Recent Developments in Botulinum Neurotoxins Detection
Abstract
1. Introduction
2. Applications of BoNT Detection
2.1. Clinical Presentation of Botulism
2.2. Laboratory Confirmation
2.3. Food Industry: Survey of Food Safety
2.4. Pharmaceutical Industry Uses, Needs for Precise Quantification of Active BoNT
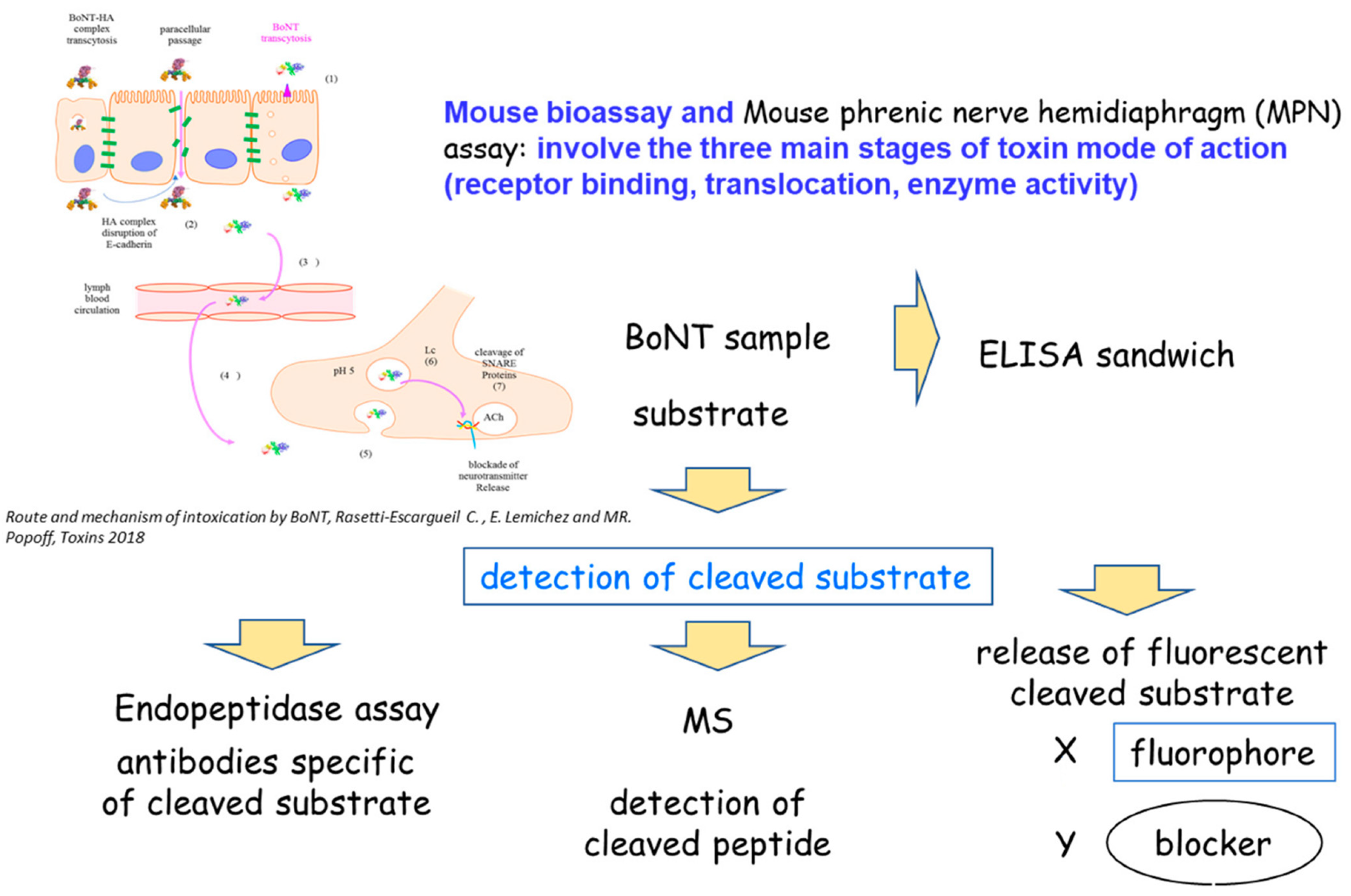
3. BoNT Detection
3.1. Physical Methods
3.1.1. Immunological Methods: Immunoassays (ELISA)
3.1.2. Mass Spectrometry Methods
3.2. Functional Methods
3.2.1. Biochemical Methods: Endopeptidase Assay
Detection of Cleavage Products by Mass Spectrometry (MS-Endopeptidase Assay)
Detection of Cleavage Products by Antibodies against Neoepitopes: Endopep-ELISA
Detection of Cleavage Products by Antibodies against Neoepitopes Using Immunosensors and Förster Resonance Energy Transfer (FRET) Assays
3.3. In Vivo and Ex Vivo Methods
3.4. Cell-Based Assays: Detection of Cleavage Products and Neurotransmitter Release Inhibition
3.5. Cell-Based Assay: Measurement of Electrical Conductance Using Multi-Electrodes Arrays
3.6. Cell-Based Assay and Bioluminescence
4. Discussion: Suitability of Each Method for Their Applications
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Peck, M.W.; Smith, T.J.; Anniballi, F.; Austin, J.W.; Bano, L.; Bradshaw, M.; Cuervo, P.; Cheng, L.W.; Derman, Y.; Dorner, B.G.; et al. Historical Perspectives and Guidelines for Botulinum Neurotoxin Subtype Nomenclature. Toxins 2017, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Masuyer, G.; Stenmark, P. Botulinum and Tetanus Neurotoxins. Annu. Rev. Biochem. 2019, 88, 811–837. [Google Scholar] [CrossRef] [PubMed]
- Pirazzini, M.; Rossetto, O.; Eleopra, R.; Montecucco, C. Botulinum Neurotoxins: Biology, Pharmacology, and Toxicology. Pharmacol. Rev. 2017, 69, 200–235. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, M.J.; Adams, J.B.; Doxey, A.C. Botulinum neurotoxin homologs in non-Clostridium species. FEBS Lett. 2015, 589, 342–348. [Google Scholar] [CrossRef]
- Zhang, S.; Masuyer, G.; Zhang, J.; Shen, Y.; Lundin, D.; Henriksson, L.; Miyashita, S.-I.; Martínez-Carranza, M.; Dong, M.; Stenmark, P. Identification and characterization of a novel botulinum neurotoxin. Nat. Commun. 2017, 8, 14130. [Google Scholar] [CrossRef]
- Zornetta, I.; Tehran, D.A.; Arrigoni, G.; Anniballi, F.; Bano, L.; Leka, O.; Zanotti, G.; Binz, T.; Montecucco, C. The first non Clostridial botulinum-like toxin cleaves VAMP within the juxtamembrane domain. Sci. Rep. 2016, 6, 30257. [Google Scholar] [CrossRef]
- Kosenina, S.; Masuyer, G.; Zhang, S.; Dong, M.; Stenmark, P. Crystal structure of the catalytic domain of the Weissella oryzae botulinum-like toxin. FEBS Lett. 2019, 593, 1403–1410. [Google Scholar] [CrossRef]
- Contreras, E.; Masuyer, G.; Qureshi, N.; Chawla, S.; Dhillon, H.S.; Lee, H.L.; Chen, J.; Stenmark, P.; Gill, S.S. A neurotoxin that specifically targets Anopheles mosquitoes. Nat. Commun. 2019, 10, 2869. [Google Scholar] [CrossRef]
- Rasetti-Escargueil, C.; Lemichez, E.; Popoff, M.R. Toxemia in Human Naturally Acquired Botulism. Toxins 2020, 12, 716. [Google Scholar] [CrossRef]
- Fenicia, L.; Anniballi, F.; Aureli, P. Intestinal toxemia botulism in Italy, 1984–2005. Eur. J. Clin. Microbiol. Infect. Dis. 2007, 26, 385–394. [Google Scholar] [CrossRef]
- Barash, J.R.; Arnon, S.S. Dual toxin-producing strain of Clostridium botulinum type Bf isolated from a California patient with infant botulism. J. Clin. Microbiol. 2004, 42, 1713–1715. [Google Scholar] [CrossRef] [PubMed]
- Dabritz, H.A.; Hill, K.K.; Barash, J.R.; Ticknor, L.O.; Helma, C.H.; Dover, N.; Payne, J.R.; Arnon, S.S. Molecular epidemiology of infant botulism in California and elsewhere, 1976–2010. J. Infect. Dis. 2014, 210, 1711–1722. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sobel, J. Botulism. Clin. Infect. Dis. 2005, 41, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- King, L.A.; Popoff, M.R.; Mazuet, C.; Espie, E.; Vaillant, V.; de Valk, H. Infant botulism in France, 1991–2009. Arch. Pediatr. 2010, 17, 1288–1292. [Google Scholar] [CrossRef]
