehensive review of symptomatic cases reported from 1944 to 2017. Clin. Infect. Dis. 2018, 67, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Opota, O.; Diene, S.M.; Bertelli, C.; Prod’hom, G.; Eckert, P.; Greub, G. Genome of the carbapenemase-producing clinical isolate Elizabethkingia miricola EM_CHUV and comparative genomics with Elizabethkingia meningoseptica and Elizabethkingia anophelis: Evidence for intrinsic multidrug resistance trait of emerging pathogens. Int. J. Antimicrob. Agents 2017, 49, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Swain, B.; Rout, S.; Otta, S.; Rakshit, A. Elizabethkingia meningoseptica: An unusual cause for septicaemia. JMM Case Rep. 2015, 2, e000005. [Google Scholar] [CrossRef]
- Lee, C.-H.; Lin, W.-C.; Chia, J.-H.; Su, L.-H.; Chien, C.-C.; Mao, A.-H.; Liu, J.-W. Community-acquired osteomyelitis caused by Chryseobacterium meningosepticum: Case report and literature review. Diagn. Microbiol. Infect. Dis. 2008, 60, 89–93. [Google Scholar] [CrossRef]
- Gupta, P.; Zaman, K.; Mohan, B.; Taneja, N. Elizabethkingia miricola: A rare non-fermenter causing urinary tract infection. World J. Clin. Cases 2017, 5, 187. [Google Scholar] [CrossRef]
- Raghavan, S.; Thomas, B.; Shastry, B. Elizabethkingia meningoseptica: Emerging multidrug resistance in a nosocomial pathogen. Case Rep. 2017, 2017, bcr-2017-221076. [Google Scholar] [CrossRef]
- Young, S.M.; Lingam, G.; Tambyah, P.A. Elizabethkingia meningoseptica Engodenous Endophthalmitis—A Case Report. Antimicrob. Resist. Infect. Control 2014, 3, 35. [Google Scholar] [CrossRef]
- Yang, J.; Xue, W.; Yu, X. Elizabethkingia meningosepticum endocarditis: A rare case and special therapy. Anatol. J. Cardiol. 2015, 15, 427. [Google Scholar] [CrossRef][Green Version]
- Chi, S.; Fekete, T. Epididymo-orchitis. In Clinical Infectious Disease, 2nd ed.; Schlossberg, D., Ed.; Cambridge University Press: Cambridge, UK, 2015; pp. 401–405. [Google Scholar]
- Gonzalez, C.; Coolen-Allou, N.; Allyn, J.; Esteve, J.; Belmonte, O.; Allou, N. Severe sepsis and pulmonary abscess with bacteremia due to Elizabethkingia miricola. Med. Mal. Infect. 2015, 46, 49–51. [Google Scholar] [CrossRef]
- Lee, C.-C.; Chen, P.-L.; Wang, L.-R.; Lee, H.-C.; Chang, C.-M.; Lee, N.-Y.; Wu, C.-J.; Shih, H.-I.; Ko, W.-C. Fatal case of community-acquired bacteremia and necrotizing fasciitis caused by Chryseobacterium meningosepticum: Case report and review of the literature. J. Clin. Microbiol. 2006, 44, 1181–1183. [Google Scholar] [CrossRef] [PubMed]
- Taufiq Kadafi, K.; Yuliarto, S.; Aji Cahyono, H.; Ratridewi, I.; Khalasha, T. Cerebral Salt Wasting Due to Bacteremia Caused by Elizabethkingia meningoseptica: A Case Report. Arch. Pediatr. Infect. Dis. 2020, 8, e44832. [Google Scholar] [CrossRef]
- Kenna, D.T.; Fuller, A.; Martin, K.; Perry, C.; Pike, R.; Burns, P.J.; Narayan, O.; Wilkinson, S.; Hill, R.; Woodford, N.; et al. rpoB gene sequencing highlights the prevalence of an E. miricola cluster over other Elizabethkingia species among UK cystic fibrosis patients. Diagn. Microbiol. Infect. Dis. 2018, 90, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Amir, A.; IC Sam, J.; Nawi, S. Elizabethkingia meningoseptica neonatal meningitis in a premature infant. Asian J. Med. Biomed. 2018, 2 (Suppl. 1), 22. [Google Scholar]
- Seong, H.; Kim, J.H.; Kim, J.H.; Lee, W.J.; Ahn, J.Y.; Ku, N.S.; Choi, J.Y.; Yeom, J.S.; Song, Y.G.; Jeong, S.J. Risk factors for mortality in patients with elizabethkingia infection and the clinical impact of the antimicrobial susceptibility patterns of elizabethkingia species. J. Clin. Med. 2020, 9, 1431. [Google Scholar] [CrossRef]
- Nori, P.; Cowman, K.; Chen, V.; Bartash, R.; Szymczak, W.; Madaline, T.; Katiyar, C.P.; Jain, R.; Aldrich, M.; Weston, G.; et al. Bacterial and fungal coinfections in COVID-19 patients hospitalized during the New York City pandemic surge. Infect. Control Hosp. Epidemiol. 2021, 42, 84–88. [Google Scholar] [CrossRef]
- Hayek, S.S.; Abd, T.T.; Cribbs, S.K.; Anderson, A.M.; Melendez, A.; Kobayashi, M.; Polito, C.; Wang, Y.F.W. Rare Elizabethkingia meningosepticum meningitis case in an immunocompetent adult. Emerg. Microbes Infect. 2013, 2, 1–4. [Google Scholar] [CrossRef]
