Corrosion of an AZ31B Magnesium Alloy by Sulfate-Reducing Prokaryotes in a Mudflat Environment
Abstract
:1. Introduction
2. Materials and Methods
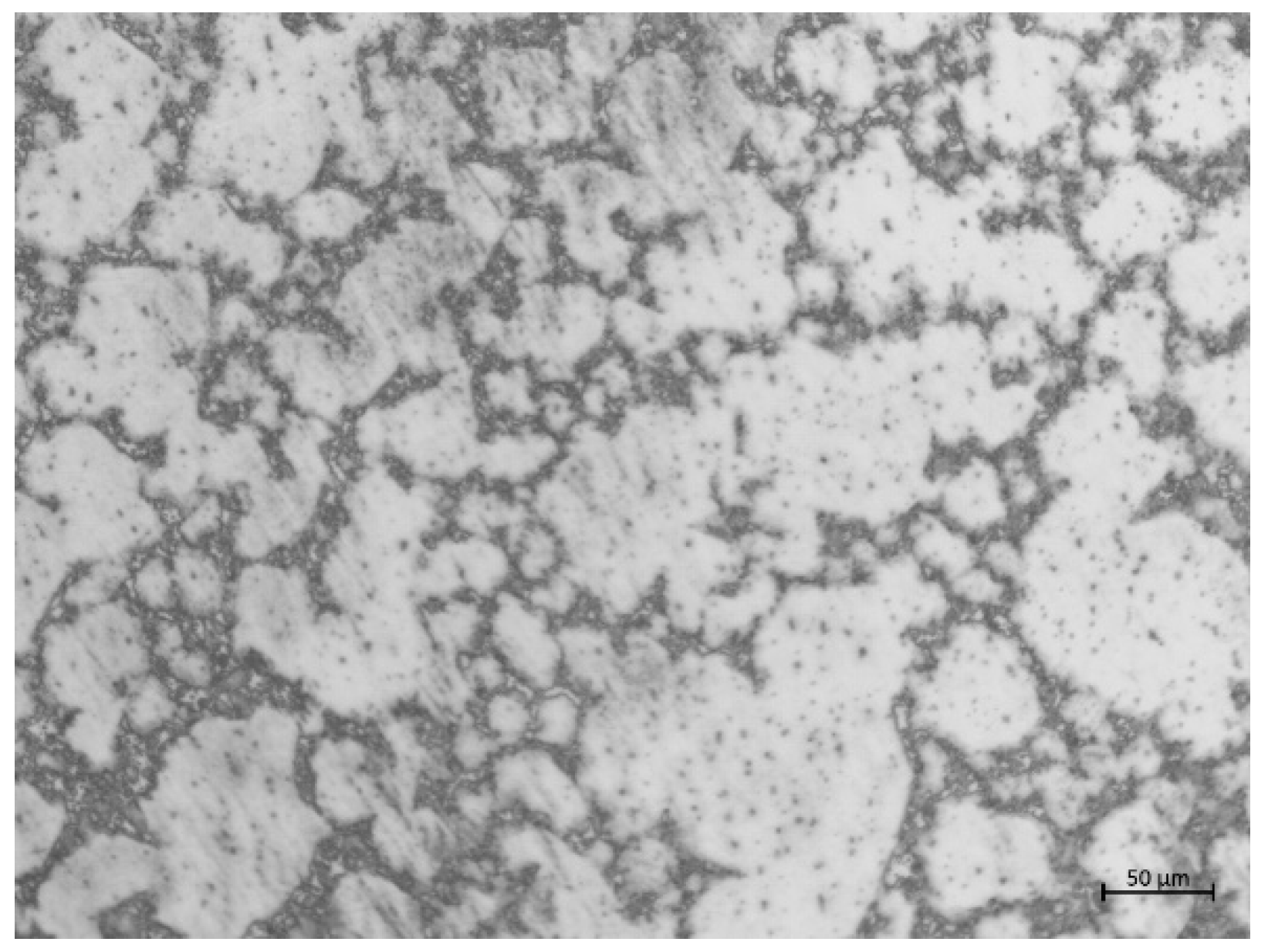
2.1. Materials and Specimens
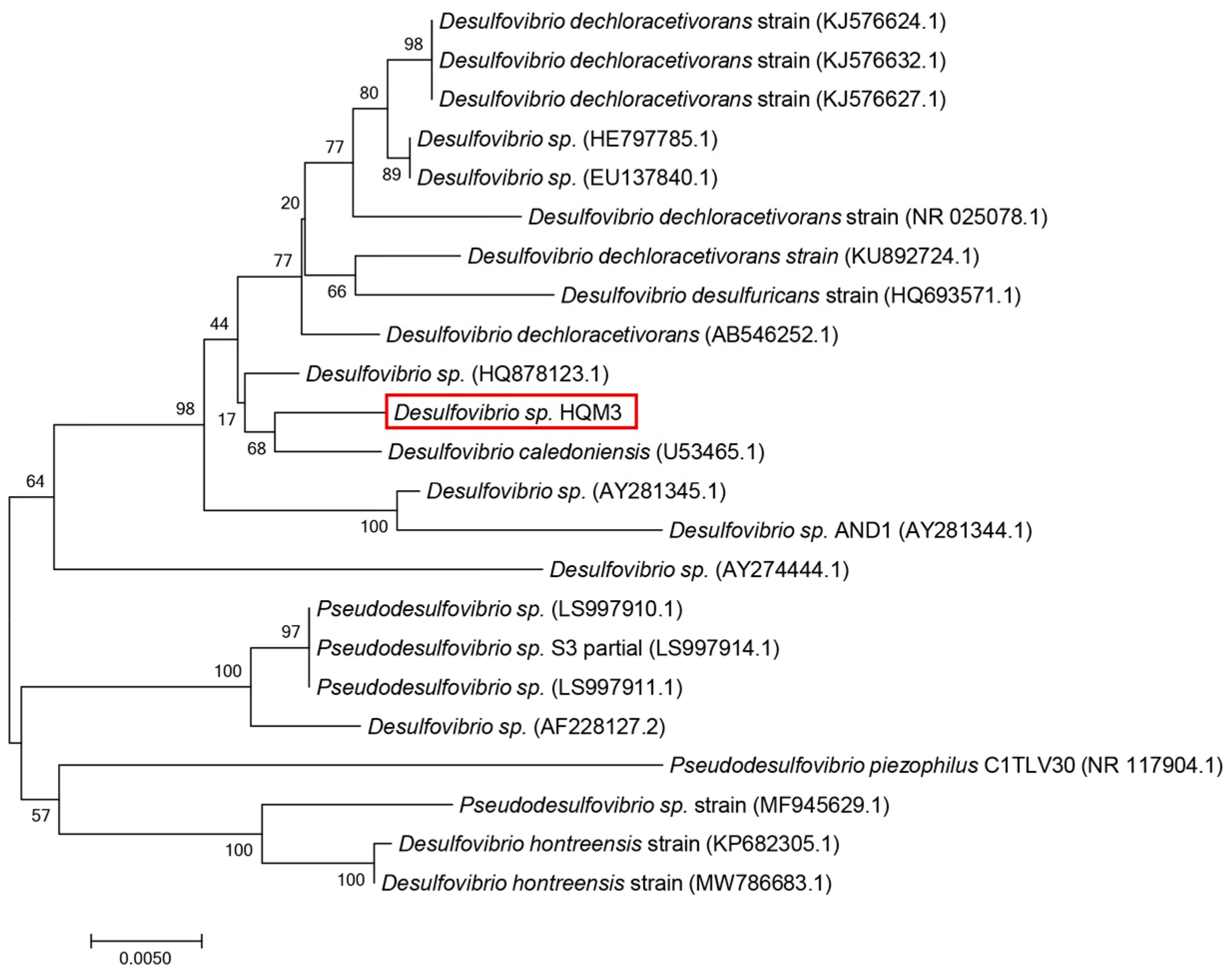
2.2. Bacterial Cultivation
2.3. Corrosion Rate by Weight Loss
2.4. Electrochemical Measurements
2.5. Surface Analysis and Corrosion Product Analysis
3. Results and Discussion
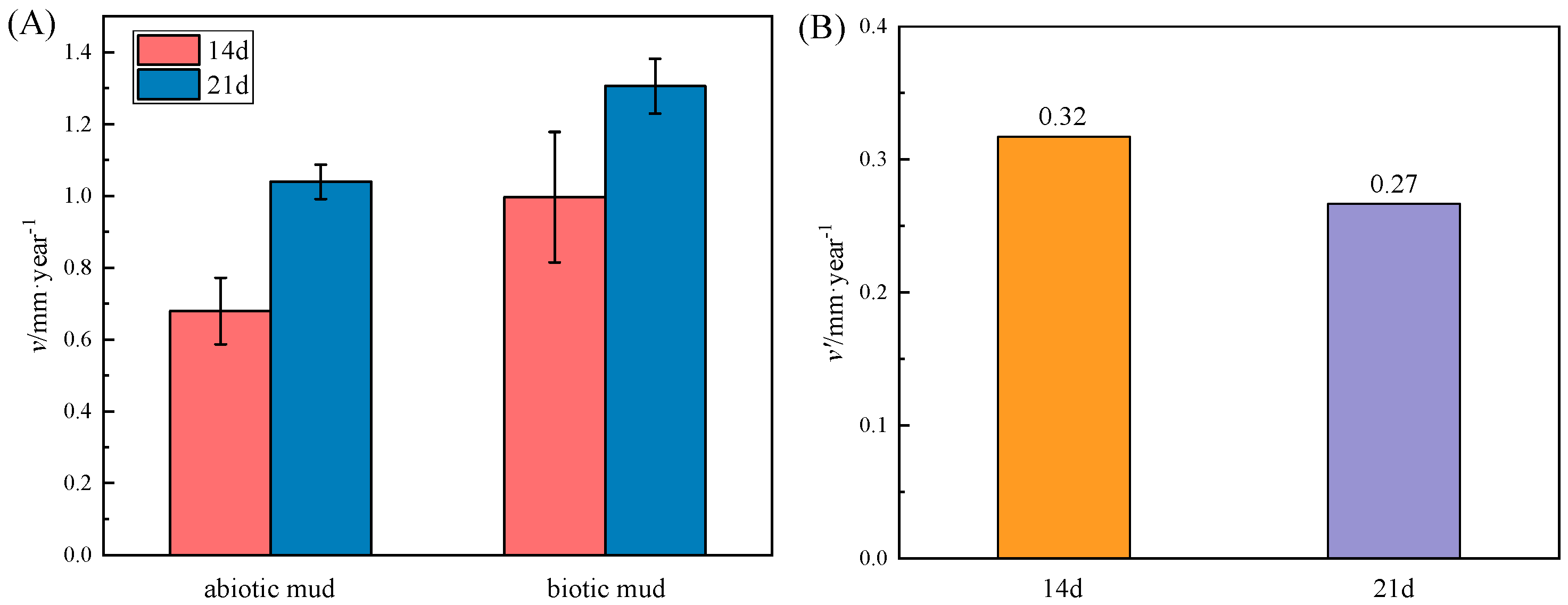
3.1. Corrosion Rate of AZ31B Determined by Weight–Loss Measurement
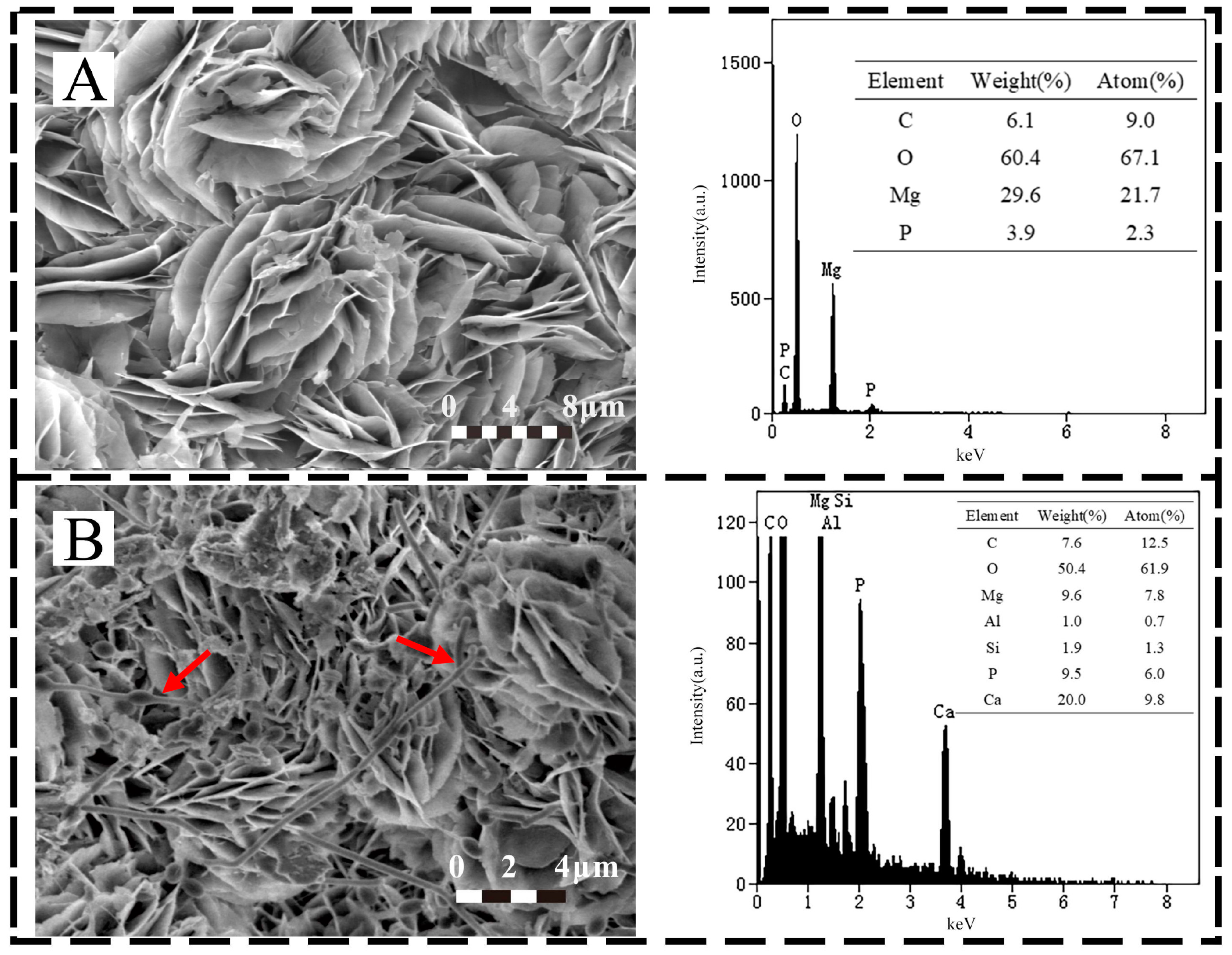
3.2. Characterization of Corrosion Products on Specimen Surfaces
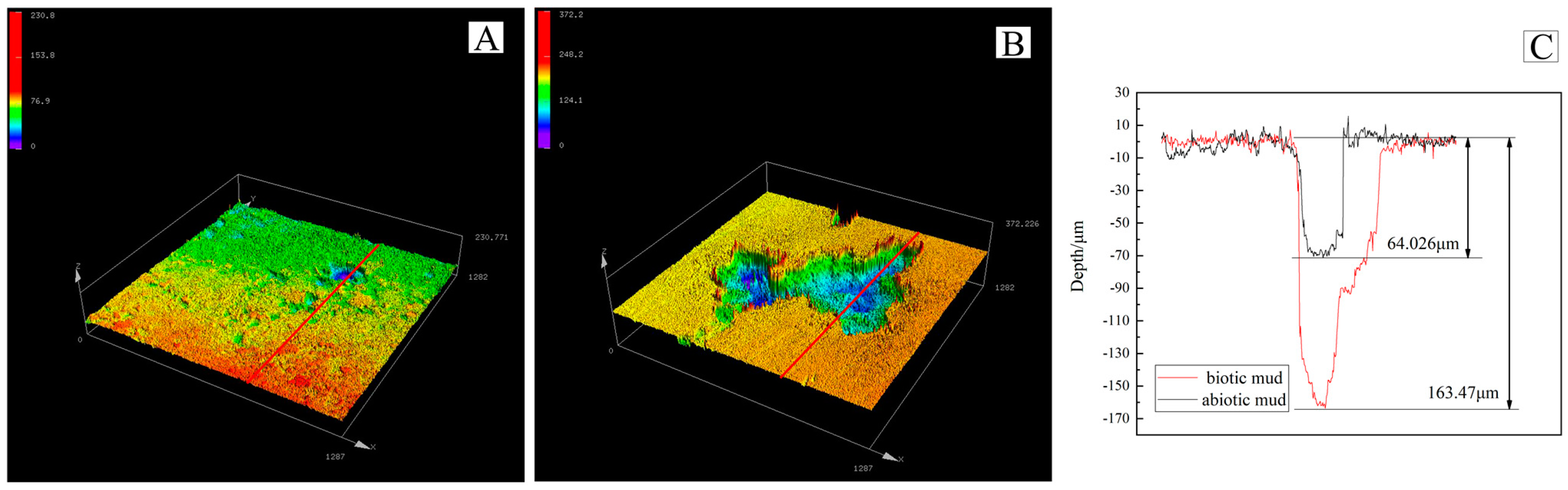
3.3. Pitting Morphology
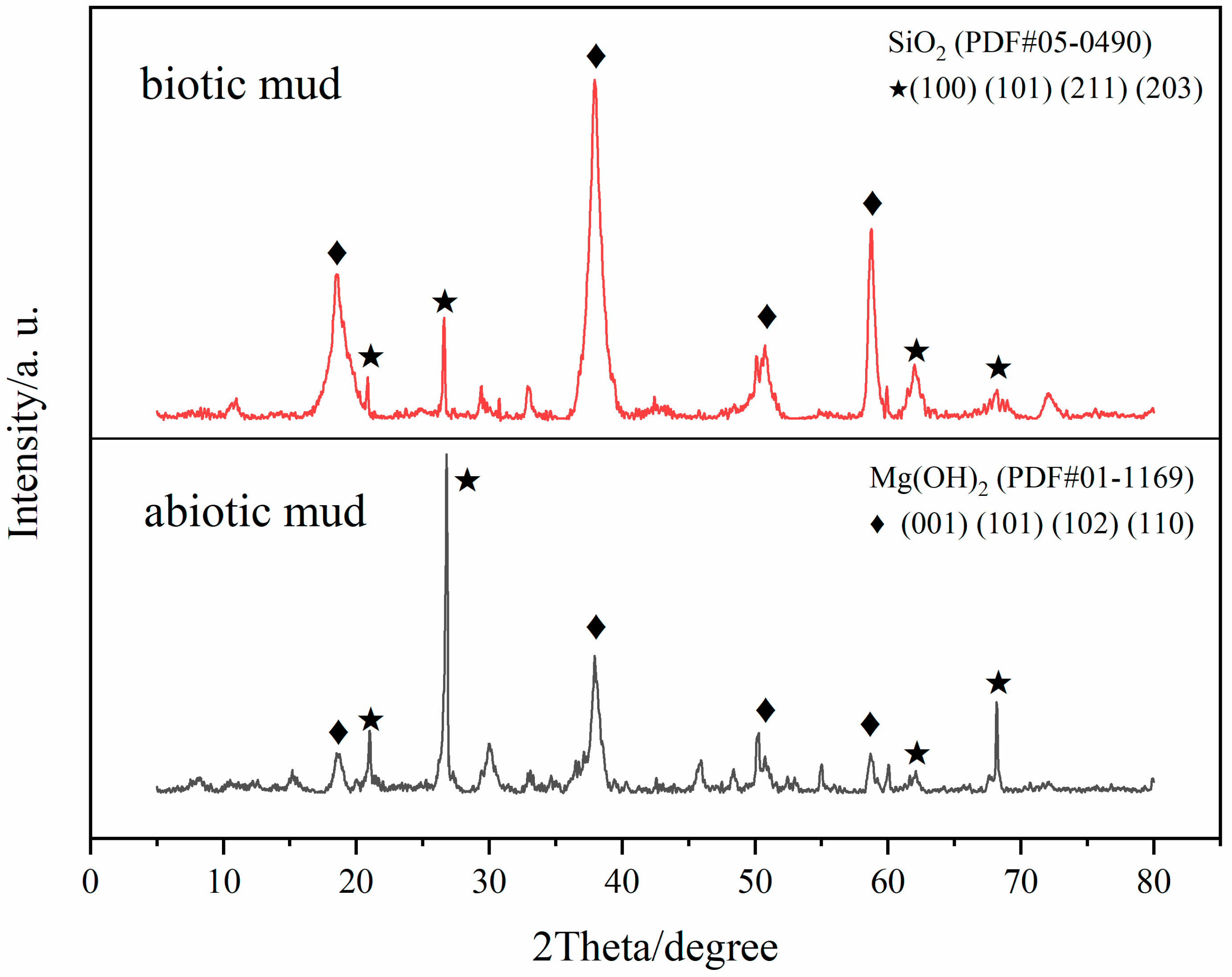
3.4. Corrosion Product Analysis
3.5. OCP Measurements
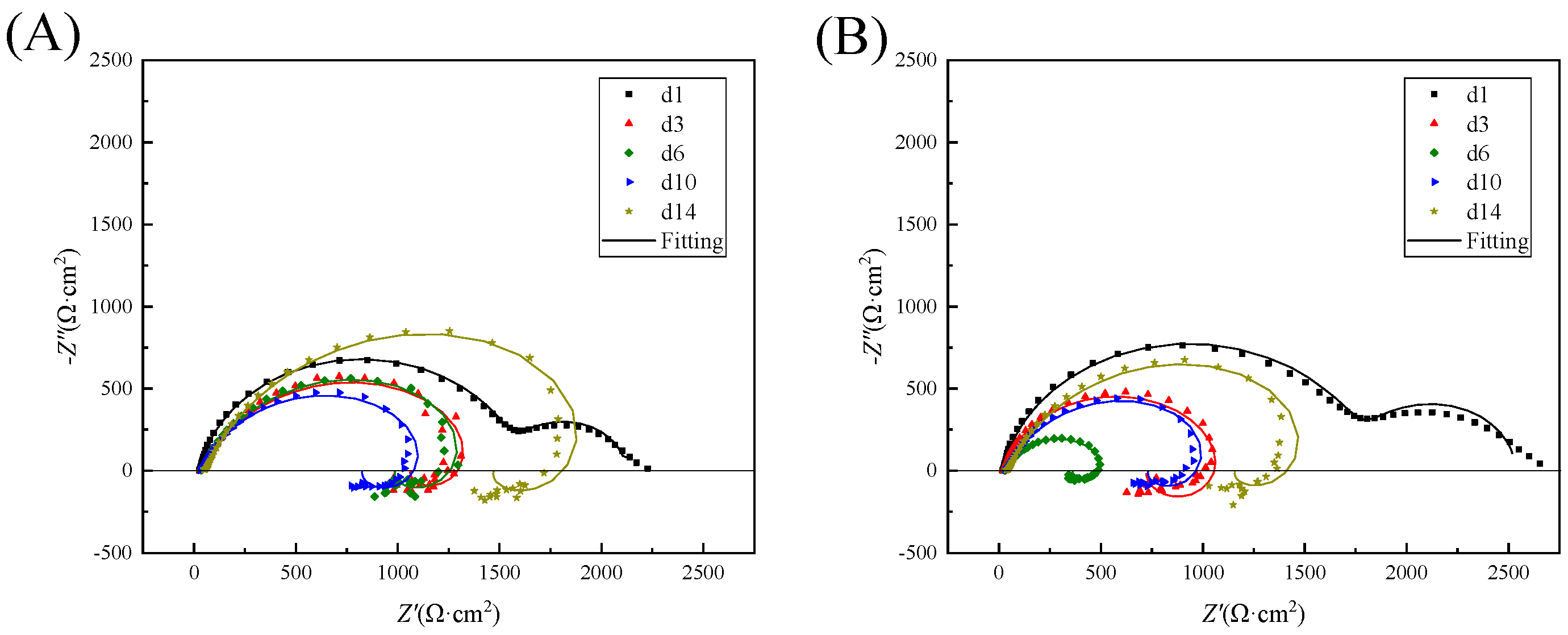
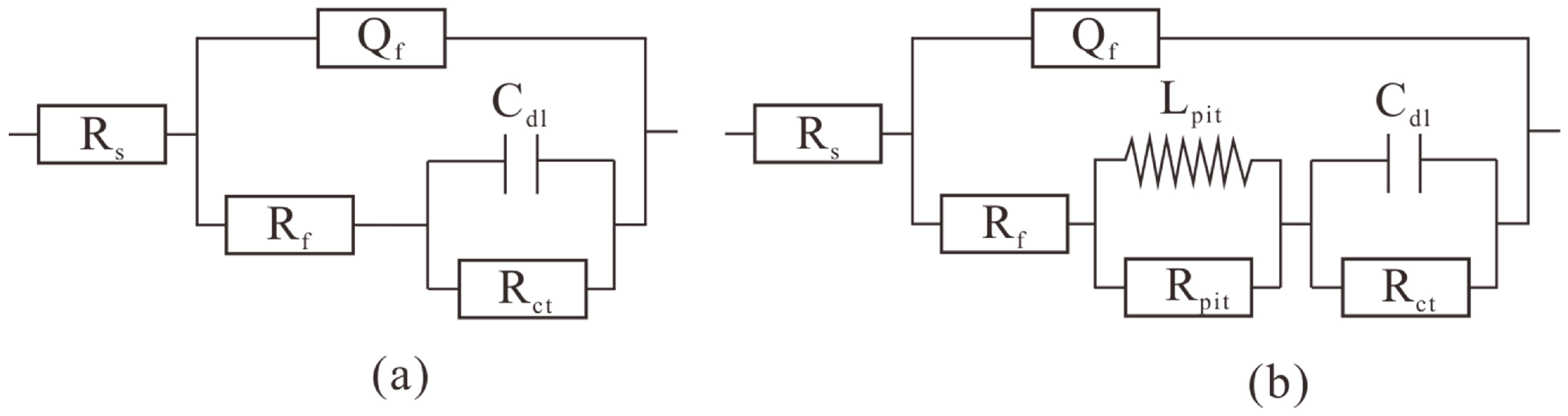
3.6. EIS Results
3.7. Discussion of Mechanism
4. Conclusions
- The corrosion of the magnesium alloy in the biotic mudflat environment is severe with a corrosion rate of 1.31 mm/year, SRP contribute around 0.3 mm/year. The pitting depth reached 163.47 μm with Mg(OH)2 as the main component of the corrosion products.
- Weight loss and electrochemical tests have shown that SRP in mudflat environment have a catalytic effect on the corrosion with uniform corrosion occurring first followed by localized corrosion such as severe pitting.
- SRP accelerate the corrosion of magnesium sacrificial anodes and are one of the causes of the abnormal failure.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karstens, S.; Buczko, U.; Jurasinski, G.; Peticzka, R.; Glatzel, S. Impact of Adjacent Land Use on Coastal Wetland Sediments. Sci. Total Environ. 2016, 550, 337–348. [Google Scholar] [CrossRef]
- Andreu, V.; Gimeno-Garcia, E.; Pascual, J.A.; Vazquez-Roig, P.; Pico, Y. Presence of Pharmaceuticals and Heavy Metals in the Waters of a Mediterranean Coastal Wetland: Potential Interactions and the Influence of the Environment. Sci. Total Environ. 2016, 540, 278–286. [Google Scholar] [CrossRef]
