The Effects of Stevia Consumption on Gut Bacteria: Friend or Foe?
Abstract
:1. Introduction
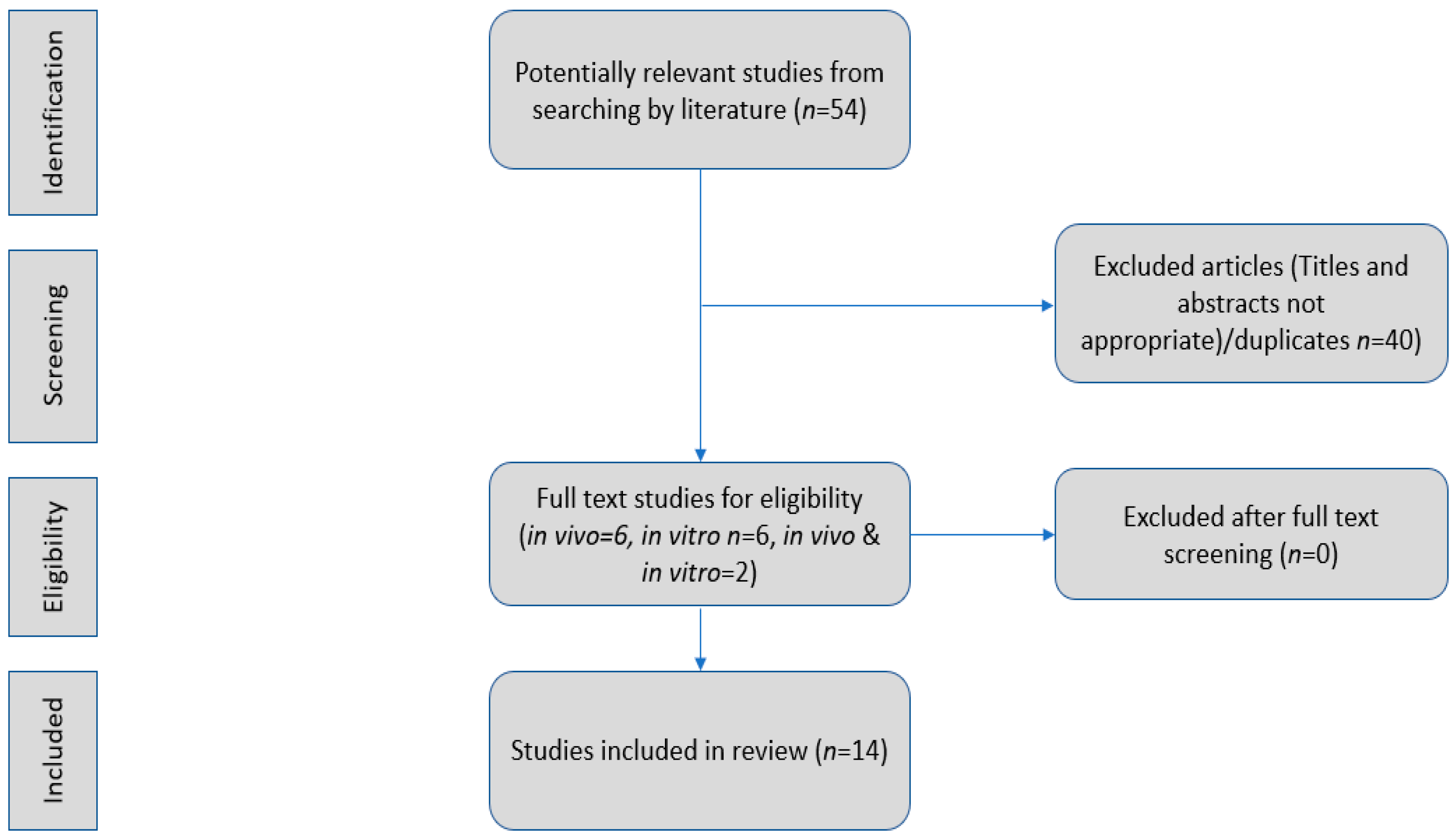
2. Materials and Methods
3. The Effects of Stevia and Steviol Glycosides on Bacterial Growth
3.1. In Vitro Studies
3.2. In Vivo Studies
4. The Effects of Stevia and Steviol Glycosides on Microbial Diversity
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ADI | Acceptable daily intake |
| A. muciniphila | Akkermansia muciniphila |
| B. bifidum | Bifidobacterium bifidum |
| B. thetaiotaomicron | Bacteroides thetaiotaomicron |
| CG | Control group |
| Chao | Chao1 richness |
| C. leptum | Clostridium leptum |
| E. coli | Escherichia coli |
| E. faecalis | Enterococcus faecalis |
| EFSA | European Food Safety Authority |
| Fa | Faith’s Phylogenetic Diversity Index |
| FDA | Food and Drug Administration |
| H’A | Shannon index OR Shannon’s diversity index OR Shannon–Wiener index (same) |
| HFD | High fat diet |
| HFS | High fat/high sucrose diet |
| J’ | Pielou’s evenness |
| LB | Lysogeny broth |
| L. rhamnosus | Lactobacillus rhamnosus |
| MAPK | Mitogen-activated protein kinase |
| MRS broth | de Man, Rogosa and Sharpe broth |
| NF-κB | Nuclear factor-kappa B |
| NMDS | Nonmetric multidimensional scaling on a Bray–Curtis dissimilarity matrix |
| NS | No significant difference |
| OTUs | Operational taxonomic units |
| P. aeruginosa | Pseudomonas aeruginosa |
| REB-A | Rebaudioside A |
| S. aureus | Staphylococcus aureus |
| SE | Shannon evenness index |
| SG | Stevia group |
| S. typhimurium | Salmonella typhimurium. |