- Sebastiampillai, B.S.; Luke, N.V.; Silva, S.; De Silva, S.T.; Premaratna, R. Septicaemia caused by Elizabethkingia-sp in a ‘healthy’Sri Lankan man. Trop. Dr. 2018, 48, 62–63. [Google Scholar] [CrossRef]
- Yang, C.; Liu, Z.; Yu, S.; Ye, K.; Li, X.; Shen, D. Comparison of three species of Elizabethkingia genus by whole-genome sequence analysis. FEMS Microbiol. Lett. 2021, 368, fnab018. [Google Scholar] [CrossRef]
- King, E.O. Studies on a group of previously unclassified bacteria associated with meningitis in infants. Am. J. Clin. Pathol. 1959, 31, 241–247. [Google Scholar] [CrossRef]
- Buttiaux, R.; Vandepitte, J. Flavobacterium in Epidemic Meningitis of New-Born Infants. Ann. Inst. Pasteur 1960, 98, 398–404. [Google Scholar]
- Chan, J.; Chong, C.; Thoon, K.; Tee, N.; Maiwald, M.; Lam, J.; Bhattacharya, R.; Chandran, S.; Yung, C.; Tan, N. Invasive paediatric Elizabethkingia meningoseptica infections are best treated with a combination of piperacillin/tazobactam and trimethoprim/sulfamethoxazole or fluoroquinolone. J. Med. Microbiol. 2019, 68, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- Saetiew, N.; Nilkate, S.; Suankratay, C. Elizabethkingia meningoseptica Infection: The First Case Series in Thailand. Presented at the 26th European Congress of Clinical Microbiology and Infectious Diseases, Bangkok, Thailand, 9–12 April 2016. [Google Scholar] [CrossRef]
- Agustini, N.M.A.; Wati, D.K.; Suparyatha, I.; Hartawan, I.N.B.; Utama, I.M.G.D.L.; Budayanti, N.N.S.; Tunas, I.K. The relationship between bacterial types and antibiotic resistance with the clinical outcomes of sepsis patients in Pediatric Intensive Care Unit at Sanglah Hospital Denpasar, Bali-Indonesia. Indones. J. Biomed. Sci. 2018, 12, 13–18. [Google Scholar] [CrossRef]
- Reed, T.A.; Watson, G.; Kheng, C.; Tan, P.; Roberts, T.; Ling, C.L.; Miliya, T.; Turner, P. Elizabethkingia anophelis Infection in Infants, Cambodia, 2012–2018. Emerg. Infect. Dis. 2020, 26, 320. [Google Scholar] [CrossRef] [PubMed]
- Trimpert, J.; Eichhorn, I.; Vladimirova, D.; Haake, A.; Schink, A.K.; Klopfleisch, R.; Lübke-Becker, A. Elizabethkingia miricola infection in multiple anuran species. Transbound. Emerg. Dis. 2020, 68, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Karl, H.; Lehmann, I.; Rehbein, H.; Schubring, R. Composition and quality attributes of conventionally and organically farmed Pangasius fillets (Pangasius hypophthalmus) on the German market. Int. J. Food Sci. Technol. 2010, 45, 56–66. [Google Scholar] [CrossRef]
- Frederiksen, W.; Ursing, J. Proposed new bacterial taxa and proposed changes of bacterial names published during 1994 and considered to be of interest to medical or veterinary bacteriology. APMIS 1995, 103, 651–654. [Google Scholar] [CrossRef]
- Kim, K.; Kim, M.; Lim, J.; Park, H.; Lee, S. Transfer of Chryseobacterium meningosepticum and Chryseobacterium miricola to Elizabethkingia gen. nov. as Elizabethkingia meningoseptica comb. nov. and Elizabethkingia miricola comb. nov. Int. J. Syst. Evol. Microbiol. 2005, 55, 1287–1293. [Google Scholar] [CrossRef]
- Nicholson, A.C.; Gulvik, C.A.; Whitney, A.M.; Humrighouse, B.W.; Graziano, J.; Emery, B.; Bell, M.; Loparev, V.; Juieng, P.; Gartin, J.; et al. Revisiting the taxonomy of the genus Elizabethkingia using whole-genome sequencing, optical mapping, and MALDI-TOF, along with proposal of three novel Elizabethkingia species: Elizabethkingia bruuniana sp. nov., Elizabethkingia ursingii sp. nov., and Elizabethkingia occulta sp. nov. Antonie Van Leeuwenhoek 2018, 111, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-H.; Kim, J.; Kim, J.-H.; Mo, S. Elizabethkingia argenteiflava sp. nov., isolated from the pod of soybean, Glycine max. Int. J. Syst. Evol. Microbiol. 2021, 71, 004767. [Google Scholar] [CrossRef]
- Hem, S.; Jarocki, V.M.; Baker, D.J.; Charles, I.G.; Drigo, B.; Aucote, S.; Donner, E.; Burnard, D.; Bauer, M.J.; Harris, P.N.; et al. Genomic analysis of Elizabethkingia species from aquatic environments: Evidence for potential clinical transmission. Curr. Res. Microb. Sci. 2022, 3, 100083. [Google Scholar] [CrossRef] [PubMed]