- Li, Y.T. Corrosion Behaviour of Steel in Beach Soil along Bohai Bay. Corros. Eng. Sci. Technol. 2009, 44, 91–95. [Google Scholar] [CrossRef]
- Yang, Y.; Wikieł, A.J.; Dall’Agnol, L.T.; Eloy, P.; Genet, M.J.; Moura, J.J.; Sand, W.; Dupont-Gillain, C.C.; Rouxhet, P.G. Proteins Dominate in the Surface Layers Formed on Materials Exposed to Extracellular Polymeric Substances from Bacterial Cultures. Biofouling 2016, 32, 95–108. [Google Scholar] [CrossRef]
- Wikieł, A.J.; Datsenko, I.; Vera, M.; Sand, W. Impact of Desulfovibrio alaskensis Biofilms on Corrosion Behaviour of Carbon Steel in Marine Environment. Bioelectrochemistry 2014, 97, 52–60. [Google Scholar] [CrossRef]
- Fathy, M.; Badawi, A.; Mazrouaa, A.M.; Mansour, N.A.; Ghazy, E.A.; Elsabee, M.Z. Styrene N-Vinylpyrrolidone Metal-Nanocomposites as Antibacterial Coatings against Sulfate Reducing Bacteria. Mater. Sci. Eng. C 2013, 33, 4063–4070. [Google Scholar] [CrossRef]
- Beck, M.; Köster, J.; Engelen, B.; Holstein, J.M.; Gittel, A.; Könneke, M.; Riedel, T.; Wirtz, K.; Cypionka, H.; Rullkötter, J. Deep Pore Water Profiles Reflect Enhanced Microbial Activity towards Tidal Flat Margins. Ocean. Dyn. 2009, 59, 371–383. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Z.B.; Qiao, Y.X.; Zheng, Y.G. Synergistic Effects of Deposits and Sulfate Reducing Bacteria on the Corrosion of Carbon Steel. Corros. Sci. 2022, 199, 110210. [Google Scholar] [CrossRef]
- Umoru, L.E.; Ige, O.O. Effects of Tin on Aluminum-Zinc-Magnesium Alloy as Sacrificial Anode in Seawater. J. Miner. Mater. Charact. Eng. 2007, 7, 105–113. [Google Scholar] [CrossRef]
- Pathak, S.S.; Mendon, S.K.; Blanton, M.D.; Rawlins, J.W. Magnesium-Based Sacrificial Anode Cathodic Protection Coatings (Mg-Rich Primers) for Aluminum Alloys. Metals 2012, 2, 353–376. [Google Scholar] [CrossRef]
