Isolation and Genetic Characterization of Streptococcus iniae Virulence Factors in Adriatic Sturgeon (Acipenser naccarii)
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish Sampling and Differential Diagnosis
2.2. Bacterial Culture and Phenotypic Identification
2.3. Kirby–Bauer Susceptibility Test
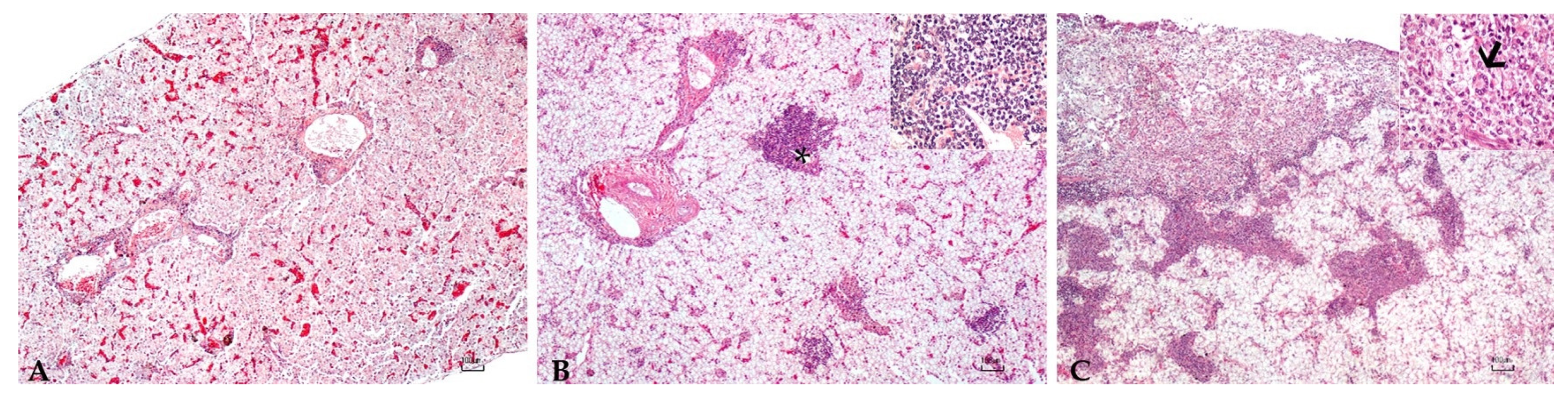
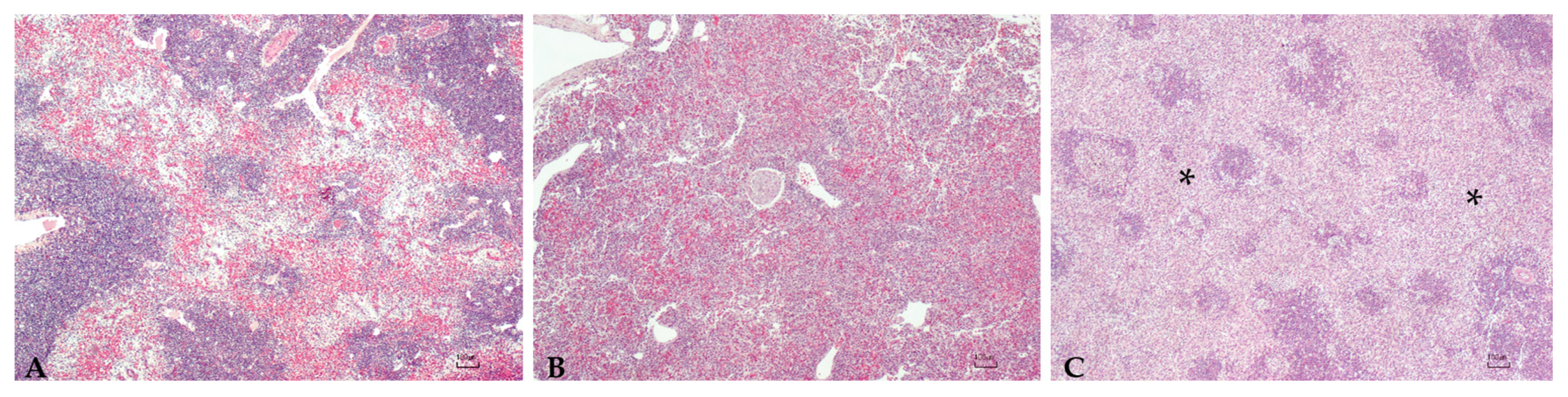
2.4. Histopathology
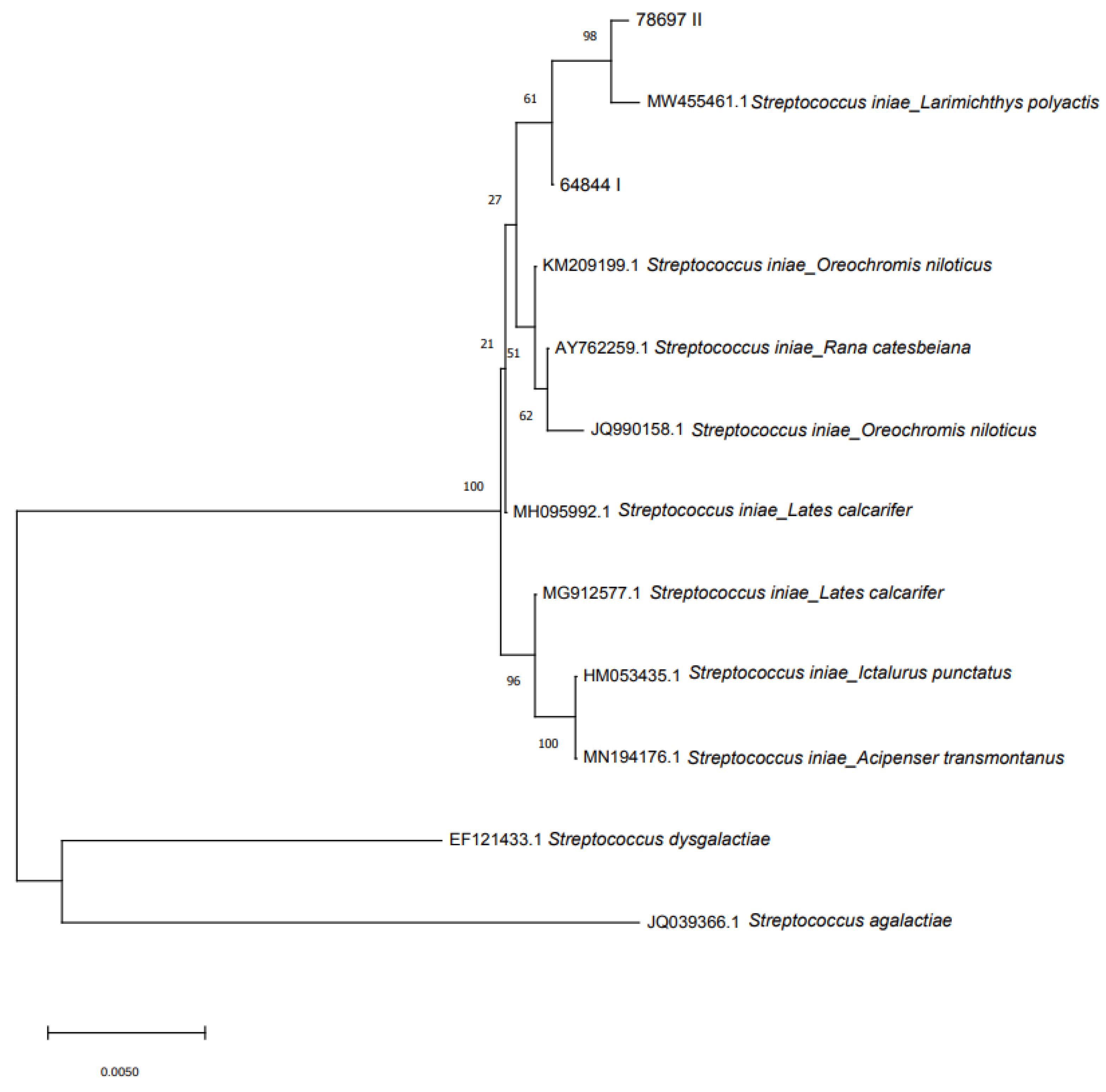
2.5. Molecular Identification, Pathogenicity and Phylogenesis
3. Results
3.1. Biometrical Features of Sturgeons
3.2. Differential Diagnosis
3.3. Bacterial Culture, Phenotypic and Proteomic Identification
3.4. Antimicrobial Resistance
3.5. Histopathology
3.6. Molecular Identification, Pathogenicity and Phylogenesis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agnew, W.; Barnes, A.C. Streptococcus iniae: An aquatic pathogen of global veterinary significance and a challenging candidate for reliable vaccination. Vet. Microbiol. 2007, 122, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Eldar, A.; Ghittino, C. Lactococcus garvieae and Streptococcus iniae infections in rainbow trout Oncorhynchus mykiss: Similar, but different diseases. Dis. Aquat. Org. 1999, 36, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Soltani, M.; Jamshidi, S.; Sharifpour, I. Streptococcosis caused by Streptococcus iniae in farmed rainbow trout (Oncorhynchus mykiss) in Iran: Biophysical characteristics and pathogenesis. Bull. Eur. Ass. Fish Pathol. 2005, 25, 95–106. [Google Scholar]
- Erfanmanesh, A.; Soltani, M.; Pirali, E.; Mohammadian, S.; Taherimirghaed, A. Genetic characterization of Streptococcus iniae in diseased farmed rainbow trout (Oncorhynchus mykiss) in Iran. Sci. World J. 2012, 2012, 594073. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shoemaker, C.A.; Evans, J.J.; Klesius, P.H. density and dose: Factors affecting mortality of Streptococcus iniae infected tilapia (Oreochromis niloticus). Aquaculture 2000, 188, 229–235. [Google Scholar] [CrossRef]
- Baums, C.G.; Hermeyer, K.; Leimbach, S.; Adamek, M.; Czerny, C.-P.; Hörstgen-Schwark, G.; Valentin-Weigand, P.; Baumgärtner, W.; Steinhagen, D. Establishment of a model of Streptococcus iniae meningoencephalitis in Nile tilapia (Oreochromis niloticus). J. Comp. Path. 2013, 149, 94–102. [Google Scholar] [CrossRef]