- Kukutla, P.; Lindberg, B.G.; Pei, D.; Rayl, M.; Yu, W.; Steritz, M.; Faye, I.; Xu, J. Insights from the genome annotation of Elizabethkingia anophelis from the malaria vector Anopheles gambiae. PLoS ONE 2014, 9, e97715. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-N.; Lai, C.-H.; Yang, C.-H.; Huang, Y.-H.; Lin, H.-F.; Lin, H.-H. Comparison of four automated microbiology systems with 16S rRNA gene sequencing for identification of Chryseobacterium and Elizabethkingia species. Sci. Rep. 2017, 7, 13824. [Google Scholar] [CrossRef] [PubMed]
- Ekcharoenkul, K.; Ngamskulrungroj, P.; Joyjamras, K.; Leelaporn, A.; Harun, A.; Kiratisin, P. Identification of Uncommon Pathogenic Bacteria by MALDI-TOF Mass Spectrometry Using a Custom Library of Siriraj Hospital. Siriraj Med. J. 2018, 70, 127–130. [Google Scholar]
- Han, M.-S.; Kim, H.; Lee, Y.; Kim, M.; Ku, N.S.; Choi, J.Y.; Yong, D.; Jeong, S.H.; Lee, K.; Chong, Y. Relative prevalence and antimicrobial susceptibility of clinical isolates of Elizabethkingia species based on 16S rRNA gene sequencing. J. Clin. Microbiol. 2017, 55, 274–280. [Google Scholar] [CrossRef]
- Burnard, D.; Gore, L.; Henderson, A.; Ranasinghe, A.; Bergh, H.; Cottrell, K.; Sarovich, D.S.; Price, E.P.; Paterson, D.L.; Harris, P.N. Comparative Genomics and Antimicrobial Resistance Profiling of Elizabethkingia Isolates Reveal Nosocomial Transmission and In Vitro Susceptibility to Fluoroquinolones, Tetracyclines, and Trimethoprim-Sulfamethoxazole. J. Clin. Microbiol. 2020, 58, e00730-20. [Google Scholar] [CrossRef]
- González, L.J.; Vila, A.J. Carbapenem resistance in Elizabethkingia meningoseptica is mediated by metallo-β-lactamase BlaB. Antimicrob. Agents Chemother. 2012, 56, 1686–1692. [Google Scholar] [CrossRef]
- Teo, J.; Tan, S.Y.-Y.; Liu, Y.; Tay, M.; Ding, Y.; Li, Y.; Kjelleberg, S.; Givskov, M.; Lin, R.T.P.; Yang, L. Comparative Genomic Analysis of Malaria Mosquito Vector-Associated Novel Pathogen Elizabethkingia anophelis. Genome Biol. Evol. 2014, 6, 1158–1165. [Google Scholar] [CrossRef]
- Breurec, S.; Criscuolo, A.; Diancourt, L.; Rendueles, O.; Vandenbogaert, M.; Passet, V.; Caro, V.; Rocha, E.P.; Touchon, M.; Brisse, S. Genomic epidemiology and global diversity of the emerging bacterial pathogen Elizabethkingia anophelis. Sci. Rep. 2016, 6, 30379. [Google Scholar] [CrossRef]
- Hu, R.; Zhang, Q.; Gu, Z. Molecular diversity of chromosomal metallo-β-lactamase genes in Elizabethkingia genus. Int. J. Antimicrob. Agents 2020, 56, 105978. [Google Scholar] [CrossRef]
- Hu, R.; Zhang, Q.; Gu, Z. Whole-genome analysis of the potentially zoonotic Elizabethkingia miricola FL160902 with two new chromosomal MBL gene variants. J. Antimicrob. Chemother. 2020, 75, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Perrin, A.; Larsonneur, E.; Nicholson, A.C.; Edwards, D.J.; Gundlach, K.M.; Whitney, A.M.; Gulvik, C.A.; Bell, M.E.; Rendueles, O.; Cury, J.; et al. Evolutionary dynamics and genomic features of the Elizabethkingia anophelis 2015 to 2016 Wisconsin outbreak strain. Nat. Commun. 2017, 8, 15483. [Google Scholar] [CrossRef]
- Lin, J.-N.; Lai, C.-H.; Yang, C.-H.; Huang, Y.-H. Elizabethkingia infections in humans: From genomics to clinics. Microorganisms 2019, 7, 295. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.-Y.; Yang, C.-H.; Lai, C.-H.; Huang, Y.-H.; Lin, J.-N. Comparative Genomics of 86 Whole-Genome Sequences in the Six Species of the Elizabethkingia Genus Reveals Intraspecific and Interspecific Divergence. Sci. Rep. 2019, 9, 19167. [Google Scholar] [CrossRef] [PubMed]
- Bellais, S.; Poirel, L.; Naas, T.; Girlich, D.; Nordmann, P. Genetic-Biochemical Analysis and Distribution of the Ambler Class A β-Lactamase CME-2, Responsible for Extended-Spectrum Cephalosporin Resistance in Chryseobacterium (Flavobacterium) meningosepticum. Antimicrob. Agents Chemother. 2000, 44, 1–9. [Google Scholar] [CrossRef]
- Chang, J.; Hsueh, P.; Wu, J.; Ho, S.; Hsieh, W.; Luh, K. Antimicrobial susceptibility of flavobacteria as determined by agar dilution and disk diffusion methods. Antimicrob. Agents Chemother. 1997, 41, 1301–1306. [Google Scholar] [CrossRef]
- Moulin, V.; Freney, J.; Hansen, W.; Philippon, A. Comportement phénotypique des Flavobacterium vis-à-vis de 39 antibiotiques. Méd. Mal. Infect. 1992, 22, 902–907. [Google Scholar] [CrossRef]