- Ghasemi, M.; Norouzi, R.; Salari, M.; Asadi, B. Iatrogenic botulism after the therapeutic use of botulinum toxin-A: A case report and review of the literature. Clin. Neuropharmacol. 2012, 35, 254–257. [Google Scholar] [CrossRef]
- Floresta, G.; Patamia, V.; Gentile, D.; Molteni, F.; Santamato, A.; Rescifina, A.; Vecchio, M. Repurposing of FDA-Approved Drugs for Treating Iatrogenic Botulism: A Paired 3D-QSAR/Docking Approach(dagger). ChemMedChem 2020, 15, 256–262. [Google Scholar] [CrossRef]
- Thirunavukkarasu, N.; Johnson, E.; Pillai, S.; Hodge, D.; Stanker, L.; Wentz, T.; Singh, B.; Venkateswaran, K.; McNutt, P.; Adler, M.; et al. Botulinum Neurotoxin Detection Methods for Public Health Response and Surveillance. Front. Bioeng. Biotechnol. 2018, 6, 80. [Google Scholar] [CrossRef]
- Bremer, P.T.; Adler, M.; Phung, C.H.; Singh, A.K.; Janda, K.D. Newly Designed Quinolinol Inhibitors Mitigate the Effects of Botulinum Neurotoxin A in Enzymatic, Cell-Based, and ex Vivo Assays. J. Med. Chem. 2017, 60, 338–348. [Google Scholar] [CrossRef]
- Iriajen, A.; Rasetti-Escargueil, C. Integrated Approach used by Government Agencies and Industry to Protect the Consumer: Food Safety Legislation. In Clostridium Botulinum: A Spore Forming Organism and a Challenge to Food Safety; Surman-Lee, S., Rasetti-Escargueil, C., Eds.; Nova Publishers: New York, NY, USA, 2012. [Google Scholar]
- Lindstrom, M.; Keto, R.; Markkula, A.; Nevas, M.; Hielm, S.; Korkeala, H. Multiplex PCR assay for detection and identification of Clostridium botulinum types A, B, E, and F in food and fecal material. Appl. Environ. Microbiol. 2001, 67, 5694–5699. [Google Scholar] [CrossRef]
- Arnon, S.S.; Schechter, R.; Inglesby, T.V.; Henderson, D.A.; Bartlett, J.G.; Ascher, M.S.; Eitzen, E.; Fine, A.D.; Hauer, J.; Layton, M.; et al. Botulinum toxin as a biological weapon: Medical and public health management. JAMA 2001, 285, 1059–1070. [Google Scholar] [CrossRef]
- Cenciarelli, O.; Riley, P.W.; Baka, A. Biosecurity Threat Posed by Botulinum Toxin. Toxins 2019, 11, 681. [Google Scholar] [CrossRef] [PubMed]
- Adler, S.; Bicker, G.; Bigalke, H.; Bishop, C.; Blümel, J.; Dressler, D.; Fitzgerald, J.; Gessler, F.; Heuschen, H.; Kegel, B.; et al. The current scientific and legal status of alternative methods to the LD50 test for botulinum neurotoxin potency testing. The report and recommendations of a ZEBET Expert Meeting. Altern. Lab. Anim. 2010, 38, 315–330. [Google Scholar] [CrossRef] [PubMed]
- Kalb, S.R.; Baudys, J.; Rees, J.C.; Smith, T.J.; Smith, L.A.; Helma, C.H.; Hill, K.; Kull, S.; Kirchner, S.; Dorner, M.B.; et al. De novo subtype and strain identification of botulinum neurotoxin type B through toxin proteomics. Anal. Bioanal. Chem. 2012, 403, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Stanker, L.H.; Sharma, S.K. Botulinum neurotoxin: Where are we with detection technologies? Crit. Rev. Microbiol. 2013, 39, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Lindstrom, M.; Kiviniemi, K.; Korkeala, H. Hazard and control of group II (non-proteolytic) Clostridium botulinum in modern food processing. Int. J. Food Microbiol. 2006, 108, 92–104. [Google Scholar] [CrossRef]
- Worbs, S.; Fiebig, U.; Zeleny, R.; Schimmel, H.; Rummel, A.; Luginbühl, W.; Dorner, B.G. Qualitative and Quantitative Detection of Botulinum Neurotoxins from Complex Matrices: Results of the First International Proficiency Test. Toxins 2015, 7, 4935–4966. [Google Scholar] [CrossRef]
- Sharma, S.K.; Ferreira, J.L.; Eblen, B.S.; Whiting, R.C. Detection of type A, B, E, and F Clostridium botulinum neurotoxins in foods by using an amplified enzyme-linked immunosorbent assay with digoxigenin-labeled antibodies. Appl. Environ. Microbiol. 2006, 72, 1231–1238. [Google Scholar] [CrossRef]
- Singh, A.; Datta, S.; Sachdeva, A.; Maslanka, S.; Dykes, J.; Skinner, G.; Burr, D.; Whiting, R.C.; Sharma, S.K. Evaluation of an enzyme-linked immunosorbent assay (ELISA) kit for the detection of botulinum neurotoxins A, B, E, and F in selected food matrices. Health Secur. 2015, 13, 37–44. [Google Scholar] [CrossRef]