- Perera, R.P.; Johnson, S.K.; Lewis, D.H. Epizootiological aspects of Streptococcus iniae affecting tilapia in Texas. Aquaculture 1997, 152, 25–33. [Google Scholar] [CrossRef]
- Deng, M.; Yu, Z.; Geng, Y.; Wang, K.; Chen, D.; Huang, X.; Ou, Y.; Chen, Z.; Zhong, Z.; Lai, W. Outbreaks of Streptococcosis associated with Streptococcus iniae in Siberian sturgeon (Acipenser baerii) in China. Aqua Res 2017, 48, 909–919. [Google Scholar] [CrossRef]
- Chen, D.; Peng, S.; Chen, D.; Yang, F.; Liu, J.; Wang, J.; Liu, Q.; Huang, X.; Ouyang, P.; Wang, K.; et al. Low lethal doses of Streptococcus iniae caused enteritis in Siberian sturgeon (Acipenser baerii). Fish Shellfish Immunol. 2020, 104, 654–662. [Google Scholar] [CrossRef]
- Chen, D.; Wang, K.; Geng, Y.; Wang, J.; Huang, X.; He, M. Pathological changes in cultured channel catfish Ictalurus punctatus spontaneously infected with Streptococcus iniae. Dis. Aquat. Org. 2011, 95, 203–208. [Google Scholar] [CrossRef]
- Bromage, E.S.; Thomas, A.; Owen, L. Streptococcus iniae, a bacterial infection in barramundi Lates calcarifer. Dis. Aquat. Org. 1999, 36, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Creeper, J.H.; Buller, N.B. An outbreak of Streptococcus iniae in barramundi (Lates calcarifera) in freshwater cage culture. Aust. Vet. J. 2006, 84, 408–411. [Google Scholar] [CrossRef] [PubMed]
- Eldar, A.; Perl, S.; Frelier, P.F.; Bercovier, H. Red drum Sciaenops ocellatus mortalities associated with Streptococcus iniae infection. Dis. Aquat. Org. 1999, 36, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Baeck, G.W.; Kim, J.H.; Gomez, D.K.; Park, S.C. Isolation and characterization of Streptococcus sp. from diseased flounder (Paralichthys olivaceus) in Jeju Island. J. Vet Sci. 2006, 7, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Nho, S.-W.; Shin, G.-W.; Park, S.-B.; Jang, H.-B.; Cha, I.-S.; Ha, M.-A.; Kim, Y.-R.; Park, Y.-K.; Dalvi, R.S.; Kang, B.-J.; et al. Phenotypic characteristics of Streptococcus iniae and Streptococcus parauberis isolated from olive flounder (Paralichthys olivaceus). FEMS Microbiol. Lett. 2009, 293, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Baiano, J.C.; Barnes, A.C. Towards control of Streptococcus iniae. Emerg. Infect. Dis. 2009, 15, 1891–1896. [Google Scholar] [CrossRef]
