A Combination of Mediterranean and Low-FODMAP Diets for Managing IBS Symptoms? Ask Your Gut!
Abstract
:1. Introduction
2. Materials and Methods
3. Results
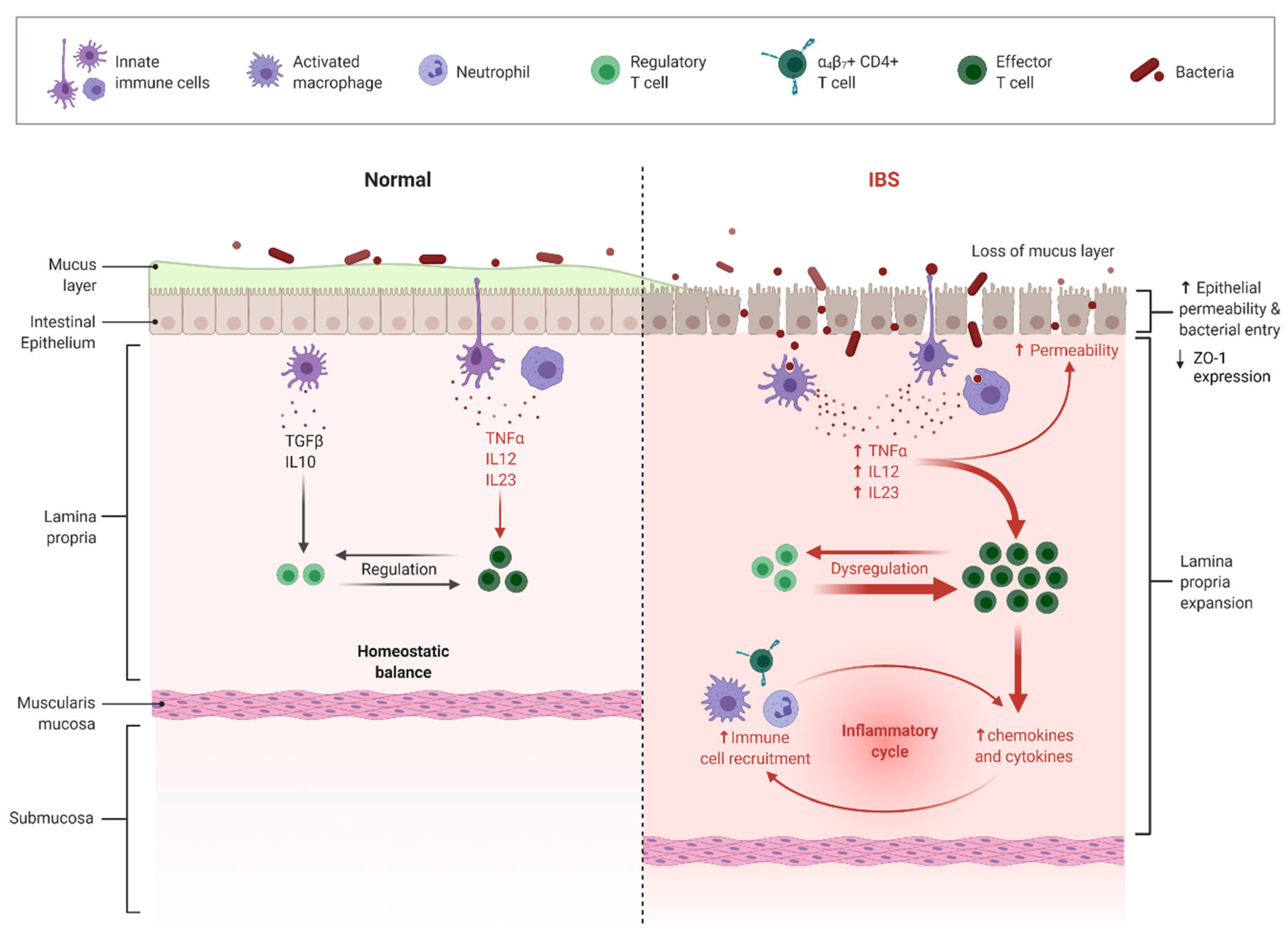
3.1. The Effect of LFD on IBS Subtypes, Gut Microbiota, and the Immune System
3.2. The Effect of MedDiet on the Immune System
3.2.1. Omega-3 Fatty Acids
3.2.2. Olive Oil and Phenolic Compounds
3.2.3. Fiber
4. Conclusions
| Food | Components | Action |
|---|---|---|
| Oat | β-Glucans (fibers) | prebiotic action [51] |
| Olives/olive oil | Hydroxy-tyrosol (phenolic compound) | anti-inflammatory action [44] |
| Walnuts | ω-3 PUFAs | prebiotic action [52] |
| Fish | ω-3 PUFAs | anti-inflammatory action [53] |
| Wine | Resveratrol (phenolic compound) | anti-inflammatory action [54] |
| Orange | Quercetin (phenolic compound) | prebiotic action [55] anti-inflammatory action [56] |
| Mandarin (Imperial, clementine) | Quercetin (phenolic compound) | anti-inflammatory action [57] |
| Tomatoes | Quercetin (phenolic compound) | prebiotic action [58] |
| Oregano, rosemary, thyme, and cumin | Phenolic compounds | anti-inflammatory action [59] |
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 5-HT | 5-Hydroxytryptamine |
| α-LA | α-Linolenic Acid |
| AA | Arachidonic Acid |
| AP-1 | Activating Protein-1 |
| CD | Crohn Disease |
| COX | Cyclooxygenase |
| CRH | Corticotropin-Releasing Hormone |
| CRP | C-Reactive Protein |
| DHA | Docosahexaenoic Acid |
| EFSA | European Food Safety Authority |
| EPA | Eicosapentaenoic Acid |
| EVOO | Extra Virgin Olive Oil |
| FCP | Fecal Calprotectin |
| FOS | Fructooligosaccharides |
| GFD | Gluten-Free Diet |
| GI | Gastrointestinal |
| GLUT-2 | Glucose Transporter 2 |
| GLUT-5 | Glucose Transporter 5 |
| GOS | Galactooligosaccharides |
| HPA | Hypothalamic–Pituitary–Adrenal |
| HT | Hydroxytyrosol |
| IBD | Inflammatory Bowel Disease |
| IBS | Irritable Bowel Syndrome |
| IBDQ | Inflammatory Bowel Disease Questionnaire |
| ICAM-1 | Intercellular Adhesion Molecule-1 |
| IL-6 | Interleukin 6 |
| iNOS | Inducible Nitric Oxide Synthase |
| LA | Linolenic Acid |
| LFD | Low-FODMAP Diet |
| LOX | Lipoxygenase |
| MAPK | Mitogen-Activated Protein Kinase |
| MedDiet | Mediterranean Diet |
| MUFAs | Monounsaturated Fatty Acids |
| NF-κΒ | Nuclear Factor–B |
| NRF2 | Nuclear Factor Erythroid 2-Related Factor |
| PI–IBS | Postinfectious Irritable Bowel Syndrome |