- Cheng, L.W.; Stanker, L.H. Detection of botulinum neurotoxin serotypes A and B using a chemiluminescent versus electrochemiluminescent immunoassay in food and serum. J. Agric. Food Chem. 2013, 61, 755–760. [Google Scholar] [CrossRef]
- von Berg, L.; Stern, D.; Weisemann, J.; Rummel, A.; Dorner, M.B.; Dorner, B.G. Optimization of SNAP-25 and VAMP-2 Cleavage by Botulinum Neurotoxin Serotypes A–F Employing Taguchi Design-of-Experiments. Toxin 2019, 11, 588. [Google Scholar] [CrossRef]
- Babrak, L.; Lin, A.; Stanker, L.H.; McGarvey, J.; Hnasko, R. Rapid Microfluidic Assay for the Detection of Botulinum Neurotoxin in Animal Sera. Toxins 2016, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Rivera, V.R.; Gamez, F.J.; Keener, W.K.; White, J.A.; Poli, M.A. Rapid detection of Clostridium botulinum toxins A, B, E, and F in clinical samples, selected food matrices, and buffer using paramagnetic bead-based electrochemiluminescence detection. Anal. Biochem. 2006, 353, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Simon, S.; Fiebig, U.; Liu, Y.; Tierney, R.; Dano, J.; Worbs, S.; Endermann, T.; Nevers, M.-C.; Volland, H.; Sesardic, D.; et al. Recommended Immunological Strategies to Screen for Botulinum Neurotoxin-Containing Samples. Toxins 2015, 7, 5011–5034. [Google Scholar] [CrossRef]
- Razai, A.; Garcia-Rodriguez, C.; Lou, J.; Geren, I.; Forsyth, C.; Robles, Y.; Tsai, R.; Smith, T.; Smith, L.; Siegel, R.; et al. Molecular evolution of antibody affinity for sensitive detection of botulinum neurotoxin type A. J. Mol. Biol. 2005, 351, 158–169. [Google Scholar] [CrossRef]
- Ferreira, J.L.; Eliasberg, S.J.; Edmonds, P.; Harrison, M.A. Comparison of the mouse bioassay and enzyme-linked immunosorbent assay procedures for the detection of type A botulinal toxin in food. J. Food Prot. 2004, 67, 203–206. [Google Scholar] [CrossRef]
- Gessler, F.; Pagel-Wieder, S.; Avondet, M.A.; Böhnel, H. Evaluation of lateral flow assays for the detection of botulinum neurotoxin type A and their application in laboratory diagnosis of botulism. Diagn. Microbiol. Infect. Dis. 2007, 57, 243–249. [Google Scholar] [CrossRef]
- Scotcher, M.C.; Cheng, L.W.; Stanker, L.H. Detection of botulinum neurotoxin serotype B at sub mouse LD(50) levels by a sandwich immunoassay and its application to toxin detection in milk. PLoS ONE 2010, 5, e11047. [Google Scholar] [CrossRef]
- Kalb, S.R.; Goodnough, M.C.; Malizio, C.J.; Pirkle, J.L.; Barr, J.R. Detection of botulinum neurotoxin A in a spiked milk sample with subtype identification through toxin proteomics. Anal. Chem. 2005, 77, 6140–6146. [Google Scholar] [CrossRef]
- Mazuet, C.; Ezan, E.; Volland, H.; Popoff, M.R.; Becher, F. Toxin detection in patients’ sera by mass spectrometry during two outbreaks of type A Botulism in France. J. Clin. Microbiol. 2012, 50, 4091–4094. [Google Scholar] [CrossRef]
- Sulaiman, I.M.; Miranda, N.; Simpson, S. MALDI-TOF Mass Spectrometry and 16S rRNA Gene Sequence Analysis for the Identification of Foodborne Clostridium spp. J. AOAC Int. 2021, 104, 1381–1388. [Google Scholar] [CrossRef]
- Kull, S.; Pauly, D.; Störmann, B.; Kirchner, S.; Stämmler, M.; Dorner, M.B.; Lasch, P.; Naumann, D.; Dorner, B.G. Multiplex detection of microbial and plant toxins by immunoaffinity enrichment and matrix-assisted laser desorption/ionization mass spectrometry. Anal. Chem. 2010, 82, 2916–2924. [Google Scholar] [CrossRef] [PubMed]
- Koike, H.; Kanda, M.; Hayashi, H.; Matsushima, Y.; Yoshikawa, S.; Ohba, Y.; Hayashi, M.; Nagano, C.; Sekimura, K.; Otsuka, K.; et al. Development of an alternative approach for detecting botulinum neurotoxin type A in honey: Analysis of non-toxic peptides with a reference labelled protein via liquid chromatography-tandem mass spectrometry. Food Addit. Contam. Part A Chem. Anal. Control Expo Risk Assess. 2020, 37, 1359–1373. [Google Scholar] [CrossRef] [PubMed]