- Kim, J.-G.; Kim, Y.-W. Advanced Mg-Mn-Ca Sacrificial Anode Materials for Cathodic Protection. Mater. Corros. 2001, 52, 137–139. [Google Scholar] [CrossRef]
- Gao, J.; Du, Y.; Tang, D.; Li, X.; Yan, D. Study on the Performance of Magnesium Sacrificial Anode. In Proceedings of the CORROSION 2015, Dallas, TX, USA, 15–19 March 2015. NACE-5647. [Google Scholar]
- Alotaibi, M.M.; Al-Shahrani, A.; Alfantazi, A. Failure Of Magnesium Anodes In Alkaline Solution. In Proceedings of the CORROSION 2011, Houston, TX, USA, 13–17 March 2011. NACE-11324. [Google Scholar]
- Hu, X.W.; Xu, C.W.; Li, J.; Wang, K. Problems in Electrochemical Anticorrosion Protection of Grounded Screen. Power Syst. Technol. 2002, 35, 75–79. [Google Scholar]
- Huang, Y.; Cao, C.; Lu, M.; Lin, H. Inhibition Effects of I- and I2 on Stress-Corrosion Cracking of Stainless-Steel in Acidic Chloride Solutions. Corrosion 1993, 49, 644–649. [Google Scholar] [CrossRef]
- Curioni, M. The Behaviour of Magnesium during Free Corrosion and Potentiodynamic Polarization Investigated by Real-Time Hydrogen Measurement and Optical Imaging. Electrochim. Acta 2014, 120, 284–292. [Google Scholar] [CrossRef] [Green Version]
- Song, G.; Cao, C. On the Linear Response of a Passivated Metallic Electrode to Potential Step Perturbation. Corros. Sci. 1992, 33, 413–423. [Google Scholar] [CrossRef]
- Li, X.; Duan, J.; Xiao, H.; Li, Y.; Liu, H.; Guan, F.; Zhai, X. Analysis of Bacterial Community Composition of Corroded Steel Immersed in Sanya and Xiamen Seawaters in China via Method of Illumina MiSeq Sequencing. Front. Microbiol. 2017, 8, 1737. [Google Scholar] [CrossRef]
- Starosvetsky, J.; Starosvetsky, D.; Armon, R. Identification of Microbiologically Influenced Corrosion (MIC) in Industrial Equipment Failures. Eng. Fail. Anal. 2007, 14, 1500–1511. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Q.; Song, Y.; Zhang, D.; Yu, S.; Zhu, X. A Study on the Corrosion Behavior of Ce-Modified Cast AZ91 Magnesium Alloy in the Presence of Sulfate-Reducing Bacteria. J. Alloys Compd. 2009, 473, 550–556. [Google Scholar] [CrossRef]