| PKC | Protein Kinase-C |
| PPRs | Pattern Recognition Receptors |
| PUFAs | Polyunsaturated Fatty Acids |
| QoL | Quality of Life |
| QUE | Quercetin |
| RCTs | Randomized Controlled Trials |
| RSV | Resveratrol |
| SCFAs | Short-Chain Fatty Acids |
| SFAs | Saturated Fatty Acids |
| SIBDQ | Short IBD Quality of Life Questionnaire |
| SIBO | Small Intestine Bacterial Overgrowth |
| TLRs | Toll-Like Receptors |
| TNBS | 2,4,6-Trinitrobenzene Sulfonic Acid |
| TNF-a | Tumor Necrosis Factor-Alpha |
| UC | Ulcerative Colitis |
| VCAM-1 | Vascular Cell Adhesion Molecule-1 |
| VOO | Virgin Olive Oil |
| ZO-1 | Zonula Occludens-1 |
References
- Hadjivasilis, A.; Tsioutis, C.; Michalinos, A.; Ntourakis, D.; Christodoulou, D.K.; Agouridis, A.P. New Insights into Irritable Bowel Syndrome: From Pathophysiology to Treatment. Ann. Gastroenterol. 2019, 32, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Saha, L. Irritable bowel syndrome: Pathogenesis, diagnosis, treatment, and evidence-based medicine. World J. Gastroenterol. 2014, 20, 6759–6773. [Google Scholar] [CrossRef] [PubMed]
- Adriani, A.; Ribaldone, D.G.; Astegiano, M.; Durazzo, M.; Saracco, G.M.; Pellicano, R. Irritable bowel syndrome: The clinical approach. Panminerva Med. 2018, 60, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Radovanovic-Dinic, B.; Tesic-Rajkovic, S.; Grgov, S.; Petrovic, G.; Zivković, V. Irritable bowel syndrome—From etiopathogenesis to therapy. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2018, 162, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacy, B.E.; Patel, N.K. Rome criteria and a diagnostic approach to irritable bowel syndrome. J. Clin. Med. 2017, 6, 99. Available online: http://www.ncbi.nlm.nih.gov/pubmed/29072609 (accessed on 20 February 2022). [CrossRef] [PubMed]
- Wang, L.; Alammar, N.; Singh, R.; Nanavati, J.; Song, Y.; Chaudhary, R. Gut Microbial Dysbiosis in the Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis of Case-Control Studies. J. Acad. Nutr. Diet 2020, 120, 565–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandeputte, D.; Joossens, M. Effects of Low and High FODMAP Diets on Human Gastrointestinal Microbiota Composition in Adults with Intestinal Diseases: A Systematic Review. Microorganisms 2020, 8, 1638. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Andrew, L.; Marlow, E.; Kunaratnam, K.; Devine, A.; Dunican, I.; Christophersen, C. Dietary Fibre Intervention for Gut Microbiota, Sleep, and Mental Health in Adults with Irritable Bowel Syndrome: A Scoping Review. Nutrients 2021, 13, 2159. [Google Scholar] [CrossRef]