| VLPs | Virus-like particles |
References
- Koyama, E.; Sakai, N.; Ohori, Y.; Kitazawa, K.; Izawa, O.; Kakegawa, K.; Fujino, A.; Ui, M. Absorption and metabolism of glycosidic sweeteners of stevia mixture and their aglycone, steviol, in rats and humans. Food Chem. Toxicol. 2003, 41, 875–883. [Google Scholar] [CrossRef]
- Halasa, B.; Walter, P.; Cai, H.; Gonzales, M.; Walter, M.; Shouppe, E.; Kosa, P.; Bielekova, B.; Hui, L.; Rother, K. Stevia Metabolites in Biosamples Ranging from Fetal Life to Adulthood. Curr. Dev. Nutr. 2020, 4, 1126. [Google Scholar] [CrossRef]
- Renwick, A.G.; Tarka, S.M. Microbial hydrolysis of steviol glycosides. Food Chem. Toxicol. 2008, 46, S70–S74. [Google Scholar] [CrossRef] [PubMed]
- Ashwell, M. Stevia, Nature’s Zero-Calorie Sustainable Sweetener: A New Player in the Fight Against Obesity. Nutr. Today 2015, 50, 129–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iatridis, N.; Kougioumtzi, A.; Vlataki, K.; Papadaki, S.; Magklara, A. Anti-Cancer Properties of Stevia rebaudiana; More than a Sweetener. Molecules 2022, 27, 1362. [Google Scholar] [CrossRef]
- Ruiz-Ojeda, F.J.; Plaza-Díaz, J.; Sáez-Lara, M.J.; Gil, A. Effects of Sweeteners on the Gut Microbiota: A Review of Experimental Studies and Clinical Trials. Adv. Nutr. 2019, 10, S31–S48. [Google Scholar] [CrossRef] [Green Version]
- Commission Regulation (EU) No 1131/2011 of 11 November 2011 Amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council with Regard to Steviol Glycosides. Available online: https://eur-lex.europa.eu/ (accessed on 17 December 2021).