- Zhou, W.; Shen, T.; Aung, N.N. Effect of Heat Treatment on Corrosion Behaviour of Magnesium Alloy AZ91D in Simulated Body Fluid. Corros. Sci. 2010, 52, 1035–1041. [Google Scholar] [CrossRef]
- Feng, H.; Wang, G.; Jin, W.; Zhang, X.; Huang, Y.; Gao, A.; Wu, H.; Wu, G.; Chu, P.K. Systematic Study of Inherent Antibacterial Properties of Magnesium-Based Biomaterials. ACS Appl. Mater. Interfaces 2016, 8, 9662–9673. [Google Scholar] [CrossRef]
- Li, Y.; Liu, G.; Zhai, Z.; Liu, L.; Li, H.; Yang, K.; Tan, L.; Wan, P.; Liu, X.; Ouyang, Z.; et al. Antibacterial Properties of Magnesium In Vitro and in an In Vivo Model of Implant-Associated Methicillin-Resistant Staphylococcus aureus Infection. Antimicrob. Agents Chemother. 2014, 58, 7586–7591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, Y.; Okita, K.; Kudo, D.; Phuong, D.N.D.; Iwamoto, Y.; Yoshioka, Y.; Ariyoshi, W.; Yamasaki, R. Magnesium Hydroxide Nanoparticles Kill Exponentially Growing and Persister Escherichia coli Cells by Causing Physical Damage. Nanomaterials 2021, 11, 1584. [Google Scholar] [CrossRef] [PubMed]
- Lv, K.; Yang, X.; Wu, T.; Xu, S.; Liu, L.; Sun, L.; Wang, X. Study on the Corrosion Behaviors of High-Silicon Chromium Iron in Acidic and Alkaline Soil Solutions. Anti-Corros. Methods Mater. 2021, 68, 182–191. [Google Scholar] [CrossRef]
- Wasim, M.; Mahmoodian, M.; Robert, D.; Li, C.-Q. Correlation Model for the Corrosion Rates of Buried Cast Iron Pipes. J. Mater. Civ. Eng. 2020, 32, 04020353. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, Y.F. Microbial Corrosion of X52 Pipeline Steel under Soil with Varied Thicknesses Soaked with a Simulated Soil Solution Containing Sulfate-Reducing Bacteria and the Associated Galvanic Coupling Effect. Electrochim. Acta 2018, 266, 312–325. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, Y.F. Corrosion of X52 Pipeline Steel in a Simulated Soil Solution with Coexistence of Desulfovibrio desulfuricans and Pseudomonas aeruginosa Bacteria. Corros. Sci. 2020, 173, 108753. [Google Scholar] [CrossRef]