- Zlotkin, A.; Chilmonczyk, S.; Eyngor, M.; Hurvitz, A.; Ghittino, C.; Eldar, A. Trojan horse effect: Phagocyte-mediated Streptococcus iniae infection of fish. Infect. Immunity 2003, 71, 2318–2325. [Google Scholar] [CrossRef]
- Locke, J.B.; Aziz, R.K.; Vicknair, M.R.; Nizet, V.; Buchanan, J.T. Streptococcus iniae M-like protein contributes to virulence in fish and is a target for live attenuated vaccine development. PLoS ONE 2008, 3, e2824. [Google Scholar] [CrossRef]
- Buchanan, J.T.; Stannard, J.A.; Lauth, X.; Ostland, V.E.; Powell, H.C.; Westerman, M.E.; Nizet, V. Streptococcus iniae phosphoglucomutase is a virulence factor and a target for vaccine development. Infect. Immun. 2005, 73, 6935–6944. [Google Scholar] [CrossRef]
- Locke, J.B.; Colvin, K.M.; Varki, N.; Vicknair, M.R.; Nizet, V.; Buchanan, J.T. Streptococcus iniae beta-hemolysin streptolysin S is a virulence factor in fish infection. Dis. Aquat. Org. 2007, 76, 17–26. [Google Scholar] [CrossRef]
- Milani, C.J.E.; Aziz, R.K.; Locke, J.B.; Dahesh, S.; Nizet, V.; Buchanan, J.T. The novel polysaccharide deacetylase homologue Pdi contributes to virulence of the aquatic pathogen Streptococcus iniae. Microbiology 2010, 156, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Eyngor, M.; Lublin, A.; Shapira, R.; Hurvitz, A.; Zlotkin, A.; Tekoah, Y.; Eldar, A. A pivotal role for the Streptococcus iniae extracellular polysaccharide in triggering proinflammatory cytokines transcription and inducing death in rainbow trout. FEMS Microbiol. Lett. 2010, 305, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.G.; Otto, G.; Colby, L.A. Selected Zoonoses. Lab. Animal Med. 2015, 1313–1370. [Google Scholar] [CrossRef]
- Muhammad, M.; Zhang, T.; Gong, S.; Bai, J.; Ju, J.; Zhao, B.; Liu, D. Streptococcus iniae: A growing threat and causative agent of disease outbreak in farmed Chinese sturgeon (Acipenser sinensis). Pak. J. Zool. 2020, 52, 1931–1939. [Google Scholar] [CrossRef]