- Kwambana-Adams, B.; Laxton, C.; Foster-Nyarko, E.; Weinstock, G.; Antonio, M. Isolation of Methicillin-resistant Staphylococcus aureus and Multidrug-resistant Elizabethkingia meningoseptica from Neonates within Minutes of Birth. Pediatric Infect. Dis. J. 2017, 36, 123–124. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, Y.; Lin, Y.; Wang, F.; Chan, Y.; Yang, T. Risk factors and outcome of levofloxacin-resistant Elizabethkingia meningoseptica bacteraemia in adult patients in Taiwan. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1–8. [Google Scholar] [CrossRef]
- Jean, S.-S.; Hsieh, T.-C.; Ning, Y.-Z.; Hsueh, P.-R. Role of vancomycin in the treatment of bacteraemia and meningitis caused by Elizabethkingia meningoseptica. Int. J. Antimicrob. Agents 2017, 50, 507–511. [Google Scholar] [CrossRef]
- Lee, E.; Robinson, M.; Thong, M.; Puthucheary, S.; Ong, T.; Ng, K. Intraventricular chemotherapy in neonatal meningitis. J. Pediatr. 1977, 91, 991–995. [Google Scholar] [CrossRef]
- Lim, V.; Halijah, M. A comparative study of the in-vitro activity of cefepime and other cephalosporins. Malays. J. Pathol. 1993, 15, 65–68. [Google Scholar] [PubMed]
- Chang, T.-Y.; Chen, H.-Y.; Chou, Y.-C.; Cheng, Y.-H.; Sun, J.-R. In vitro activities of imipenem, vancomycin, and rifampicin against clinical Elizabethkingia species producing BlaB and GOB metallo-beta-lactamases. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 2045–2052. [Google Scholar] [CrossRef] [PubMed]
- Santona, A.; Paglietti, B.; Al-Qahtani, A.A.; Bohol, M.F.F.; Senok, A.; Deligios, M.; Rubino, S.; Al-Ahdal, M.N. Novel type of VanB2 teicoplanin-resistant hospital-associated Enterococcus faecium. Int. J. Antimicrob. Agents 2014, 44, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Sader, H.S.; Jones, R.N.; Pfaller, M.A. Relapse of catheter-related Flavobacterium meningosepticum bacteremia demonstrated by DNA macrorestriction analysis. Clin. Infect. Dis. 1995, 21, 997–1000. [Google Scholar] [CrossRef] [PubMed]
- Ozkalay, N.; Anil, M.; Agus, N.; Helvaci, M.; Sirti, S. Community-acquired meningitis and sepsis caused by Chryseobacterium meningosepticum in a patient diagnosed with thalassemia major. J. Clin. Microbiol. 2006, 44, 3037–3039. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Soehnlen, M.; Blom, J.; Terrapon, N.; Henrissat, B.; Walker, E.D. Comparative genomic analyses reveal diverse virulence factors and antimicrobial resistance mechanisms in clinical Elizabethkingia meningoseptica strains. PLoS ONE 2019, 14, e0222648. [Google Scholar] [CrossRef]
- Lin, J.-N.; Lai, C.-H.; Yang, C.-H.; Huang, Y.-H.; Lin, H.-H. Genomic features, phylogenetic relationships, and comparative genomics of Elizabethkingia anophelis strain EM361-97 isolated in Taiwan. Sci. Rep. 2017, 7, 14317. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Chew, S.C.; Tay, M.; Salido, M.M.S.; Teo, J.; Lauro, F.M.; Givskov, M.; Yang, L. Complete genome sequence and transcriptomic analysis of the novel pathogen Elizabethkingia anophelis in response to oxidative stress. Genome Biol. Evol. 2015, 7, 1676–1685. [Google Scholar] [CrossRef]
- Lau, S.K.; Wu, A.K.; Teng, J.L.; Tse, H.; Curreem, S.O.; Tsui, S.K.; Huang, Y.; Chen, J.H.; Lee, R.A.; Yuen, K.-Y.; et al. Evidence for Elizabethkingia anophelis transmission from mother to infant, Hong Kong. Emerg. Infect. Dis. 2015, 21, 232. [Google Scholar] [CrossRef]
- Jacobs, A.; Chenia, H.Y. Biofilm formation and adherence characteristics of an Elizabethkingia meningoseptica isolate from Oreochromis mossambicus. Ann. Clin. Microbiol. Antimicrob. 2011, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Alav, I.; Sutton, J.M.; Rahman, K.M. Role of bacterial efflux pumps in biofilm formation. J. Antimicrob. Chemother. 2018, 73, 2003–2020. [Google Scholar] [CrossRef] [PubMed]
- Puah, S.M.; Fong, S.P.; Kee, B.P.; Puthucheary, S.; Chua, K.H. Molecular identification and biofilm-forming ability of Elizabethkingia species. Microb. Pathog. 2022, 162, 105345. [Google Scholar] [CrossRef] [PubMed]
- Dunn, M.; Ramirez-Trujillo, J.; Hernández-Lucas, I. Major roles of isocitrate lyase and malate synthase in bacterial and fungal pathogenesis. Microbiology 2009, 155, 3166–3175. [Google Scholar] [CrossRef]