- Prescott, L.M. Microbiology, 5th ed.; The McGraw-Hill: New York, NY, USA, 2002; ISBN 0-07-282905-2. [Google Scholar]
- Yang, Y.; Li, Y.; Liang, Z.; Bai, P.; Nie, J.; Liu, S.; Chen, B.; Wei, S.; Guan, Q.; Cai, J. Continuous Hot Corrosion Behaviour of an FeCrAlSi Coating Prepared by Laser Cladding. Surf. Coat. Technol. 2021, 421, 127424. [Google Scholar] [CrossRef]
- Yang, L.; Lin, C.; Gao, H.; Xu, W.; Li, Y.; Hou, B.; Huang, Y. Corrosion Behaviour of AZ63 Magnesium Alloy in Natural Seawater and 3.5 Wt.% NaCl Aqueous Solution. Int. J. Electrochem. Sci. 2018, 13, 8084–8093. [Google Scholar] [CrossRef]
- Chen, S.; Wang, P.; Zhang, D. Corrosion Behavior of Copper under Biofilm of Sulfate-Reducing Bacteria. Corros. Sci. 2014, 87, 407–415. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, Y.; Wang, Q.; Liu, J. Influence of Sulfate-Reducing Bacteria on the Corrosion Residual Strength of an AZ91D Magnesium Alloy. Materials 2014, 7, 7118–7129. [Google Scholar] [CrossRef] [Green Version]
- Dubey, D.; Kadali, K.; Kancharla, H.; Zindal, A.; Jain, J.; Mondal, K.; Singh, S.S. Effect of Precipitate Characteristics on the Corrosion Behavior of a AZ80 Magnesium Alloy. Met. Mater. Int. 2021, 27, 3282–3292. [Google Scholar] [CrossRef]
- Jang, Y.; Collins, B.; Sankar, J.; Yun, Y. Effect of Biologically Relevant Ions on the Corrosion Products Formed on Alloy AZ31B: An Improved Understanding of Magnesium Corrosion. Acta Biomater. 2013, 9, 8761–8770. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Unsal, T.; Kumseranee, S.; Punpruk, S.; Mohamed, M.E.; Saleh, M.A.; Gu, T. Sulfate Reducing Bacterium Desulfovibrio vulgaris Caused Severe Microbiologically Influenced Corrosion of Zinc and Galvanized Steel. Int. Biodeterior. Biodegrad. 2021, 157, 105160. [Google Scholar] [CrossRef]