- Soto, E.; Richey, C.; Stevens, B.; Yun, S.; Kenelty, K.; Reichley, S.; Griffin, M.; Kurobe, T.; Camus, A. Co-infection of Acipenserid herpesvirus 2 (AciHV-2) and Streptococcus iniae in cultured white sturgeon Acipenser transmontanus. Dis. Aquat. Org. 2017, 124, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Pierezan, F.; Shahin, K.; Heckman, T.I.; Ang, J.; Byrne, B.A.; Soto, E. Outbreaks of severe myositis in cultured white sturgeon (Acipenser transmontanus L.) associated with Streptococcus iniae. J. Fish Dis. 2020, 43, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, L.; Wang, J.; Wang, S.; Cao, H. Isolation, identification and drug sensitivity of Streptococcus iniae from hybrid sturgeons (Huso dauricus female x Acipenser schrencki male). Acta Microbiol. Sin. 2014, 54, 442–448. [Google Scholar]
- Mugetti, D.; Colussi, S.; Pastorino, P.; Varello, K.; Tomasoni, M.; Menconi, V.; Pedron, C.; Bozzetta, E.; Acutis, P.L.; Prearo, M. Episode of mortality associated with isolation of Streptococcus iniae in Adriatic sturgeon (Acipenser naccarii Bonaparte, 1836) reared in Northern Italy. J. Fish Dis. 2022. [Google Scholar] [CrossRef]
- Santi, M.; Pastorino, P.; Foglini, C.; Righetti, M.; Pedron, C.; Prearo, M. A survey of bacterial infections in sturgeon farming in Italy. J. Appl. Ichthyol. 2018, 35, 275–282. [Google Scholar] [CrossRef]
- Jensen, S.; Bergh, O.; Enger, O.; Hjeltnes, B. Use of PCR-RFLP for genotyping 16S rRNA and characterizing bacteria cultured from halibut fry. Can. J. Microbiol. 2002, 48, 379–386. [Google Scholar] [CrossRef]
- Mata, A.I.; Blanco, M.M.; Domínguez, L.; Fernández-Garayzábal, J.F.; Gibello, A. Development of a PCR assay for Streptococcus iniae based on the lactate oxidase (lctO) gene with potential diagnostic value. Vet. Microbiol. 2004, 101, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Bigarré, L.; Lesne, M.; Lautraite, A.; Chesneau, V.; Leroux, A.; Jamin, M.; Boitard, P.M.; Toffan, A.; Prearo, M.; Labrut, S.; et al. Molecular identification of iridoviruses infecting various sturgeon species in Europe. J. Fish Dis. 2017, 40, 105–118. [Google Scholar] [CrossRef] [PubMed]