- Rajilić-Stojanović, M.; Jonkers, D.M.; Salonen, A.; Hanevik, K.; Raes, J.; Jalanka, J.; de Vos, W.M.; Manichanh, C.; Golic, N.; Enck, P.; et al. Intestinal Microbiota and Diet in IBS: Causes, Consequences, or Epiphenomena? Am. J. Gastroenterol. 2015, 110, 278–287. [Google Scholar] [CrossRef] [Green Version]
- Chumpitazi, B.P.; Hollister, E.B.; Oezguen, N.; Tsai, C.M.; McMeans, A.R.; Luna, R.A.; Savidge, T.C.; Versalovic, J.; Shulman, R.J. Gut microbiota influences low fermentable substrate diet efficacy in children with irritable bowel syndrome. Gut Microbes 2014, 5, 165–175. [Google Scholar] [CrossRef] [Green Version]
- Spiller, R.C.; Jenkins, D.; Thornley, J.P.; Hebden, J.M.; Wright, T.; Skinner, M.; Neal, K.R. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut 2000, 47, 804–811. [Google Scholar] [CrossRef]
- Gwee, K.A.; Leong, Y.L.; Graham, C.; McKendrick, M.W.; Collins, S.M.; Walters, S.J. The role of psychological and biological factors in postinfective gut dysfunction. Gut 1999, 44, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Vannucchi, M.G.; Evangelista, S. Experimental Models of Irritable Bowel Syndrome and the Role of the Enteric Neurotransmission. J. Clin. Med. 2018, 3, 4. [Google Scholar] [CrossRef] [Green Version]
- Barrett, J.S.; Gearry, R.B.; Muir, J.G.; Irving, P.M.; Rose, R.; Rosella, O. Dietary poorly absorbed, short-chain carbohydrates increase delivery of water and fermentable substrates to the proximal colon. Aliment. Pharmacol. Ther. 2010, 31, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Altobelli, E.; Del Negro, V.; Angeletti, P.M.; Latella, G. Low-FODMAP Diet Improves Irritable Bowel Syndrome Symptoms: A Meta-Analysis. Nutrients 2017, 9, 940. [Google Scholar] [CrossRef] [PubMed]
- Basnayake, C. Treatment of irritable bowel syndrome. Aust. Prescr. 2018, 41, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.T.; Shah, N.D.; Sauk, J.; Limketkai, B.N. Popular Diet Trends for Inflammatory Bowel Diseases: Claims and Evidence. Curr. Treat. Options Gastroenterol. 2019, 17, 564–576. [Google Scholar] [CrossRef]
- Tuck, K.L.; Hayball, P.J. Major phenolic compounds in olive oil: Metabolism and health effects. J. Nutr. Biochem. 2002, 13, 636–644. [Google Scholar] [CrossRef]