- Unsal, T.; Wang, D.; Kumseranee, S.; Punpruk, S.; Mohamed, M.E.-S.; Saleh, M.A.; Gu, T. Assessment of 2,2-Dibromo-3-Nitrilopropionamide Biocide Enhanced by D-Tyrosine against Zinc Corrosion by a Sulfate Reducing Bacterium. Ind. Eng. Chem. Res. 2021, 60, 4009–4018. [Google Scholar] [CrossRef]
- Ma, L.; Li, W.; Zhu, S.; Wang, L.; Guan, S. Corrosion Inhibition of Schiff Bases for Mg-Zn-Y-Nd Alloy in Normal Saline: Experimental and Theoretical Investigations. Corros. Sci. 2021, 184, 109268. [Google Scholar] [CrossRef]
- Little, B.J.; Hinks, J.; Blackwood, D.J. Microbially Influenced Corrosion: Towards an Interdisciplinary Perspective on Mechanisms. Int. Biodeterior. Biodegrad. 2020, 154, 105062. [Google Scholar] [CrossRef]
- AlAbbas, F.M.; Bhola, R.; Spear, J.R.; Olson, D.L.; Mishra, B. Electrochemical Characterization of Microbiologically Influenced Corrosion on Linepipe Steel Exposed to Facultative Anaerobic Desulfovibrio sp. Int. J. Electrochem. Sci. 2013, 8, 859–871. [Google Scholar]
- Shi, Z.; Cao, F.; Song, G.-L.; Liu, M.; Atrens, A. Corrosion Behaviour in Salt Spray and in 3.5% NaCl Solution Saturated with Mg(OH)2 of as-Cast and Solution Heat-Treated Binary Mg–RE Alloys: RE = Ce, La, Nd, Y, Gd. Corros. Sci. 2013, 76, 98–118. [Google Scholar] [CrossRef]
- Liu, T.; Cheng, Y.F. The Influence of Cathodic Protection Potential on the Biofilm Formation and Corrosion Behaviour of an X70 Steel Pipeline in Sulfate Reducing Bacteria Media. J. Alloys Compd. 2017, 729, 180–188. [Google Scholar] [CrossRef]
- Gao, X.; Wu, H.; Liu, M.; Zhang, Y.; Gao, F. Corrosion Behavior of High Strength C71500 Cu-Ni Alloy Pipe in Simulated High Sulfide Polluted Seawater at Different Temperatures. Int. J. Electrochem. Sci. 2021, 16, 150932. [Google Scholar] [CrossRef]
- Gopi, K.R.; Nayaka, H.S.; Sahu, S. Corrosion Behavior of ECAP-Processed AM90 Magnesium Alloy. Arab. J. Sci. Eng. 2018, 43, 4871–4878. [Google Scholar] [CrossRef]