- Morineaux, V.; Mazuet, C.; Hilaire, D.; Enche, J.; Popoff, M.R. Characterization of botulinum neurotoxin type A subtypes by immunocapture enrichment and liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2015, 407, 5559–5570. [Google Scholar] [CrossRef] [PubMed]
- Kalb, S.R.; Moura, H.; Boyer, A.E.; McWilliams, L.G.; Pirkle, J.L.; Barr, J.R. The use of Endopep-MS for the detection of botulinum toxins A, B, E, and F in serum and stool samples. Anal. Biochem. 2006, 351, 84–92. [Google Scholar] [CrossRef]
- Rosen, O.; Feldberg, L.; Gura, S.; Zichel, R. A new peptide substrate for enhanced botulinum neurotoxin type B detection by endopeptidase-liquid chromatography-tandem mass spectrometry/multiple reaction monitoring assay. Anal. Biochem. 2015, 473, 7–10. [Google Scholar] [CrossRef]
- Rosen, O.; Feldberg, L.; Gura, S.; Zichel, R. Improved detection of botulinum type E by rational design of a new peptide substrate for endopeptidase-mass spectrometry assay. Anal. Biochem. 2014, 456, 50–52. [Google Scholar] [CrossRef]
- Kalb, S.R.; Baudys, J.; Wang, D.; Barr, J.R. Recommended mass spectrometry-based strategies to identify botulinum neurotoxin-containing samples. Toxins 2015, 7, 1765–1778. [Google Scholar] [CrossRef]
- Kalb, S.R.; Baudys, J.; Smith, T.J.; Smith, L.A.; Barr, J.R. Characterization of Hemagglutinin Negative Botulinum Progenitor Toxins. Toxins 2017, 9, 193. [Google Scholar] [CrossRef]
- Rosen, O.; Feldberg, L.; Yamin, T.S.; Dor, E.; Barnea, A.; Weissberg, A.; Zichel, R. Development of a multiplex Endopep-MS assay for simultaneous detection of botulinum toxins A, B and E. Sci. Rep. 2017, 7, 14859. [Google Scholar] [CrossRef]
- Kalb, S.R.; Baudys, J.; Kiernan, K.; Wang, D.; Becher, F.; Barr, J.R. Proposed BoNT/A and /B Peptide Substrates Cannot Detect Multiple Subtypes in the Endopep-MS Assay. J. Anal. Toxicol. 2020, 44, 173–179. [Google Scholar] [CrossRef]
- Wang, D.; Baudys, J.; Hoyt, K.M.; Barr, J.R.; Kalb, S.R. Further optimization of peptide substrate enhanced assay performance for BoNT/A detection by MALDI-TOF mass spectrometry. Anal. Bioanal. Chem. 2017, 409, 4779–4786. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Baudys, J.; Hoyt, K.; Barr, J.R.; Kalb, S.R. Sensitive detection of type G botulinum neurotoxin through Endopep-MS peptide substrate optimization. Anal. Bioanal. Chem. 2019, 411, 5489–5497. [Google Scholar] [CrossRef] [PubMed]
- Tevell Aberg, A.; Karlsson, I.; Hedeland, M. Modification and validation of the Endopep-mass spectrometry method for botulinum neurotoxin detection in liver samples with application to samples collected during animal botulism outbreaks. Anal. Bioanal. Chem. 2021, 413, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.G.A.; Ochiai, M.; Liu, Y.; Ekong, T.; Sesardic, D. Development of improved SNAP25 endopeptidase immuno-assays for botulinum type A and E toxins. J. Immunol. Methods 2008, 329, 92–101. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Rigsby, P.; Sesardic, D.; Marks, J.D.; Jones, R.G. A functional dual-coated (FDC) microtiter plate method to replace the botulinum toxin LD50 test. Anal. Biochem. 2012, 425, 28–35. [Google Scholar] [CrossRef]
- Nuss, J.E.; Ruthel, G.; Tressler, L.E.; Wanner, L.M.; Torres-Melendez, E.; Hale, M.L.; Bavari, S. Development of cell-based assays to measure botulinum neurotoxin serotype A activity using cleavage-sensitive antibodies. J. Biomol. Screen. 2010, 15, 42–51. [Google Scholar] [CrossRef]
- Rhéaume, C.; Cai, B.B.; Wang, J.; Fernandez-Salas, E.; Aoki, K.R.; Francis, J.; Broide, R.S. A Highly Specific Monoclonal Antibody for Botulinum Neurotoxin Type A-Cleaved SNAP25. Toxins 2015, 7, 2354–2370. [Google Scholar] [CrossRef]
- von Berg, L.; Stern, D.; Pauly, D.; Mahrhold, S.; Weisemann, J.; Jentsch, L.; Hansbauer, E.; Müller, C.; Avondet, M.A.; Rummel, A.; et al. Functional detection of botulinum neurotoxin serotypes A to F by monoclonal neoepitope-specific antibodies and suspension array technology. Sci. Rep. 2019, 9, 5531. [Google Scholar] [CrossRef]