- Casas, R.; Sacanella, E.; Estruch, R. The immune protective effect of the Mediterranean diet against chronic low-grade inflammatory diseases. Endocr. Metab. Immune. Disord. Drug Targets 2014, 14, 245–254. [Google Scholar] [CrossRef] [Green Version]
- Minelli, P.; Montinari, M.R. The Mediterranean Diet and Cardioprotection: Historical Overview and Current Research. J. Multidiscip. Healthc. 2019, 12, 805–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nani, A.; Murtaza, B.; Khan, A.S.; Khan, N.; Hichami, A. Antioxidant and Anti-Inflammatory Potential of Polyphenols Contained in Mediterranean Diet in Obesity: Molecular Mechanisms. Molecules 2021, 26, 985. [Google Scholar] [CrossRef] [PubMed]
- Bellini, M.; Tonarelli, S.; Nagy, A.G.; Pancetti, A.; Costa, F.; Ricchiuti, A.; de Bortoli, N.; Mosca, M.; Marchi, S.; Rossi, A. Low FODMAP Diet: Evidence, Doubts, and Hopes. Nutrients 2020, 12, 148. [Google Scholar] [CrossRef] [Green Version]
- Nybacka, S.; Störsrud, S.; Lindqvist, H.M.; Törnblom, H.; Simrén, M.; Winkvist, A. Habitual FODMAP Intake in Relation to Symptom Severity and Pattern in Patients with Irritable Bowel Syndrome. Nutrients 2020, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Marsh, A.; Eslick, E.M.; Eslick, G.D. Does a diet low in FODMAPs reduce symptoms associated with functional gastrointestinal disorders? A comprehensive systematic review and meta-analysis. Eur. J. Nutr. 2016, 55, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.S.C.; Yu, S.; Fedewa, A. Systematic review: Dietary fibre and FODMAP-restricted diet in the management of constipation and irritable bowel syndrome. Aliment. Pharmacol. Ther. 2015, 41, 1256–1270. [Google Scholar] [CrossRef]
- Van Lanen, A.-S.; de Bree, A.; Greyling, A. Efficacy of a low-FODMAP diet in adult irritable bowel syndrome: A systematic review and meta-analysis. Eur. J. Nutr. 2021, 60, 3505–3522. [Google Scholar] [CrossRef]
- Venter, C.; Eyerich, S.; Sarin, T.; Klatt, K.C. Nutrition and the Immune System: A Complicated Tango. Nutrients 2020, 12, 818. [Google Scholar] [CrossRef] [Green Version]
- Cox, S.R.; Prince, A.C.; Myers, C.E.; Irving, P.M.; Lindsay, J.O.; Lomer, M.C. Fermentable Carbohydrates [FODMAPs] Exacerbate Functional Gastrointestinal Symptoms in Patients with Inflammatory Bowel Disease: A Randomised, Double-blind, Placebo-controlled, Cross-over, Re-challenge Trial. J. Crohn’s Colitis 2017, 11, 1420–1429. [Google Scholar] [CrossRef] [Green Version]