- Peteliuk, V.; Rybchuk, L.; Bayliak, M.; Storey, K.B.; Lushchak, O. Natural sweetener Stevia rebaudiana: Functionalities, health benefits and potential risks. EXCLI J. 2021, 20, 1412–1430. [Google Scholar] [CrossRef]
- Takahashi, K.; Matsuda, M.; Ohashi, K.; Taniguchi, K.; Nakagomi, O.; Abe, Y.; Mori, S.; Sato, N.; Okutani, K.; Shigeta, S. Analysis of anti-rotavirus activity of extract from Stevia rebaudiana. Antivir. Res. 2001, 49, 15–24. [Google Scholar] [CrossRef]
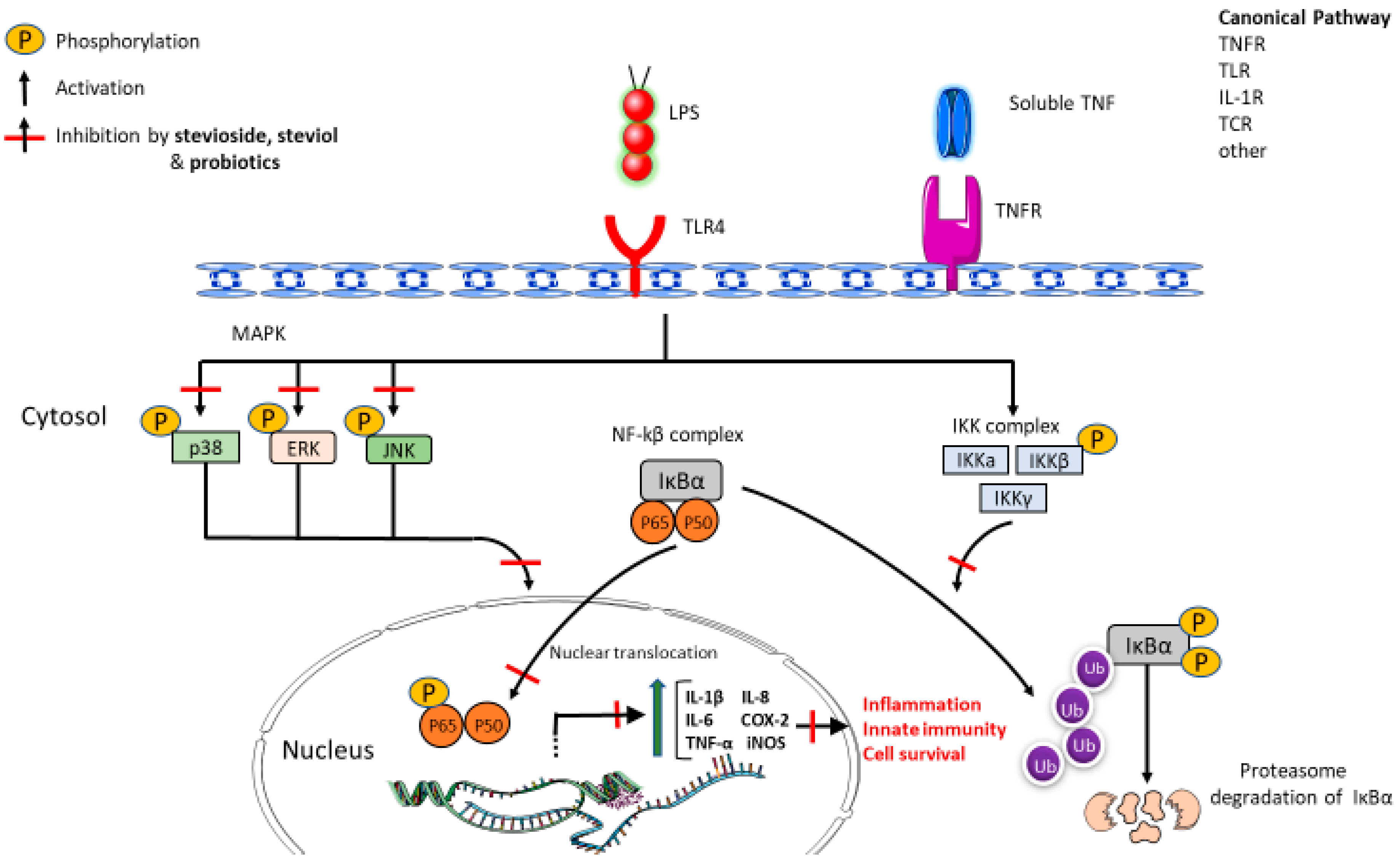
- Fengyang, L.; Yunhe, F.; Bo, L.; Zhicheng, L.; Depeng, L.; Dejie, L.; Wen, Z.; Yongguo, C.; Naisheng, Z.; Xichen, Z.; et al. Stevioside suppressed inflammatory cytokine secretion by downregulation of NF-κB and MAPK signaling pathways in LPS-stimulated RAW264.7 cells. Inflammation 2012, 35, 1669–1675. [Google Scholar] [CrossRef]
- Sehar, I.; Kaul, A.; Bani, S.; Pal, H.C.; Saxena, A.K. Immune up regulatory response of a non-caloric natural sweetener, stevioside. Chem. Biol. Interact. 2008, 173, 115–121. [Google Scholar] [CrossRef]
- Alavala, S.; Sangaraju, R.; Nalban, N.; Sahu, B.D.; Jerald, M.K.; Kilari, E.K.; Sistla, R. Stevioside, a diterpenoid glycoside, shows anti-inflammatory property against Dextran Sulphate Sodium-induced ulcerative colitis in mice. Eur. J. Pharmacol. 2019, 855, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Qi, L.; Lv, Z.; Jin, S.; Wei, X.; Shi, F. Dietary Stevioside Supplementation Alleviates Lipopolysaccharide-Induced Intestinal Mucosal Damage through Anti-Inflammatory and Antioxidant Effects in Broiler Chickens. Antioxidants 2019, 8, 575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohd-Radzman, N.H.; Ismail, W.I.W.; Adam, Z.; Jaapar, S.S.; Adam, A. Potential Roles of Stevia rebaudiana Bertoni in Abrogating Insulin Resistance and Diabetes: A Review. Evid. Based Complement. Alternat. Med. 2013, 2013, 718049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeppesen, P.B.; Gregersen, S.; Rolfsen, S.E.D.; Jepsen, M.; Colombo, M.; Agger, A.; Xiao, J.; Kruhøffer, M.; Orntoft, T.; Hermansen, K. Antihyperglycemic and blood pressure-reducing effects of stevioside in the diabetic Goto-Kakizaki rat. Metabolism 2003, 52, 372–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geeraert, B.; Crombé, F.; Hulsmans, M.; Benhabilès, N.; Geuns, J.M.; Holvoet, P. Stevioside inhibits atherosclerosis by improving insulin signaling and antioxidant defense in obese insulin-resistant mice. Int. J. Obes. 2010, 34, 569–577. [Google Scholar] [CrossRef] [Green Version]
- Holvoet, P.; Rull, A.; García-Heredia, A.; López-Sanromà, S.; Geeraert, B.; Joven, J.; Camps, J. Stevia-derived compounds attenuate the toxic effects of ectopic lipid accumulation in the liver of obese mice: A transcriptomic and metabolomic study. Food Chem. Toxicol. 2015, 77, 22–33. [Google Scholar] [CrossRef]
- Shivanna, N.; Naika, M.; Khanum, F.; Kaul, V.K. Antioxidant, anti-diabetic and renal protective properties of Stevia rebaudiana. J. Diabetes Complicat. 2013, 27, 103–113. [Google Scholar] [CrossRef]