- Lévêque, C.; Ferracci, G.; Maulet, Y.; Grand-Masson, C.; Blanchard, M.-P.; Seagar, M.; El Far, O. A substrate sensor chip to assay the enzymatic activity of Botulinum neurotoxin A. Biosens. Bioelectron. 2013, 49, 276–281. [Google Scholar] [CrossRef]
- Ferracci, G.; Marconi, S.; Mazuet, C.; Jover, E.; Blanchard, M.-P.; Seagar, M.; Popoff, M.; Lévêque, C. A label-free biosensor assay for botulinum neurotoxin B in food and human serum. Anal. Biochem. 2011, 410, 281–288. [Google Scholar] [CrossRef]
- Cheng, H.P.; Chuang, H.S. Rapid and Sensitive Nano-Immunosensors for Botulinum. ACS Sens. 2019, 4, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, J.; Sharma, M.K.; Ponmariappan, S.; Sarita Shaik, M.; Upadhyay, S. Electrochemical immunosensor for botulinum neurotoxin type-E using covalently ordered graphene nanosheets modified electrodes and gold nanoparticles-enzyme conjugate. Biosens. Bioelectron. 2015, 69, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.G. Detection of biologically active botulinum neurotoxin—A in serum using high-throughput FRET-assay. J. Pharmacol. Toxicol. Methods 2012, 65, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Xu, C.; Li, X.; Chen, S. A simple, rapid and sensitive FRET assay for botulinum neurotoxin serotype B detection. PLoS ONE 2014, 9, e114124. [Google Scholar] [CrossRef]
- Bagramyan, K.; Kaplan, B.E.; Cheng, L.W.; Strotmeier, J.; Rummel, A.; Kalkum, M. Substrates and controls for the quantitative detection of active botulinum neurotoxin in protease-containing samples. Anal. Chem. 2013, 85, 5569–5576. [Google Scholar] [CrossRef]
- Weingart, O.G.; Eyer, K.; Luchtenborg, C.; Sachsenheimer, T.; Brugger, B.; van Oostrum, M.; Wollscheid, B.; Dittrich, P.; Loessner, M.S. In vitro quantification of botulinum neurotoxin type A1 using immobilized nerve cell-mimicking nanoreactors in a microfluidic platform. Analyst 2019, 144, 5755–5765. [Google Scholar] [CrossRef]
- Das, R.G.; Sesardic, D. Alternatives to the LD50 assay for botulinum toxin potency testing: Strategies and progress towards refinement, reduction and replacement. AATEX 14. In Proceedings of the 6th World Congress on Alternatives & Animal Use in the Life Sciences, Tokyo, Janpan, 21–25 August 2007; pp. 581–585. [Google Scholar]
- Pellett, S.; Tepp, W.H.; Johnson, E.A. Critical Analysis of Neuronal Cell and the Mouse Bioassay for Detection of Botulinum. Neurotoxins 2019, 11, 713. [Google Scholar] [CrossRef]
- Broide, R.S.; Rubino, J.; Nicholson, G.S.; Ardila, M.C.; Brown, M.S.; Aoki, K.R.; Francis, J. The rat Digit Abduction Score (DAS) assay: A physiological model for assessing botulinum neurotoxin-induced skeletal muscle paralysis. Toxicon 2013, 71, 18–24. [Google Scholar] [CrossRef]
- Aoki, K.R. A comparison of the safety margins of botulinum neurotoxin serotypes A, B, and F in mice. Toxicon 2001, 39, 1815–1820. [Google Scholar] [CrossRef]
- Rasetti-Escargueil, C.; Jones, R.G.A.; Liu, Y.; Sesardic, D. Measurement of botulinum types A, B and E neurotoxicity using the phrenic nerve-hemidiaphragm: Improved precision with in-bred mice. Toxicon 2009, 53, 503–511. [Google Scholar] [CrossRef]
- Whitemarsh, R.C.M.; Strathman, M.J.; Chase, L.G.; Stankewicz, C.; Tepp, W.H.; Johnson, E.A.; Pellett, S. Novel application of human neurons derived from induced pluripotent stem cells for highly sensitive botulinum neurotoxin detection. Toxicol. Sci. 2012, 126, 426–435. [Google Scholar] [CrossRef] [PubMed]
- McNutt, P.; Celver, J.; Hamilton, T.; Mesngon, M. Embryonic stem cell-derived neurons are a novel, highly sensitive tissue culture platform for botulinum research. Biochem. Biophys. Res. Commun. 2011, 405, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Kiris, E.; Nuss, J.E.; Burnett, J.C.; Kota, K.P.; Koh, D.C.; Wanner, L.M.; Torres-Melendez, E.; Gussio, R.; Tessarollo, L.; Bavari, S. Embryonic stem cell-derived motoneurons provide a highly sensitive cell culture model for botulinum neurotoxin studies, with implications for high-throughput drug discovery. Stem Cell Res. 2011, 6, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Pellett, S. Progress in cell based assays for botulinum neurotoxin detection. Curr. Top. Microbiol. Immunol. 2013, 364, 257–285. [Google Scholar] [PubMed]