- Wilson, B.; Cox, S.R.; Whelan, K. Challenges of the low FODMAP diet for managing irritable bowel syndrome and approaches to their minimisation and mitigation. Proc. Nutr. Soc. 2021, 80, 19–28. [Google Scholar] [CrossRef]
- Sloan, T.J.; Jalanka, J.; Major, G.A.D.; Krishnasamy, S.; Pritchard, S.; Abdelrazig, S. A low FODMAP diet is associated with changes in the microbiota and reduction in breath hydrogen but not colonic volume in healthy subjects. PLoS ONE 2018, 13, e0201410. [Google Scholar] [CrossRef] [Green Version]
- Hanning, N.; Edwinson, A.L.; Ceuleers, H.; Peters, S.A.; De Man, J.G.; Hassett, L.C.; De Winter, B.Y.; Grover, M. Intestinal barrier dysfunction in irritable bowel syndrome: A systematic review. Ther. Adv. Gastroenterol. 2021, 14, 1756284821993586. [Google Scholar] [CrossRef]
- Scuderi, S.A.; Casili, G.; Lanza, M.; Filippone, A.; Paterniti, I.; Esposito, E.; Campolo, M. Modulation of NLRP3 Inflammasome Attenuated Inflammatory Response Associated to Diarrhea-Predominant Irritable Bowel Syndrome. Biomedicines 2020, 8, 519. [Google Scholar] [CrossRef] [PubMed]
- Raskov, H.; Burcharth, J.; Pommergaard, H.-C.; Rosenberg, J. Irritable bowel syndrome, the microbiota and the gut-brain axis. Gut Microbes 2016, 7, 365–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, S.-Y.; Gillilland, M.; Wu, X.; Leelasinjaroen, P.; Zhang, G.; Zhou, H.; Ye, B.; Lu, Y.; Owyang, C. FODMAP diet modulates visceral nociception by lipopolysaccharide-mediated intestinal inflammation and barrier dysfunction. J. Clin. Investig. 2017, 128, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Staudacher, H.M.; Scholz, M.; Lomer, M.C.; Ralph, F.S.; Irving, P.M.; Lindsay, J.O.; Fava, F.; Tuohy, K.; Whelan, K. Gut microbiota associations with diet in irritable bowel syndrome and the effect of low FODMAP diet and probiotics. Clin. Nutr. 2021, 40, 1861–1870. [Google Scholar] [CrossRef] [PubMed]
- So, D.; Yao, C.K.; Ardalan, Z.S.; Thwaites, P.A.; Kalantar-Zadeh, K.; Gibson, P.R.; Muir, J.G. Supplementing Dietary Fibers with a Low-FODMAP Diet in Irritable Bowel Syndrome: A Randomized Controlled Crossover Trial. Clin. Gastroenterol. Hepatol. 2021. Available online: http://www.ncbi.nlm.nih.gov/pubmed/34929392 (accessed on 20 February 2022).