- Qu, Q.; Li, S.; Li, L.; Zuo, L.; Ran, X.; Qu, Y.; Zhu, B. Adsorption and Corrosion Behaviour of Trichoderma harzianum for AZ31B Magnesium Alloy in Artificial Seawater. Corros. Sci. 2017, 118, 12–23. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, J.; Sun, C.; Yu, Z.; Hou, B. The Corrosion of Two Aluminium Sacrificial Anode Alloys in SRB-Containing Sea Mud. Corros. Sci. 2014, 83, 375–381. [Google Scholar] [CrossRef]
- Dong, X.; Sun, Q.; Zhou, Y.; Qu, Y.; Shi, H.; Zhang, B.; Xu, S.; Liu, W.; Li, N.; Yan, J. Influence of Microstructure on Corrosion Behavior of Biomedical Co-Cr-Mo-W Alloy Fabricated by Selective Laser Melting. Corros. Sci. 2020, 170, 108688. [Google Scholar] [CrossRef]
- Wei, B.; Xu, J.; Gao, L.; Feng, H.; Wu, J.; Sun, C.; Wang, Z.; Ke, W. Nanosecond Pulsed Laser-Assisted Modified Copper Surface Structure: Enhanced Surface Microhardness and Microbial Corrosion Resistance. J. Mater. Sci. Technol. 2022, 107, 111–123. [Google Scholar] [CrossRef]
- Persson, D.; Thierry, D.; Karlsson, O. Corrosion and Corrosion Products of Hot Dipped Galvanized Steel during Long Term Atmospheric Exposure at Different Sites World-Wide. Corros. Sci. 2017, 126, 152–165. [Google Scholar] [CrossRef]
- Song, G.-L. Corrosion of Magnesium Alloys; Woodhead: New Delhi, India, 2011. [Google Scholar]
- Atrens, A.; Song, G.-L.; Liu, M.; Shi, Z.; Cao, F.; Dargusch, M.S. Review of Recent Developments in the Field of Magnesium Corrosion. Adv. Eng. Mater. 2015, 17, 400–453. [Google Scholar] [CrossRef]
- Gu, T.; Zhao, K.; Nesic, S. A New Mechanistic Model for MIC Based on a Biocatalytic Cathodic Sulfate Reduction Theory. In Proceedings of the Paper presented at the CORROSION 2009, Atlanta, GA, USA, 22–26 March 2009; NACE-09390. Available online: https://onepetro.org/NACECORR/proceedings-abstract/CORR09/All-CORR09/NACE-09390/128779 (accessed on 6 March 2022).