- McKinney, J.D.; Zu Bentrup, K.H.; Muñoz-Elías, E.J.; Miczak, A.; Chen, B.; Chan, W.-T.; Swenson, D.; Sacchettini, J.C.; Jacobs, W.R.; Russell, D.G. Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature 2000, 406, 735–738. [Google Scholar] [CrossRef]
- Roberts, M.F.; Khan, H.M.; Goldstein, R.; Reuter, N.; Gershenson, A. Search and subvert: Minimalist bacterial phosphatidylinositol-specific phospholipase C enzymes. Chem. Rev. 2018, 118, 8435–8473. [Google Scholar] [CrossRef]
- Monturiol-Gross, L.; Villalta-Romero, F.; Flores-Díaz, M.; Alape-Girón, A. Bacterial phospholipases C with dual activity: Phosphatidylcholinesterase and sphingomyelinase. FEBS Open Bio 2021, 11, 3262–3275. [Google Scholar] [CrossRef]
- Vicente, C.S.; Nascimento, F.X.; Ikuyo, Y.; Cock, P.J.; Mota, M.; Hasegawa, K. The genome and genetics of a high oxidative stress tolerant Serratia sp. LCN16 isolated from the plant parasitic nematode Bursaphelenchus xylophilus. BMC Genom. 2016, 17, 301. [Google Scholar] [CrossRef]
- Hassett, D.J.; Alsabbagh, E.; Parvatiyar, K.; Howell, M.L.; Wilmott, R.W.; Ochsner, U.A. A protease-resistant catalase, KatA, released upon cell lysis during stationary phase is essential for aerobic survival of a Pseudomonas aeruginosa oxyR mutant at low cell densities. J. Bacteriol. 2000, 182, 4557–4563. [Google Scholar] [CrossRef]
- Wang, M.; Gao, H.; Lin, N.; Zhang, Y.; Huang, N.; Walker, E.D.; Ming, D.; Chen, S.; Hu, S. The antibiotic resistance and pathogenicity of a multidrug-resistant Elizabethkingia anophelis isolate. Microbiologyopen 2019, 8, e804. [Google Scholar] [CrossRef]
- Yang, C.; Liu, Z.; Yu, S.; Ye, K.; Li, X.; Shen, D. Comparison of Whole-Genome Sequences for Three Species of the Elizabethkingia Genus. 2020. Available online: https://www.researchsquare.com/article/rs-61004/v1 (accessed on 21 April 2022).
- Heberle, H.; Meirelles, G.V.; da Silva, F.R.; Telles, G.P.; Minghim, R. InteractiVenn: A web-based tool for the analysis of sets through Venn diagrams. BMC Bioinform. 2015, 16, 169. [Google Scholar] [CrossRef] [PubMed]
- Laith, A.; Mazlan, A.; Ambak, M.; Jabar, A.; Najiah, M. Isolation and Identification of Elizabethkingia meningoseptica from Diseased African Catfish Clarias gariepinus. J. Microbiol. Biotechnol. Food Sci. 2017, 6, 1070–1076. [Google Scholar] [CrossRef]
- Tew, L.-S.; She, L.-Y.; Chew, C.-H. Isolation, Antimicrobial Susceptibility Profile and Detection of Sul1, blaTEM, and blaSHV in Amoxicillin-Clavulanate-Resistant Bacteria Isolated From Retail Sausages in Kampar, Malaysia. Jundishapur J. Microbiol. 2016, 9, e37897. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, Y.; Wang, F. Risk factors of healthcare-associated Elizabethkingia meningoseptica infections in Taiwan medical center. Int. J. Antimicrob. Agents 2017, 50, S141. [Google Scholar]
- Khan, I.; Lall, M.; Sen, S.; Ninawe, S.; Chandola, P. Multiresistant Elizabethkingia meningoseptica infections in tertiary care. Med. J. Armed Forces India 2015, 71, 282. [Google Scholar] [CrossRef]
- Kämpfer, P.; Matthews, H.; Glaeser, S.P.; Martin, K.; Lodders, N.; Faye, I. Elizabethkingia anophelis sp. nov., isolated from the midgut of the mosquito Anopheles gambiae. Int. J. Syst. Evol. Microbiol. 2011, 61, 2670–2675. [Google Scholar] [CrossRef]
- Chen, S.; Johnson, B.K.; Yu, T.; Nelson, B.N.; Walker, E.D. Elizabethkingia anophelis: Physiologic and transcriptomic responses to iron stress. Front. Microbiol. 2020, 11, 804. [Google Scholar] [CrossRef]
- Onyango, M.; Payne, A.; Stout, J.; Dieme, C.; Kuo, L.; Kramer, L.; Ciota, A. Potential for transmission of Elizabethkingia anophelis by Aedes albopictus and the role of microbial interactions in Zika virus competence. bioRxiv 2020, 702464. [Google Scholar] [CrossRef]
- Akhouayri, I.; Habtewold, T.; Christophides, G. Melanotic pathology and vertical transmission of the gut commensal Elizabethkingia meningoseptica in the major malaria vector Anopheles gambiae. PLoS ONE 2013, 8, e77619. [Google Scholar] [CrossRef]