- Khare, N.; Chandra, S. Stevioside mediated chemosensitization studies and cytotoxicity assay on breast cancer cell lines MDA-MB-231 and SKBR3. Saudi J. Biol. Sci. 2019, 26, 1596–1601. [Google Scholar] [CrossRef]
- Gupta, E.; Kaushik, S.; Purwar, S.; Sharma, R.; Balapure, A.K.; Sundaram, S. Anticancer potential of steviol in MCF-7 human breast cancer cells. Pharmacogn. Mag. 2017, 13, 345–350. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Rojo, E.; Cariño-Cortés, R.; Berumen, L.C.; García-Alcocer, G.; Escobar-Cabrera, J. Stevia eupatoria and Stevia pilosa extracts inhibit the proliferation and migration of prostate cancer cells. Medicina 2020, 56, 90. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Xia, Y.; Sui, X.; Peng, Q.; Zhang, T.; Li, J.; Zhang, J. Steviol, a natural product inhibits proliferation of the gastrointestinal cancer cells intensively. Oncotarget 2018, 9, 26299–26308. [Google Scholar] [CrossRef] [PubMed]
- Bessler, H.; Djaldetti, M. The impact of three commercial sweeteners on cytokine expression by mononuclears impelled by colon carcinoma cells. Int. J. Food Sci. Nutr. 2019, 70, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, L.; Duffy, A. Factors Influencing the Gut Microbiota, Inflammation, and Type 2 Diabetes. J. Nutr. 2017, 147, 1468S–1475S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, S.L.; Chiang, E.; Plantinga, A.; Carey, H.V.; Suen, G.; Swoap, S.J. Effect of stevia on the gut microbiota and glucose tolerance in a murine model of diet-induced obesity. FEMS Microbiol. Ecol. 2020, 96, fiaa079. [Google Scholar] [CrossRef]
- Li, S.; Chen, T.; Dong, S.; Xiong, Y.; Wei, H.; Xu, F. The Effects of Rebaudioside A on Microbial Diversity in Mouse Intestine. Food Sci. Technol. Res. 2014, 20, 459–467. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.-P.; Browman, D.; Herzog, H.; Neely, G.G. Non-nutritive sweeteners possess a bacteriostatic effect and alter gut microbiota in mice. PLoS ONE 2018, 13, e0199080. [Google Scholar] [CrossRef]
- Deniņa, I.; Semjonovs, P.; Fomina, A.; Treimane, R.; Linde, R. The influence of stevia glycosides on the growth of Lactobacillus reuteri strains. Lett. Appl. Microbiol. 2014, 58, 278–284. [Google Scholar] [CrossRef]
- Kunová, G.; Rada, V.; Vidaillac, A.; Lisova, I. Utilisation of steviol glycosides from Stevia rebaudiana (Bertoni) by lactobacilli and bifidobacteria in in vitro conditions. Folia Microbiol. 2014, 59, 251–255. [Google Scholar] [CrossRef]
- Boling, L.; Cuevas, D.A.; Grasis, J.A.; Kang, H.S.; Knowles, B.; Levi, K.; Maughan, H.; McNair, K.; Rojas, M.I.; Sanchez, S.E.; et al. Dietary prophage inducers and antimicrobials: Toward landscaping the human gut microbiome. Gut Microbes 2020, 11, 721–734. [Google Scholar] [CrossRef] [Green Version]