- Catassi, G.; Lionetti, E.; Gatti, S.; Catassi, C. The Low FODMAP Diet: Many Question Marks for a Catchy Acronym. Nutrients 2017, 9, 292. [Google Scholar] [CrossRef]
- Mori, T.A. Marine OMEGA-3 fatty acids in the prevention of cardiovascular disease. Fitoterapia 2017, 123, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bi, X.; Wang, S.; Zhang, Z.; Li, F.; Zhao, A.Z. Therapeutic Potential of ω-3 Polyunsaturated Fatty Acids in Human Autoimmune Diseases. Front. Immunol. 2019, 10, 2241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrea, L.; Caprio, M.; Tuccinardi, D.; Moriconi, E.; Di Renzo, L.; Muscogiuri, G. Could ketogenic diet “starve” cancer? Emerging evidence. Crit. Rev. Food Sci. Nutr. 2022, 62, 1800–1821. [Google Scholar] [CrossRef]
- Calder, P.C. n-3 fatty acids, inflammation and immunity: New mechanisms to explain old actions. Proc. Nutr. Soc. 2013, 72, 326–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez, S.; Svahn, S.L.; Johansson, M.E. Effects of Omega-3 Fatty Acids on Immune Cells. Int. J. Mol. Sci. 2019, 20, 5028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cariello, M.; Contursi, A.; Gadaleta, R.M.; Piccinin, E.; De Santis, S.; Piglionica, M.; Spaziante, A.F.; Sabbà, C.; Villani, G.; Moschetta, A. Extra-Virgin Olive Oil from Apulian Cultivars and Intestinal Inflammation. Nutrients 2020, 12, 1084. [Google Scholar] [CrossRef] [Green Version]
- García-Montero, C.; Fraile-Martínez, O.; Gómez-Lahoz, A.M.; Pekarek, L.; Castellanos, A.J.; Noguerales-Fraguas, F. Nutritional Components in Western Diet Versus Mediterranean Diet at the Gut Microbiota-Immune System Interplay. Implications for Health and Disease. Nutrients 2021, 13, 699. [Google Scholar] [CrossRef]
- Lucas, L.; Russell, A.; Keast, R. Molecular Mechanisms of Inflammation. Anti-Inflammatory Benefits of Virgin Olive Oil and the Phenolic Compound Oleocanthal. Curr. Pharm. Des. 2011, 17, 754–768. [Google Scholar] [CrossRef] [PubMed]
- Taticchi, A.; Urbani, S.; Albi, E.; Servili, M.; Codini, M.; Traina, G. In Vitro Anti-Inflammatory Effects of Phenolic Compounds from Moraiolo Virgin Olive Oil (MVOO) in Brain Cells via Regulating the TLR4/NLRP3 Axis. Molecules 2019, 24, 4523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Gallego, J.; García-Mediavilla, M.V.; Sánchez-Campos, S.; Tuñón, M.J. Fruit polyphenols, immunity and inflammation. Br. J. Nutr. 2010, 104 (Suppl. S104), S15–S27. [Google Scholar] [CrossRef] [Green Version]
- Nanayakkara, W.S.; Skidmore, P.M.; O’Brien, L.; Wilkinson, T.J.; Gearry, R.B. Efficacy of the low FODMAP diet for treating irritable bowel syndrome: The evidence to date. Clin. Exp. Gastroenterol. 2016, 9, 131–142. [Google Scholar]
- Żyła, E.; Dziendzikowska, K.; Kamola, D.; Wilczak, J.; Sapierzyński, R.; Harasym, J. Anti-Inflammatory Activity of Oat Beta-Glucans in a Crohn’s Disease Model: Time- and Molar Mass-Dependent Effects. Int. J. Mol. Sci. 2021, 22, 4485. [Google Scholar] [CrossRef]
- Kristek, A.; Wiese, M.; Heuer, P.; Kosik, O.; Schär, M.Y.; Soycan, G. Oat bran, but not its isolated bioactive β-glucans or polyphenols, have a bifidogenic effect in an in vitro fermentation model of the gut microbiota. Br. J. Nutr. 2019, 121, 549–559. [Google Scholar] [CrossRef]