- Rani, A.; Sharma, A.; Rajagopal, R.; Adak, T.; Bhatnagar, R.K. Bacterial diversity analysis of larvae and adult midgut microflora using culture-dependent and culture-independent methods in lab-reared and field-collected Anopheles stephensi-an Asian malarial vector. BMC Microbiol. 2009, 9, 96. [Google Scholar] [CrossRef]
- Ngwa, C.; Glöckner, V.; Abdelmohsen, U.R.; Scheuermayer, M.; Fischer, R.; Hentschel, U.; Pradel, G. 16S rRNA gene-based identification of Elizabethkingia meningoseptica (Flavobacteriales: Flavobacteriaceae) as a dominant midgut bacterium of the Asian malaria vector Anopheles stephensi (Dipteria: Culicidae) with antimicrobial activities. J. Med. Entomol. 2013, 50, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Lindh, J.; Borg-Karlson, A.; Faye, I. Transstadial and horizontal transfer of bacteria within a colony of Anopheles gambiae (Diptera: Culicidae) and oviposition response to bacteria-containing water. Acta Trop. 2008, 107, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gilbreath, T.M., III; Kukutla, P.; Yan, G.; Xu, J. Dynamic gut microbiome across life history of the malaria mosquito Anopheles gambiae in Kenya. PLoS ONE 2011, 6, e24767. [Google Scholar] [CrossRef] [PubMed]
- Boissière, A.; Tchioffo, M.; Bachar, D.; Abate, L.; Marie, A.; Nsango, S.; Shahbazkia, H.; Awono-Ambene, P.; Levashina, E.; Christen, R.; et al. Midgut microbiota of the malaria mosquito vector Anopheles gambiae and interactions with Plasmodium falciparum infection. PLoS Pathog. 2012, 8, e1002742. [Google Scholar] [CrossRef]
- Wang, S.; Ghosh, A.K.; Bongio, N.; Stebbings, K.A.; Lampe, D.J.; Jacobs-Lorena, M. Fighting malaria with engineered symbiotic bacteria from vector mosquitoes. Proc. Natl. Acad. Sci. USA 2012, 109, 12734–12739. [Google Scholar] [CrossRef]
- Dong, Y.; Manfredini, F.; Dimopoulos, G. Implication of the mosquito midgut microbiota in the defense against malaria parasites. PLoS Pathog. 2009, 5, e1000423. [Google Scholar] [CrossRef]
- Tainchum, K.; Dupont, C.; Chareonviriyaphap, T.; Jumas-Bilak, E.; Bangs, M.J.; Manguin, S. Bacterial microbiome in wild-caught Anopheles mosquitoes in western Thailand. Front. Microbiol. 2020, 11, 965. [Google Scholar] [CrossRef]
- Thong, M.; Puthucheary, S.; Lee, E. Flavobacterium meningosepticum infection: An epidemiological study in a newborn nursery. J. Clin. Pathol. 1981, 34, 429–433. [Google Scholar] [CrossRef]
- Chew, K.L.; Cheng, B.; Lin, R.T.; Teo, J.W. Elizabethkingia anophelis is the dominant Elizabethkingia species found in blood cultures in Singapore. J. Clin. Microbiol. 2018, 56, e01445-17. [Google Scholar] [CrossRef]
- Yung, C.-F.; Maiwald, M.; Loo, L.H.; Soong, H.Y.; Tan, C.B.; Lim, P.K.; Li, L.; Tan, N.W.; Chong, C.-Y.; Tee, N.; et al. Elizabethkingia anophelis and association with tap water and handwashing, Singapore. Emerg. Infect. Dis. 2018, 24, 1730. [Google Scholar] [CrossRef]
- Loo, L.W.; Liew, Y.X.; Choong, H.L.L.; Tan, A.L.; Chlebicki, P. Microbiology and audit of vascular access-associated bloodstream infections in multi-ethnic Asian hemodialysis patients in a tertiary hospital. Infect. Dis. 2015, 47, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Sooklin, L.; Anand, A.J.; Rajadurai, V.S.; Chandran, S. Management of large congenital chylous ascites in a preterm infant: Fetal and neonatal interventions. BMJ Case Rep. CP 2020, 13, e235849. [Google Scholar] [CrossRef]
- Liestiadi, D.E.F.; Azlin, E.; Nafianti, S. A hematologic scoring system and C-reactive protein compared to blood cultures for diagnosing bacterial neonatal sepsis. Paediatr. Indones 2017, 57, 71. [Google Scholar] [CrossRef][Green Version]
- Lee, E.; Robinson, M.; Thong, M.; Puthucheary, S. Rifamycin in Neonatal Flavobacteria meningitis. Arch. Dis. Child. 1976, 51, 209–213. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Raimondi, A.; Moosdeen, F.; Williams, J. Antibiotic resistance pattern of Flavobacterium meningosepticum. Eur. J. Clin. Microbiol. Infect. Dis. 1986, 5, 461–463. [Google Scholar] [CrossRef]
- Zakaria, Z.; Idris, B. Intraoperative Cerebrospinal Fluid Sample from First Ventriculoperitoneal Shunt Operation: Is it Indicated? Malays. J. Med. Sci. MJMS 2013, 20, 102. [Google Scholar]