- Pawar, R.S.; Krynitsky, A.J.; Rader, J.I. Sweeteners from plants--with emphasis on Stevia rebaudiana (Bertoni) and Siraitia grosvenorii (Swingle). Anal. Bioanal. Chem. 2013, 405, 4397–4407. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial Activity of Terpenes and Terpenoids Present in Essential Oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahalak, K.K.; Firrman, J.; Tomasula, P.M.; Nuñez, A.; Lee, J.-J.; Bittinger, K.; Rinaldi, W.; Liu, L.S. Impact of Steviol Glycosides and Erythritol on the Human and Cebus apella Gut Microbiome. J. Agric. Food Chem. 2020, 68, 13093–13101. [Google Scholar] [CrossRef] [PubMed]
- Gerasimidis, K.; Bryden, K.; Chen, X.; Papachristou, E.; Verney, A.; Roig, M.; Hansen, R.; Nichols, B.; Papadopoulou, R.; Parrett, A. The impact of food additives, artificial sweeteners and domestic hygiene products on the human gut microbiome and its fibre fermentation capacity. Eur. J. Nutr. 2020, 59, 3213–3230. [Google Scholar] [CrossRef] [Green Version]
- Markus, V.; Share, O.; Teralı, K.; Ozer, N.; Marks, R.S.; Kushmaro, A.; Golberg, K. Anti-Quorum Sensing Activity of Stevia Extract, Stevioside, Rebaudioside A and Their Aglycon Steviol. Molecules 2020, 25, 5480. [Google Scholar] [CrossRef]
- Yu, M.; Gao, T.; Liu, Z.; Diao, X. Effects of Dietary Supplementation with High Fiber (Stevia Residue) on the Fecal Flora of Pregnant Sows. Animals 2020, 10, 2247. [Google Scholar] [CrossRef]
- Nettleton, J.E.; Klancic, T.; Schick, A.; Choo, A.C.; Shearer, J.; Borgland, S.L.; Chleilat, F.; Mayengbam, S.; Reimer, R.A. Low-Dose Stevia (Rebaudioside A) Consumption Perturbs Gut Microbiota and the Mesolimbic Dopamine Reward System. Nutrients 2019, 11, 1248. [Google Scholar] [CrossRef] [Green Version]
- Nettleton, J.E.; Cho, N.A.; Klancic, T.; Nicolucci, A.C.; Shearer, J.; Borgland, S.L.; Johnston, L.A.; Ramay, H.R.; Noye Tuplin, E.; Chleilat, F.; et al. Maternal low-dose aspartame and stevia consumption with an obesogenic diet alters metabolism, gut microbiota and mesolimbic reward system in rat dams and their offspring. Gut 2020, 69, 1807–1817. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Nettleton, J.E.; Gänzle, M.G.; Reimer, R.A. A Metagenomics Investigation of Intergenerational Effects of Non-nutritive Sweeteners on Gut Microbiome. Front. Nutr. 2021, 8, 795848. [Google Scholar] [CrossRef]
- de la Garza, A.L.; Romero-Delgado, B.; Martínez-Tamez, A.M.; Cárdenas-Tueme, M.; Camacho-Zamora, B.D.; Matta-Yee-Chig, D.; Sánchez-Tapia, M.; Torres, N.; Camacho-Morales, A. Maternal Sweeteners Intake Modulates Gut Microbiota and Exacerbates Learning and Memory Processes in Adult Male Offspring. Front. Pediatr. 2021, 9, 746437. [Google Scholar] [CrossRef]
- Willis, A.D. Rarefaction, Alpha Diversity, and Statistics. Front. Microbiol. 2019, 10, 2407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boonkaewwan, C.; Burodom, A. Anti-inflammatory and immunomodulatory activities of stevioside and steviol on colonic epithelial cells. J. Sci. Food Agric. 2013, 93, 3820–3825. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Singh, B.P.; Sandhu, N.; Singh, N.; Kaur, R.; Rokana, N.; Singh, K.S.; Chaudhary, V.; Panwar, H. Probiotic mediated NF-κB regulation for prospective management of type 2 diabetes. Mol. Biol. Rep. 2020, 47, 2301–2313. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.M.; Versalovic, J. Probiotics-host communication: Modulation of signaling pathways in the intestine. Gut Microbes 2020, 1, 148–163. [Google Scholar] [CrossRef] [PubMed]