- Fernandez-Salas, E.; Wang, J.; Molina, Y.; Nelson, J.B.; Jacky, B.P.; Aoki, K.R. Botulinum neurotoxin serotype A specific cell-based potency assay to replace the mouse bioassay. PLoS ONE 2012, 7, e49516. [Google Scholar] [CrossRef]
- Maslanka, S.E.; Luquez, C.; Dykes, J.K.; Tepp, W.H.; Pier, C.L.; Pellett, S.; Raphael, B.H.; Kalb, S.R.; Barr, J.R.; Rao, A.; et al. A Novel Botulinum Neurotoxin, Previously Reported as Serotype H, Has a Hybrid-Like Structure With Regions of Similarity to the Structures of Serotypes A and F and Is Neutralized With Serotype A Antitoxin. J. Infect. Dis. 2016, 213, 379–385. [Google Scholar] [CrossRef]
- Whitemarsh, R.C.; Pier, C.L.; Tepp, W.H.; Pellett, S.; Johnson, E.A. Model for studying Clostridium botulinum neurotoxin using differentiated motor neuron-like NG108-15 cells. Biochem. Biophys. Res. Commun. 2012, 427, 426–430. [Google Scholar] [CrossRef]
- Keller, J.E.; Neale, E.A. The role of the synaptic protein snap-25 in the potency of botulinum neurotoxin type A. J. Biol. Chem. 2001, 276, 13476–13482. [Google Scholar] [CrossRef]
- Sheridan, R.E.; Smith, T.J.; Adler, M. Primary cell culture for evaluation of botulinum neurotoxin antagonists. Toxicon 2005, 45, 377–382. [Google Scholar] [CrossRef]
- Rasetti-Escargueil, C.C.M.; R Preneta-Blanc, R. Fleck and D. Sesardic. Enhanced sensitivity to Botulinum type A neurotoxin of human neuroblastoma SH-SY5Y cells after differentiation into mature neuronal cells. Botulinum J. 2011, 2, 30–48. [Google Scholar] [CrossRef]
- Tegenge, M.A.; Bohnel, H.; Gessler, F.; Bicker, G. Neurotransmitter vesicle release from human model neurons (NT2) is sensitive to botulinum toxin A. Cell Mol. Neurobiol. 2012, 32, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.O.; Rosenfield, J.; Tzipori, S.; Park, J.B. M17 human neuroblastoma cell as a cell model for investigation of botulinum neurotoxin A activity and evaluation of BoNT/A specific antibody. Botulinum J. 2008, 1, 135–152. [Google Scholar] [CrossRef]
- Purkiss, J.R.; Friis, L.M.; Doward, S.; Quinn, C.P. Clostridium botulinum neurotoxins act with a wide range of potencies on SH-SY5Y human neuroblastoma cells. Neurotoxicology 2001, 22, 447–453. [Google Scholar] [CrossRef]
- Hong, W.S.; Pezzi, H.M.; Schuster, A.R.; Berry, S.M.; Sung, K.E.; Beebe, D.J. Development of a Highly Sensitive Cell-Based Assay for Detecting Botulinum Neurotoxin Type A through Neural Culture Media Optimization. J. Biomol. Screen. 2016, 21, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Diamant, E.; Torgeman, A.; Epstein, E.; Mechaly, A.; Ben David, A.; Levin, L.; Schwartz, A.; Dor, E.; Girshengorn, M.; Barnea, A.; et al. A cell-based alternative to the mouse potency assay for pharmaceutical type E botulinum antitoxins. ALTEX 2022, 39, 113–122. [Google Scholar] [CrossRef]
- Torgeman, A.; Diamant, E.; Levin, L.; Ben David, A.; Epstein, E.; Girshengorn, M.; Mazor, O.; Rosenfeld, R.; Zichel, R. An in vitro cell-based potency assay for pharmaceutical type A botulinum antitoxins. Vaccine 2017, 35, 7213–7216. [Google Scholar] [CrossRef] [PubMed]
- Stahl, A.M.; Ruthel, G.; Torres-Melendez, E.; Kenny, T.A.; Panchal, R.G.; Bavari, S. Primary cultures of embryonic chicken neurons for sensitive cell-based assay of botulinum neurotoxin: Implications for therapeutic discovery. J. Biomol. Screen. 2007, 12, 370–377. [Google Scholar] [CrossRef]
- Keller, J.E.; Cai, F.; Neale, E.A. Uptake of botulinum neurotoxin into cultured neurons. Biochemistry 2004, 43, 526–532. [Google Scholar] [CrossRef]
- Pellett, S.; Tepp, W.H.; Clancy, C.M.; Borodic, G.E.; Johnson, E.A. A neuronal cell-based botulinum neurotoxin assay for highly sensitive and specific detection of neutralizing serum antibodies. FEBS Lett. 2007, 581, 4803–4808. [Google Scholar] [CrossRef]