- Wan Hassan, W.M.N.; Paramasivam, R.P.; Kandasamy, R.; Hassan, M.H.; Zaini, R.H.M. An uncommon Elizabethkingia meningoseptica septicemia in hemorrhagic stroke with septic shock patient during prolonged neuro-intensive care management. Anaesth. Pain Intensive Care 2017, 21, 268–271. [Google Scholar]
- Ali, N.A.M.; Reddy, S.C. Bilateral simultaneous infectious keratitis secondary to contact lens wear: An unusual case report with rare organisms. Eye Contact Lens 2007, 33, 338–340. [Google Scholar] [CrossRef]
- Phoon, H.Y.; Hussin, H.; Hussain, B.M.; Lim, S.Y.; Woon, J.J.; Er, Y.X.; Thong, K.L. Distribution, genetic diversity and antibiotic resistance of clinically important bacteria from the environment of a tertiary hospital. J. Glob. Antimicrob. Resist. 2018, 14, 132–140. [Google Scholar] [CrossRef]
- Haller, L.; Chen, H.; Ng, C.; Le, T.H.; Koh, T.H.; Barkham, T.; Sobsey, M.; Gin, K.Y.-H. Occurrence and characteristics of extended-spectrum β-lactamase-and carbapenemase-producing bacteria from hospital effluents in Singapore. Sci. Total Environ. 2018, 615, 1119–1125. [Google Scholar] [CrossRef]
- Balm, M.; Salmon, S.; Jureen, R.; Teo, C.; Mahdi, R.; Seetoh, T.; Teo, J.; Lin, R.; Fisher, D. Bad design, bad practices, bad bugs: Frustrations in controlling an outbreak of Elizabethkingia meningoseptica in intensive care units. J. Hosp. Infect. 2013, 85, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, I.; Teo, J.; Balm, M.N.; Fisher, D.A.; Jureen, R.; Lin, R.T. Klebsiella pneumoniae carbapenemase-producing enterobacteria in hospital, Singapore. Emerg. Infect. Dis. 2012, 18, 1381. [Google Scholar] [CrossRef] [PubMed]
- Zainuri, N.; Ransangan, J.; Lal, T.; Jintoni, B.; Chung, V. Identification of Elizabethkingia meningoseptica from American bullfrog (Rana catesbeiana) farmed in Sabah, Malaysia using PCR method and future management of outbreak. Malays. J. Microbiol. 2013, 9, 13–23. [Google Scholar] [CrossRef]
- Tee, L.; Najiah, M. Antibiogram and heavy metal tolerance of bullfrog bacteria in Malaysia. Open Vet. J. 2011, 1, 39–45. [Google Scholar]
- Surat, W.; Mhuantong, W.; Sangsrakru, D.; Chareonviriyaphap, T.; Arunyawat, U.; Kubera, A.; Sittivicharpinyo, T.; Siripan, O.; Pootakham, W. Gut Bacterial Diversity in Plasmodium-infected and Plasmodium-uninfected Anopheles minimus. Chiang Mai J. Sci. 2016, 43, 427–440. [Google Scholar]
- Thi, A.N.T.; Noseda, B.; Samapundo, S.; Nguyen, B.L.; Broekaert, K.; Rasschaert, G.; Heyndrickx, M.; Devlieghere, F. Microbial ecology of Vietnamese Tra fish (Pangasius hypophthalmus) fillets during processing. Int. J. Food Microbiol. 2013, 167, 144–152. [Google Scholar]
- Kim, M.; Singh, D.; Lai-Hoe, A.; Go, R.; Rahim, R.A.; Ainuddin, A.; Chun, J.; Adams, J.M. Distinctive phyllosphere bacterial communities in tropical trees. Microb. Ecol. 2012, 63, 674–681. [Google Scholar] [CrossRef]
- Oh, Y.M.; Kim, M.; Lee-Cruz, L.; Lai-Hoe, A.; Go, R.; Ainuddin, N.; Rahim, R.A.; Shukor, N.; Adams, J.M. Distinctive bacterial communities in the rhizoplane of four tropical tree species. Microb. Ecol. 2012, 64, 1018–1027. [Google Scholar] [CrossRef]
- Boo, N.; Wong, Y.; Lim, V. Pattern of neonatal septicemia in a Malaysian maternity hospital. Med. J. Malays. 1989, 44, 189–193. [Google Scholar]
- Habsah, H.; Zakuan, Z. Profiles of Imipenem Resistance Organism in an Adult ICU in a Teaching Hospital in Malaysia. Int. J. Infect. Dis. 2008, 12, e118–e119. [Google Scholar] [CrossRef][Green Version]
- Lowbridge, C.; Fadhil, S.A.; Krishnan, G.D.; Schimann, E.; Karuppan, R.M.; Sriram, N.; Rajahram, G.S.; Menon, J.; Patel, A.; William, T.; et al. How can gastro-intestinal tuberculosis diagnosis be improved? A prospective cohort study. BMC Infect. Dis. 2020, 20, 255. [Google Scholar] [CrossRef]
- Teo, J.; Tan, S.Y.-Y.; Tay, M.; Ding, Y.; Kjelleberg, S.; Givskov, M.; Lin, R.T.; Yang, L. First case of E anophelis outbreak in an intensive-care unit. Lancet 2013, 382, 855–856. [Google Scholar] [CrossRef]
- Lew, K.Y.; Ng, T.M.; Tan, M.; Tan, S.H.; Lew, E.L.; Ling, L.M.; Ang, B.; Lye, D.; Teng, C.B. Safety and clinical outcomes of carbapenem de-escalation as part of an antimicrobial stewardship programme in an ESBL-endemic setting. J. Antimicrob. Chemother. 2015, 70, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-L.; Liu, K.-M.; Chang, H.-L.; Liao, Y.-C.; Lin, J.-S.; Kung, F.-Y.; Ho, C.-M.; Lin, K.-H.; Chen, Y.-T. The Evolutionary Trend and Genomic Features of an Emerging Lineage of Elizabethkingia anophelis Strains in Taiwan. Microbiol. Spectr. 2022, 10, e01682-21. [Google Scholar] [CrossRef] [PubMed]