| Reference | Strains | Intervention | Control | Beneficial or No Effect on Bacterial Populations Growth | Adverse Effects on Bacterial Populations Growth |
|---|---|---|---|---|---|
| Markus et al. 2020 | E. coli K802NR-pSB1075 | REB-A Stevioside Steviol | LB | Possible interruption of Gram-negative bacterial communication | |
| Li et al. 2014 | E. coli O157:H7 S. typhimurium ATCC 13311 S. aureus CGMCC 26001 Listeria monocytogenes CMCC 54007 Bifidobacterium longum ATCC 15707 Lactobacillus plantarum ACCC 11095 | REB-A | Saline buffer | No effect E. coli, S. typhimurium, Listeria monocytogenes Bifidobacterium longum, Lactobacillus plantarum Reduced S. aureus CG * | |
| Wang et al. 2018 | E. coli HB101 and K-12 | REB-A | LB with agar | Reduced E. coli HB101 No effect E. coli K-12 | |
| Deniņa et al. 2014 | Lactobacillus reuteri (six strains) | REB-A Stevioside | Acetic acid and lactic acid | Inhibit Lactobacillus reuteri strains | |
| Boling et al. 2020 | B. thetaiotaomicron VPI-5482 E. faecalis S. aureus | REB-A | None | Reduced S. aureus | Increased E. faecalis Reduced B. thetaiotaomicron VPI-5482 |
| Gerasimidis et al. 2020 | Bacteroides/Prevotella Bifidobacterium Blautia coccoides C. leptum E. coli | Stevia | None | No effect Bifidobacterium, Blautia coccoides, Bacteroides/Prevotella, C. leptum, E. coli | |
| Mahalak et al. 2020 | Human gut microbiota | Steviol glycosides + erythritol | None | Increased B. thetaiotaomicron No effect E. coli Enterococcus caccae L. rhamnosus Ruminococcus gauvreauii Bacteroides galacturonicus | |
| Kunová et al. 2014 | Bifidobacteria (longum subsp. CCDM 219, animalis subsp. lactis CCDM 94, dentium CCDM 318, breve CCDM 562, bifidum CCDM 559, adolescentis AVNB3-P1, bifidum JKM, bifidum JOV) Lactobacilli (brevis CCDM 202, delbrueckii subsp., bulgaricus CCDM 66, acidophilus CCDM 151, paracasei subsp. CCDM 212, mucosae SP1TA2) | Stevioside and REB-A (purity ≥ 95% of steviol glycosides), medium containing REB-A | MRS broth and D-glucose | Increased Lactobacillus mucosae SP1TA2 Increased Bifidobacterium bifidum CCDM 559, Bifidobacterium breve CCDM 562, Bifidobacterium adolescentis AVNB3- P1 No effect B. longum subsp. longum CCDM 219 B. animalis subsp. lactis CCDM 94 B. dentium CCDM 318 B. bifidum JKM B. bifidum JOV No effect L. brevis CCDM 202 L. delbrueckii subsp. bulgaricus CCDM 66 L. acidophilus CCDM 151 L. paracasei subsp. paracasei CCDM 212 |
| Reference | Type of Study | Model/Samples | Intervention | Control | Beneficial or No Effect on Bacterial Populations Growth | Adverse Effects on Bacterial Populations Growth |
|---|---|---|---|---|---|---|