- Chen, X.; Wang, G.; Gao, F.; Wang, Y.; He, C. Effects of Sulphate-Reducing Bacteria on Crevice Corrosion in X70 Pipeline Steel under Disbonded Coatings. Corros. Sci. 2015, 101, 1–11. [Google Scholar] [CrossRef]
- Cord-Ruwisch, R.; Widdel, F. Corroding Iron as a Hydrogen Source for Sulphate Reduction in Growing Cultures of Sulphate-Reducing Bacteria. Appl. Microbiol. Biotechnol. 1986, 25, 169–174. [Google Scholar] [CrossRef]
- von Wolzogen Kühr, C.A.H. De Grafiteering van Gietijzer Als Electrobiochemisch Proces in Anaerobe Gronden. Water 1934, 18, 147–165. [Google Scholar]
- Gu, T.; Jia, R.; Unsal, T.; Xu, D. Toward a Better Understanding of Microbiologically Influenced Corrosion Caused by Sulfate Reducing Bacteria. J. Mater. Sci. Technol. 2019, 35, 631–636. [Google Scholar] [CrossRef]
- Jia, R.; Unsal, T.; Xu, D.; Lekbach, Y.; Gu, T. Microbiologically Influenced Corrosion and Current Mitigation Strategies: A State of the Art Review. Int. Biodeterior. Biodegrad. 2019, 137, 42–58. [Google Scholar] [CrossRef]
- Videla, H.A. An Overview of Mechanisms by Which Sulphate-Reducing Bacteria Influence Corrosion of Steel in Marine Environments. Biofouling 2000, 15, 37–47. [Google Scholar] [CrossRef] [PubMed]


| Al | Si | Ca | Zn | Fe | Be | Mn | Cu | Mg |
|---|---|---|---|---|---|---|---|---|
| 3.19 | 0.02 | 0.04 | 0.81 | 0.005 | 0.1 | 0.334 | 0.05 | Bal |
| Time (Day) | Rs (Ω·cm2) | Qf (F/cm2) | n | Rf (Ω·cm2) | Lpit (H·cm2) | Rpit (Ω·cm2) | Cdl (F/cm2) | Rct (Ω·cm2) |
|---|---|---|---|---|---|---|---|---|
| 1 | 19.3 | 2.70 × 10−5 | 8.96 × 10−1 | 1.82 × 103 | \ | \ | 3.04 × 10−3 | 696 |
| 3 | 20.6 | 2.88 × 10−5 | 8.58 × 10−1 | 1.15 × 10−22 | 134 | 428 | 2.56 × 10−6 | 701 |
| 6 | 31.1 | 6.89 × 10−5 | 7.81 × 10−1 | 2.21 × 10−3 | 32.0 | 215 | 5.02 × 10−6 | 331 |
| 10 | 34.1 | 7.43 × 10−5 | 7.69 × 10−1 | 3.72 × 10−3 | 79.4 | 619 | 4.16 × 10−6 | 700 |
| 14 | 41.0 | 6.73 × 10−5 | 7.71 × 10−1 | 6.18 × 10−8 | 147 | 907 | 3.06 × 10−6 | 1120 |
| Time (Day) | Rs (Ω·cm2) | Qf (F/cm2) | n | Rf (Ω·cm2) | Lpit (H·cm2) | Rpit (Ω·cm2) | Cdl (F/cm2) | Rct (Ω·cm2) |
|---|---|---|---|---|---|---|---|---|
| 1 | 28.6 | 2.20 × 10−5 | 9.04 × 10−1 | 1.58 × 103 | \ | \ | 3.19 × 10−3 | 509 |
| 3 | 32.7 | 3.82 × 10−5 | 7.65 × 10−1 | 1.42 × 10−3 | 89.3 | 543 | 2.93 × 10−6 | 1030 |
| 6 | 38.5 | 5.18 × 10−5 | 7.82 × 10−1 | 2.49 × 10−3 | 98.2 | 700 | 2.44 × 10−6 | 951 |
| 10 | 33.7 | 5.11 × 10−5 | 7.88 × 10−1 | 1.48 × 10−3 | 90.7 | 520 | 3.63 × 10−6 | 793 |
| 14 | 52.7 | 5.57 × 10−5 | 7.81 ×10−1 | 1.00 × 10−2 | 212 | 1120 | 2.52 × 10−6 | 1420 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, X.; Zhang, J.; Wang, Z.; Zhang, R.; Sand, W.; Zhang, L.; Duan, J.; Zhu, Q.; Hou, B. Corrosion of an AZ31B Magnesium Alloy by Sulfate-Reducing Prokaryotes in a Mudflat Environment. Microorganisms 2022, 10, 839. https://doi.org/10.3390/microorganisms10050839
Lan X, Zhang J, Wang Z, Zhang R, Sand W, Zhang L, Duan J, Zhu Q, Hou B. Corrosion of an AZ31B Magnesium Alloy by Sulfate-Reducing Prokaryotes in a Mudflat Environment. Microorganisms. 2022; 10(5):839. https://doi.org/10.3390/microorganisms10050839
Chicago/Turabian StyleLan, Xiao, Jie Zhang, Zaifeng Wang, Ruiyong Zhang, Wolfgang Sand, Liang Zhang, Jizhou Duan, Qingjun Zhu, and Baorong Hou. 2022. "Corrosion of an AZ31B Magnesium Alloy by Sulfate-Reducing Prokaryotes in a Mudflat Environment" Microorganisms 10, no. 5: 839. https://doi.org/10.3390/microorganisms10050839
APA StyleLan, X., Zhang, J., Wang, Z., Zhang, R., Sand, W., Zhang, L., Duan, J., Zhu, Q., & Hou, B. (2022). Corrosion of an AZ31B Magnesium Alloy by Sulfate-Reducing Prokaryotes in a Mudflat Environment. Microorganisms, 10(5), 839. https://doi.org/10.3390/microorganisms10050839