- CLSI Document VET03-A; Methods for Antimicrobial Disk Susceptibility Testing of Bacteria Isolated from Aquatic Animals. Approved Quidelines; Clinical Laboratory Standards Institute: Wayne, PA, USA, 2006.
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing of Bacteria Isolated from Aquatic Animals; Second Informational Supplement; Clinical Laboratory Standards Institute: Wayne, PA, USA, 2014. [Google Scholar]
- Pastorino, P.; Colussi, S.; Pizzul, E.; Varello, K.; Menconi, V.; Mugetti, D.; Tomasoni, M.; Esposito, G.; Bertoli, M.; Bozzetta, E.; et al. The unusual isolation of carnobacteria in eyes of healthy salmonids in high-mountain lakes. Sci. Rep. 2021, 11, 2314. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Tamura, K.; Nei, M.; Kumar, S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc. Natl. Acad. Sci. USA 2004, 101, 11030–11035. [Google Scholar] [CrossRef]
- Liu, J.; Yang, F.; Peng, S.; Geng, Y.; Huang, X.; Ouyang, P.; Li, Z.; Chen, D. Molecular characterization and pathogenicity of Streptococcus iniae in yellow catfish (Pelteobagrus fulvidraco). Aquac. Res. 2020, 51, 5259–5264. [Google Scholar] [CrossRef]
- Nawawi, R.A.; Baiano, J.C.; Kvennefors, E.C.; Barnes, A.C. Host-directed evolution of a novel lactate oxidase in Streptococcus iniae isolates from barramundi (Lates calcarifer). Appl. Environ. Microbiol. 2009, 75, 2908–2919. [Google Scholar] [CrossRef]
- Feng, Y.; Bai, M.; Geng, Y.; Chen, D.; Huang, X.; Ouyang, P.; Zuo, Z.; Huang, C.; Lai, W. The potential risk of antibiotic resistance of Streptococcus iniae in sturgeon cultivation in Sichuan, China. Environ. Sci. Pollut. Res. 2021, 28, 69171–69180. [Google Scholar] [CrossRef]
- Evans, J.J.; Klesius, P.H.; Pasnik, D.J.; Bohnsack, J.F. Human Streptococcus agalactiae isolate in Nile tilapia (Oreochromis niloticus). Emerg. Infect. Dis. 2009, 15, 774–776. [Google Scholar] [CrossRef]
- Ludwig, A. A sturgeon view on conservation genetics. Eur. J. Wildl. Res. 2006, 52, 3–8. [Google Scholar] [CrossRef]

| ID | Total Length (cm) | Weight (g) |
|---|---|---|
| 78697.1 | 94.5 | 4100 |
| 78697.2 | 81.5 | 2800 |
| 78697.3 | 96.2 | 3700 |
| 78697.4 | 98.5 | 3800 |