| Becker et al. 2020 | Preclinical RCT | Mice (feces) | HFS + stevia | Saccharin | Increased Firmicutes/Bacteroidetes ratio | |
| Li et al. 2014 | Preclinical RCT | Mice (feces Enterococci Enterobacteria Lactobacilli) | Low dose REB-A (0.5 mg/mL) High dose REB-A (5.0 mg/mL) | None | Increased Lactobacilli (high dose only) No effect Enterococci Enterobacteria | |
| Nettleton et al. 2019 | Preclinical RCT | Rats (feces Bifidobacteriaceae Enterobacteriaceae) | REB-A and REB-A + prebiotic | Water | Increased A. muciniphila (in both groups), Bacteroides goldsteinii (REB-A group) B. thetaiotaomicron (REB-A group) (correlated with intestinal angiogenesis) Reduced Clostridiales family XIII (in both groups), Lactobacillus intestinalis (REB-A group) | Reduced Ruminococcaceae UCG 005 (in both groups), Bifidobacteriaceae (REB-A group) |
| Nettleton et al. 2020 | Preclinical | Obese rats during pregnancy and lactation and their offspring (feces) | HFS + REB-A | Lean rats during pregnancy and lactation and their offspring: control diet | Obese rats and offspring Reduced Bifidobacterium | Obese rats Increased C. leptum Obese rats and offspring Increased Porphyromonadaceae (metabolic syndrome development) Sporobacter (type-2 diabetes development) Enterobacteriaceae (proinflammatory) |
| Wang et al. 2022 | Preclinical RCT | Obese rats during pregnancy and lactation and their offspring (Distal jejunum, ileum tissue, cecal digesta) | HFS + stevia | Rats during pregnancy and lactation: HFS + water Offspring: control diet | Increased 14_Bacteroidaceae unclassified Reduced A. muciniphila Limosilactobacillus reuteri | |
| de la Garza et al. 2022 | Preclinical RCT | Rats during pregnancy and lactation and their male offspring (feces) | In prenatal period: cafeteria diet. In gestation and lactation: Stevia + control diet Offspring: control diet | Control diet | Maternal and male offspring group Reduced Bacteroidetes, Cyanobacteria Increased Firmicutes Elusimicrobia (correlated with decreased blood glucose levels) | Maternal and male offspring group Increased Firmicutes/Bacteroidetes ratio, Bacteroidales Clostridiales (contribute to cognitive dysfunction) |
| Mahalak et al. 2020 | Preclinical | Monkey (Cebus apella) (feces) | Steviol glycosides + erythritol | - | No effect in the microbial community | |
| Yu et al. 2020 | Preclinical RCT | Pregnant sows (feces) | Corn–soybean-meal diets + stevia residue 20%, 30%, 40% | Control diet | Increased Lachnospiraceae_XPB1014, Christensenellaceae_R-7_ Ruminococcaceae_UCG-005 Reduced Treponema_2 |
| Reference | Target Group | Evaluate | Alpha Diversity | Beta Diversity |
|---|---|---|---|---|
| Li et al. 2014 | Mice | a-diversity measures: Richness, H’A and SE |
| - |
| Nettleton et al. 2019 | Mice |
| NS difference in alpha diversity measures between CG and SG | NS difference in beta diversity measures between CG and SG |
| Nettleton et al. 2020 | Mice |
| Significantly higher a-diversity measures in SG compared to CG | NS difference in beta diversity measures between CG and SG |