- Verderio, C.; Grumelli, C.; Raiteri, L.; Coco, S.; Paluzzi, S.; Caccin, P.; Rossetto, O.; Bonanno, G.; Montecucco, C.; Matteoli, M. Traffic of botulinum toxins A and E in excitatory and inhibitory neurons. Traffic 2007, 8, 142–153. [Google Scholar] [CrossRef]
- Schiavo, G.; Rossetto, O.; Benfenati, F.; Poulain, B.; Montecucco, C. Tetanus and botulinum neurotoxins are zinc proteases specific for components of the neuroexocytosis apparatus. Ann. N. Y. Acad. Sci. 1994, 710, 65–75. [Google Scholar] [CrossRef]
- Yowler, B.C.; Kensinger, R.D.; Schengrund, C.L. Botulinum neurotoxin A activity is dependent upon the presence of specific gangliosides in neuroblastoma cells expressing synaptotagmin I. J. Biol. Chem. 2002, 277, 32815–32819. [Google Scholar] [CrossRef]
- Pellett, S.; Du, Z.W.; Pier, C.L.; Tepp, W.H.; Zhang, S.C.; Johnson, E.A. Sensitive and quantitative detection of botulinum neurotoxin in neurons derived from mouse embryonic stem cells. Biochem. Biophys. Res. Commun. 2011, 404, 388–392. [Google Scholar] [CrossRef]
- Pellett, S.; Schwartz, M.P.; Tepp, W.H.; Josephson, R.; Scherf, J.M.; Pier, C.L.; Thomson, J.A.; Murphy, W.; Johnson, E.A. Human Induced Pluripotent Stem Cell Derived Neuronal Cells Cultured on Chemically-Defined Hydrogels for Sensitive In Vitro Detection of Botulinum Neurotoxin. Sci. Rep. 2015, 5, 14566. [Google Scholar] [CrossRef]
- Kiris, E.; Kota, K.P.; Burnett, J.C.; Soloveva, V.; Kane, C.D.; Bavari, S. Recent developments in cell-based assays and stem cell technologies for botulinum neurotoxin research and drug discovery. Expert Rev. Mol. Diagn. 2014, 14, 153–168. [Google Scholar] [CrossRef]
- Schenke, M.; Prause, H.C.; Bergforth, W.; Przykopanski, A.; Rummel, A.; Klawonn, F.; Seeger, B. Human-Relevant Sensitivity of iPSC-Derived Human Motor Neurons to BoNT/A1 and B1. Toxins 2021, 13, 585. [Google Scholar] [CrossRef]
- Rust, A.; Doran, C.; Hart, R.; Binz, T.; Stickings, P.; Sesardic, D.; Peden, A.A.; Davletov, B. A Cell Line for Detection of Botulinum Neurotoxin Type B. Front. Pharmacol. 2017, 8, 796. [Google Scholar] [CrossRef]
- de Lamotte, J.D.; Polentes, J.; Roussange, F.; Lesueur, L.; Feurgard, P.; Perrier, A.; Nicoleau, C.; Martinat, C. Optogenetically controlled human functional motor endplate for testing botulinum neurotoxins. Stem Cell Res. Ther. 2021, 12, 599. [Google Scholar] [CrossRef]
- Jenkinson, S.P.; Grandgirard, D.; Heidemann, M.; Tscherter, A.; Avondet, M.A.; Leib, S.L. Embryonic Stem Cell-Derived Neurons Grown on Multi-Electrode Arrays as a Novel In vitro Bioassay for the Detection of Clostridium botulinum Neurotoxins. Front. Pharmacol. 2017, 8, 73. [Google Scholar] [CrossRef]
- Scarlatos, A.; Cadotte, A.J.; DeMarse, T.B.; Welt, B.A. Cortical networks grown on microelectrode arrays as a biosensor for botulinum toxin. J. Food Sci. 2008, 73, E129–E136. [Google Scholar] [CrossRef]
- Black, B.J.; Atmaramani, R.; Pancrazio, J.J. Spontaneous and Evoked Activity from Murine Ventral Horn Cultures on Microelectrode Arrays. Front. Cell Neurosci. 2017, 11, 304. [Google Scholar] [CrossRef]
- Beske, P.H.; Bradford, A.B.; Grynovicki, J.O.; Glotfelty, E.J.; Hoffman, K.M.; Hubbard, K.S.; Tuznik, K.M.; McNutt, P.M. Botulinum and Tetanus Neurotoxin-Induced Blockade of Synaptic Transmission in Networked Cultures of Human and Rodent Neurons. Toxicol. Sci. 2016, 149, 503–515. [Google Scholar] [CrossRef]
- Neuschafer-Rube, F.; Pathe-Neuschafer-Rube, A.; Puschel, G.P. Discrimination of the Activity of Low-Affinity Wild-Type and High-Affinity Mutant Recombinant BoNT/B by a SIMA Cell-Based Reporter Release Assay. Toxins 2022, 14, 65. [Google Scholar] [CrossRef]
- Pathe-Neuschafer-Rube, A.; Neuschafer-Rube, F.; Haas, G.; Langoth-Fehringer, N.; Puschel, G.P. Cell-Based Reporter Release Assay to Determine the Potency of Proteolytic Bacterial Neurotoxins. Toxins 2018, 10, 360. [Google Scholar] [CrossRef]
- Tam, C.C.; Flannery, A.R.; Cheng, L.W. A Rapid, Sensitive, and Portable Biosensor Assay for the Detection of Botulinum Neurotoxin Serotype A in Complex Food Matrices. Toxins 2018, 10, 476. [Google Scholar] [CrossRef]