- Bamberger, C.; Rossmeier, A.; Lechner, K.; Wu, L.; Waldmann, E.; Fischer, S.; Stark, R.G.; Altenhofer, J.; Henze, K.; Parhofer, K.G. A Walnut-Enriched Diet Affects Gut Microbiome in Healthy Caucasian Subjects: A Randomized, Controlled Trial. Nutrients 2018, 10, 244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wall, R.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Fatty acids from fish: The anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr. Rev. 2010, 68, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Chalons, P.; Amor, S.; Courtaut, F.; Cantos-Villar, E.; Richard, T.; Auger, C.; Chabert, P.; Schni-Kerth, V.; Aires, V.; Delmas, D. Study of Potential Anti-Inflammatory Effects of Red Wine Extract and Resveratrol through a Modulation of Interleukin-1-Beta in Macrophages. Nutrients 2018, 10, 1856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costabile, A.; Walton, G.E.; Tzortzis, G.; Vulevic, J.; Charalampopoulos, D.; Gibson, G.R. Effects of Orange Juice Formulation on Prebiotic Functionality Using an In Vitro Colonic Model System. PLoS ONE 2015, 10, e0121955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coelho, R.C.L.A.; Hermsdorff, H.H.M.; Bressan, J. Anti-inflammatory Properties of Orange Juice: Possible Favorable Molecular and Metabolic Effects. Mater. Veg. 2013, 68, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Zhao, S.; Ning, Z.; Zeng, H.; Shu, Y.; Tao, O.; Xiao, C.; Lu, C.; Liu, Y. Citrus fruits as a treasure trove of active natural metabolites that potentially provide benefits for human health. Chem. Cent. J. 2015, 9, 1–14. [Google Scholar] [CrossRef] [Green Version]
- García-Alonso, F.J.; González-Barrio, R.; Martín-Pozuelo, G.; Hidalgo, N.; Navarro-González, I.; Masuero, D.; Soini, E.; Vrhovsek, U.; Periago, M.J. A study of the prebiotic-like effects of tomato juice consumption in rats with diet-induced non-alcoholic fatty liver disease (NAFLD). Food Funct. 2017, 8, 3542–3552. [Google Scholar] [CrossRef]
- Gutiérrez-Grijalva, E.P.; Picos-Salas, M.A.; Leyva-López, N.; Criollo-Mendoza, M.S.; Vazquez-Olivo, G.; Heredia, J.B. Flavonoids and Phenolic Acids from Oregano: Occurrence, Biological Activity and Health Benefits. Plants 2017, 7, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Lingen, E. A Mediterranean Diet-Based Lifestyle Intervention in Inflammatory Bowel Disease Patients with Quiescent Disease; A Pilot Study. Am. J. Biomed. Sci. Res. 2021, 13, 47–53. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasti, A.; Petsis, K.; Lambrinou, S.; Katsas, K.; Nikolaki, M.; Papanikolaou, I.S.; Hatziagelaki, E.; Triantafyllou, K. A Combination of Mediterranean and Low-FODMAP Diets for Managing IBS Symptoms? Ask Your Gut! Microorganisms 2022, 10, 751. https://doi.org/10.3390/microorganisms10040751
Kasti A, Petsis K, Lambrinou S, Katsas K, Nikolaki M, Papanikolaou IS, Hatziagelaki E, Triantafyllou K. A Combination of Mediterranean and Low-FODMAP Diets for Managing IBS Symptoms? Ask Your Gut! Microorganisms. 2022; 10(4):751. https://doi.org/10.3390/microorganisms10040751
Chicago/Turabian StyleKasti, Arezina, Konstantinos Petsis, Sophia Lambrinou, Konstantinos Katsas, Maroulla Nikolaki, Ioannis S. Papanikolaou, Erifili Hatziagelaki, and Konstantinos Triantafyllou. 2022. "A Combination of Mediterranean and Low-FODMAP Diets for Managing IBS Symptoms? Ask Your Gut!" Microorganisms 10, no. 4: 751. https://doi.org/10.3390/microorganisms10040751
APA StyleKasti, A., Petsis, K., Lambrinou, S., Katsas, K., Nikolaki, M., Papanikolaou, I. S., Hatziagelaki, E., & Triantafyllou, K. (2022). A Combination of Mediterranean and Low-FODMAP Diets for Managing IBS Symptoms? Ask Your Gut! Microorganisms, 10(4), 751. https://doi.org/10.3390/microorganisms10040751