| Wang et al. 2018 | Mice | a-diversity measure: H’A | NS difference in alpha diversity measures between CG and sucralose in normal chow or HFD-fed mice | - |
| Gerasimidis et al. 2020 | 13 healthy volunteers | a-diversity measures: OTUs, Chao, Rarefied richness, H’A, J’ | Addition of stevia significantly increased H’A, J’ and Rarefied richness (compared to CG) | - |
| de la Garza et al. 2022 | Mice (male) | a-diversity measure: H’A |
| - |
| Mahalak et al. 2020 | In vitro |
| NS difference in alpha diversity measures over time between CG, Erythritol group and SN Stevia group | No consistent pattern was observed between each group |
| 1 volunteer in vivo | Consumption of SN Stevia & Erythritol increased alpha diversity measures significantly over time (p < 0.05) | NS difference in beta diversity measures over time | ||
| Yu et al. 2020 | Sows | a-diversity measures: OTUs, Sobs, Chao1, Ace, H’A, Simpson, Coverage index | NS difference in alpha diversity measure between CG and experimental groups fed with stevia residue |
| Ref | Beneficial Effect | Harmful Effect | ||
|---|---|---|---|---|
| Beneficial Strains Growth | Suppression of Pathogens | Suppression of Beneficial Strains | Pathogen Growth | |
| [5] | Lactobacilli | S. aureus CG | ||
| [6] | A. muciniphila Bacteroides goldsteinii B. thetaiotaomicron | Clostridiales family XIII Lactobacillus intestinalis | Ruminococcaceae UCG 005 Bifidobacteriaceae | |
| [7] | E. coli HB101 | |||
| [8] | Lactobacillus reuteri (six strains) | |||
| [10] | S. aureus | B. thetaiotaomicron VPI-5482 | E. faecalis | |
| [14] | B. thetaiotaomicron | |||
| [15] | Lactobacillus mucosae SP1TA2 Bifidobacterium bifidum CCDM 559, Bifidobacterium breve CCDM 562, Bifidobacterium adolescentis AVNB3-P1 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasti, A.N.; Nikolaki, M.D.; Synodinou, K.D.; Katsas, K.N.; Petsis, K.; Lambrinou, S.; Pyrousis, I.A.; Triantafyllou, K. The Effects of Stevia Consumption on Gut Bacteria: Friend or Foe? Microorganisms 2022, 10, 744. https://doi.org/10.3390/microorganisms10040744
Kasti AN, Nikolaki MD, Synodinou KD, Katsas KN, Petsis K, Lambrinou S, Pyrousis IA, Triantafyllou K. The Effects of Stevia Consumption on Gut Bacteria: Friend or Foe? Microorganisms. 2022; 10(4):744. https://doi.org/10.3390/microorganisms10040744
Chicago/Turabian StyleKasti, Arezina N., Maroulla D. Nikolaki, Kalliopi D. Synodinou, Konstantinos N. Katsas, Konstantinos Petsis, Sophia Lambrinou, Ioannis A. Pyrousis, and Konstantinos Triantafyllou. 2022. "The Effects of Stevia Consumption on Gut Bacteria: Friend or Foe?" Microorganisms 10, no. 4: 744. https://doi.org/10.3390/microorganisms10040744
APA StyleKasti, A. N., Nikolaki, M. D., Synodinou, K. D., Katsas, K. N., Petsis, K., Lambrinou, S., Pyrousis, I. A., & Triantafyllou, K. (2022). The Effects of Stevia Consumption on Gut Bacteria: Friend or Foe? Microorganisms, 10(4), 744. https://doi.org/10.3390/microorganisms10040744