- Hobbs, R.J.; Thomas, C.A.; Halliwell, J.; Gwenin, C.D. Rapid Detection of Botulinum Neurotoxins-A Review. Toxins 2019, 11, 418. [Google Scholar] [CrossRef]
- Ambrin, G.; Cai, S.; Singh, B.R. Critical analysis in the advancement of cell-based assays for botulinum neurotoxin. Crit. Rev. Microbiol. 2022, 1–17, ahead of print. [Google Scholar] [CrossRef]
| BoNT Type | Method | Sample | Sensitivity | Reference |
|---|---|---|---|---|
| A | microfluidic double sandwich immunoassay | clinical serum samples | 3 pg/mL (8 MLD/mL) | [32] |
| A, B, E, F | electrochemiluminescence with biotinylated antibodies bound to streptavidin-coated beads | food serum, urine, | 50–100 pg/mL A, E 50 pg/mL B 100 pg/mL F 400 pg/mL | [33] |
| A, B, E | sandwich ELISA lateral flow immunoassay | buffer | A 10 pg/mL B 12 pg/mL E 107 pg/mL | [34] |
| A | flow cytometry with yeast displaying increased affinity scFv | buffer | 15 pg/mL | [35] |
| A | ELISA | food sample | 156–165 ng/mL | [36] |
| A | lateral flow immunoassay | buffer | 10–50 ng/mL | [37] |
| B | sandwich ELISA | buffermilk | 5 pg/mL 39 pg/mL | [38] |
| A, B | electrochemiluminescent assay | buffer | A 3–12 pg/mL B 13–17 pg/mL | [30] |
| Method Principles | Analysis Time | BoNT Toxinotype | Sensitivity | Benefits/Limitations | References |
|---|---|---|---|---|---|
| Immunological methods: sandwich ELISA, electro-chemiluminescent assay | 6–7 h | A–F | 2–176 pg/mL | Rapid detection/detection of active and inactive BoNTs, detection hampered by neurotoxin associated proteins | [25,26,27,28,29,30,31] |
| Immunological methods: lateral flow assay, | 30 min | A–B | 10–50 ng/mL (10,000–50,000 pg/mL) | Rapid detection/detection of active and inactive BoNTs, detection hampered by neurotoxin associated proteins | [25,34,37] |
| Mass spectrometry | 5–8 h | A–F | 0.1–1 pg/mL pg/mL | Rapid detection/detection of active and inactive BoNTs | [39,40,41,42,43,44] |
| Endopeptidase ELISA based or MS based | 7–8 h | A–G | 0.1–1000 pg/mL | Rapid detection/detection of cleavage only | [44,45,46,47,48,49,50,51,52,53,54,55,56] |
| Immunosensors and FRET assays | 2–5 h | A | 0.1–20 pg/mL | Rapid detection/detection of active and inactive BoNTs | [60,61,62,63,64,65] |
| In vivo mouse bioassay | 4 days | A–F | 1–10 pg/mL | Sensitive method detecting functional toxin but ethical concern, variability and duration | [23,68,69] |
| Ex vivo methods hemidiaphragm test | 9–5 h | A–F | 1–10 pg/mL | Sensitive method detecting functional toxin but ethical concern and technically demanding | [70,71,72] |
| Cell-based assays human neurons from induced pluripotent cells and monitoring of SNAP-25 cleavage by Western blot | 3–5 days | A–E | 0.003 pM–10 pM (0.55–1500 pg/mL) | Sensitive method detecting functional toxin but technically demanding | [73,74,75,76,80,81,89,90,95,96,97,98] |
| Cell-based assays using differentiated cell lines | 3–5 days | A–E | 5.5 pM–10 nM (825–150,000 pg/mL) | Sensitive method detecting functional toxin but technically demanding | [77,82,83,84,85,86,87,88,94,99,105,106] |
| Electrical conductance assays | 1–3 days | A | 25,000 pg/mL | Method detecting functional toxin but long and technically demanding | [86,87] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasetti-Escargueil, C.; Popoff, M.R. Recent Developments in Botulinum Neurotoxins Detection. Microorganisms 2022, 10, 1001. https://doi.org/10.3390/microorganisms10051001
Rasetti-Escargueil C, Popoff MR. Recent Developments in Botulinum Neurotoxins Detection. Microorganisms. 2022; 10(5):1001. https://doi.org/10.3390/microorganisms10051001
Chicago/Turabian StyleRasetti-Escargueil, Christine, and Michel R. Popoff. 2022. "Recent Developments in Botulinum Neurotoxins Detection" Microorganisms 10, no. 5: 1001. https://doi.org/10.3390/microorganisms10051001
APA StyleRasetti-Escargueil, C., & Popoff, M. R. (2022). Recent Developments in Botulinum Neurotoxins Detection. Microorganisms, 10(5), 1001. https://doi.org/10.3390/microorganisms10051001