| ID | Liver | Spleen | Gut |
|---|---|---|---|
| 78697.1 | Severe inflammation | Severe inflammation | Severe inflammation |
| 78697.2 | Moderate inflammation | Mild inflammation/severe congestion | Mild inflammation |
| 78697.3 | Mild inflammation/severe congestion | Moderate inflammation | No lesion |
| 78697.4 | Moderate inflammation | No lesion | No lesion |
| scpI (822 bp) | simA (994 bp) | pdi (381 bp) | sagA (190 bp) | cpsD (534 bp) | pgm (713 bp) | cfi (328 bp) | |
|---|---|---|---|---|---|---|---|
| 64844 | − | + | + | + | + | + | + |
| 78697 | − | + | + | + | − | + | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colussi, S.; Pastorino, P.; Mugetti, D.; Antuofermo, E.; Sciuto, S.; Esposito, G.; Polinas, M.; Tomasoni, M.; Burrai, G.P.; Fernández-Garayzábal, J.F.; et al. Isolation and Genetic Characterization of Streptococcus iniae Virulence Factors in Adriatic Sturgeon (Acipenser naccarii). Microorganisms 2022, 10, 883. https://doi.org/10.3390/microorganisms10050883
Colussi S, Pastorino P, Mugetti D, Antuofermo E, Sciuto S, Esposito G, Polinas M, Tomasoni M, Burrai GP, Fernández-Garayzábal JF, et al. Isolation and Genetic Characterization of Streptococcus iniae Virulence Factors in Adriatic Sturgeon (Acipenser naccarii). Microorganisms. 2022; 10(5):883. https://doi.org/10.3390/microorganisms10050883
Chicago/Turabian StyleColussi, Silvia, Paolo Pastorino, Davide Mugetti, Elisabetta Antuofermo, Simona Sciuto, Giuseppe Esposito, Marta Polinas, Mattia Tomasoni, Giovanni Pietro Burrai, José Francisco Fernández-Garayzábal, and et al. 2022. "Isolation and Genetic Characterization of Streptococcus iniae Virulence Factors in Adriatic Sturgeon (Acipenser naccarii)" Microorganisms 10, no. 5: 883. https://doi.org/10.3390/microorganisms10050883
APA StyleColussi, S., Pastorino, P., Mugetti, D., Antuofermo, E., Sciuto, S., Esposito, G., Polinas, M., Tomasoni, M., Burrai, G. P., Fernández-Garayzábal, J. F., Acutis, P. L., Pedron, C., & Prearo, M. (2022). Isolation and Genetic Characterization of Streptococcus iniae Virulence Factors in Adriatic Sturgeon (Acipenser naccarii). Microorganisms, 10(5), 883. https://doi.org/10.3390/microorganisms10050883