- Santoso, M.S.; Yohan, B.; Denis, D.; Hayati, R.F.; Haryanto, S.; Trianty, L.; Noviyanti, R.; Hibberd, M.L.; Sasmono, R.T. Diagnostic accuracy of 5 different brands of dengue virus non-structural protein 1 (NS1) antigen rapid diagnostic tests (RDT) in Indonesia. Diagn. Microbiol. Infect. Dis. 2020, 98, 115116. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Soehnlen, M.; Downes, F.; Walker, E. Insights from the draft genome into the pathogenicity of a clinical isolate of Elizabethkingia meningoseptica Em3. Stand. Genom. Sci. 2017, 12, 56. [Google Scholar] [CrossRef] [PubMed]
- Amladi, A.; Jacob, J.J.; Anandan, S.; Veeraraghavan, B. Draft genome sequence of carbapenem-resistant Elizabethkingia anophelis strain BP8467 clinical isolate from India. J. Glob. Antimicrob. Resist. 2020, 21, 200–202. [Google Scholar] [CrossRef]
- Yum, J.H.; Lee, E.Y.; Hur, S.-H.; Jeong, S.H.; Lee, H.; Yong, D.; Chong, Y.; Lee, E.-W.; Nordmann, P.; Lee, K. Genetic diversity of chromosomal metallo-β-lactamase genes in clinical isolates of Elizabethkingia meningoseptica from Korea. J. Microbiol. 2010, 48, 358–364. [Google Scholar] [CrossRef]
- Bellais, S.; Aubert, D.; Naas, T.; Nordmann, P. Molecular and biochemical heterogeneity of class B carbapenem-hydrolyzing β-lactamases in Chryseobacterium meningosepticum. Antimicrob. Agents Chemother. 2000, 44, 1878–1886. [Google Scholar] [CrossRef]
- Matyi, S.A.; Hoyt, P.R.; Hosoyama, A.; Yamazoe, A.; Fujita, N.; Gustafson, J.E. Draft genome sequences of Elizabethkingia meningoseptica. Genome Announc. 2013, 1, e00444-13. [Google Scholar] [CrossRef]
- Sun, G.; Wang, L.; Bao, C.; Li, T.; Ma, L.; Chen, L. Complete genome sequence of Elizabethkingia meningoseptica, isolated from a T-cell non-Hodgkin’s lymphoma patient. Genome Announc. 2015, 3, e00673-15. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, J.P.; Burd, E.M. Other gram-negative and gram-variable bacilli. Princ. Pract. Infect. Dis. 2010, 2, 2751–2768. [Google Scholar]
- Rossolini, G.M.; Franceschini, N.; Lauretti, L.; Caravelli, B.; Riccio, M.L.; Galleni, M.; Frère, J.-M.; Amicosante, G. Cloning of a Chryseobacterium (Flavobacterium) meningosepticum chromosomal gene (blaA CME) encoding an extended-spectrum class A β-lactamase related to the Bacteroides cephalosporinases and the VEB-1 and PER β-lactamases. Antimicrob. Agents Chemother. 1999, 43, 2193–2199. [Google Scholar] [CrossRef] [PubMed]
- Ghafoori, S.M.; Robles, A.M.; Arada, A.M.; Shirmast, P.; Dranow, D.M.; Mayclin, S.J.; Lorimer, D.D.; Myler, P.J.; Edwards, T.E.; Kuhn, M.L. Structural characterization of a Type B chloramphenicol acetyltransferase from the emerging pathogen Elizabethkingia anophelis NUHP1. Sci. Rep. 2021, 11, 9453. [Google Scholar] [CrossRef]
- Naidenov, B.; Lim, A.; Willyerd, K.; Torres, N.J.; Johnson, W.L.; Hwang, H.J.; Hoyt, P.; Gustafson, J.E.; Chen, C. Pan-genomic and polymorphic driven prediction of antibiotic resistance in Elizabethkingia. Front. Microbiol. 2019, 10, 1446. [Google Scholar] [CrossRef]
- Lin, J.-N.; Lai, C.-H.; Yang, C.-H.; Huang, Y.-H.; Lin, H.-H. Clinical manifestations, molecular characteristics, antimicrobial susceptibility patterns and contributions of target gene mutation to fluoroquinolone resistance in Elizabethkingia anophelis. J. Antimicrob. Chemother. 2018, 73, 2497–2502. [Google Scholar] [CrossRef]
- Johnson, W.L.; Ramachandran, A.; Torres, N.J.; Nicholson, A.C.; Whitney, A.M.; Bell, M.; Villarma, A.; Humrighouse, B.W.; Sheth, M.; Dowd, S.E. The draft genomes of Elizabethkingia anophelis of equine origin are genetically similar to three isolates from human clinical specimens. PLoS ONE 2018, 13, e0200731. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, D.; Wang, Y.; Yan, H.; Shi, L.; Zhou, L. Occurrence of antimicrobial resistance genes sul and dfrA 12 in hospital environmental isolates of Elizabethkingia meningoseptica. World J. Microbiol. Biotechnol. 2012, 28, 3097–3102. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).