KI and WU Polyomaviruses: Seroprevalence Study and DNA Prevalence in SARS-CoV-2 RNA Positive and Negative Respiratory Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Serum Samples for Seroepidemiology
2.2. Expression and Purification of Recombinant Proteins
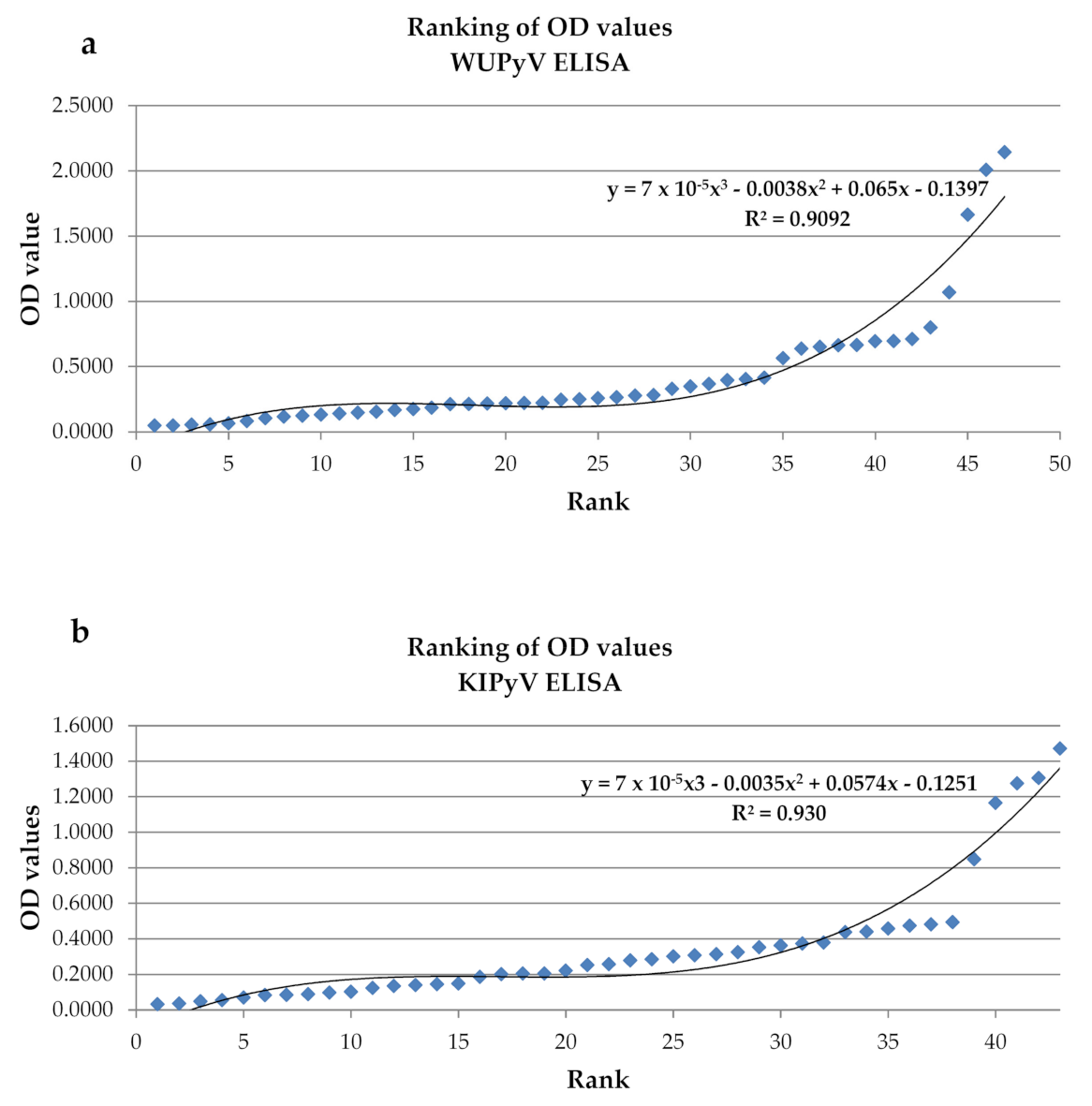
2.3. ELISA and Cut-Off Determination
2.4. Samples for the DNA Prevalence Study
2.5. Viral DNA Detection
2.6. Statistical Analysis
3. Results and Discussion
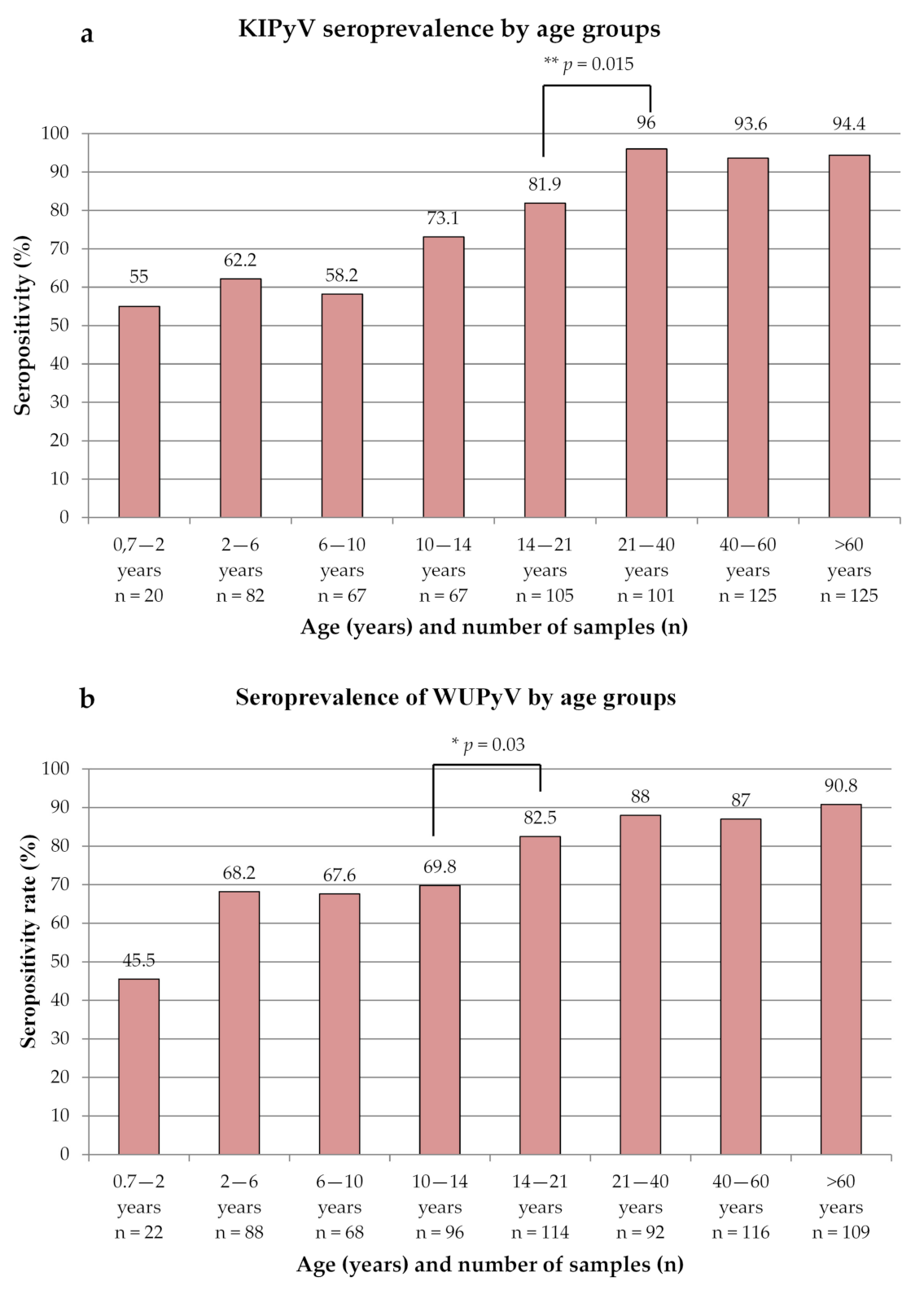
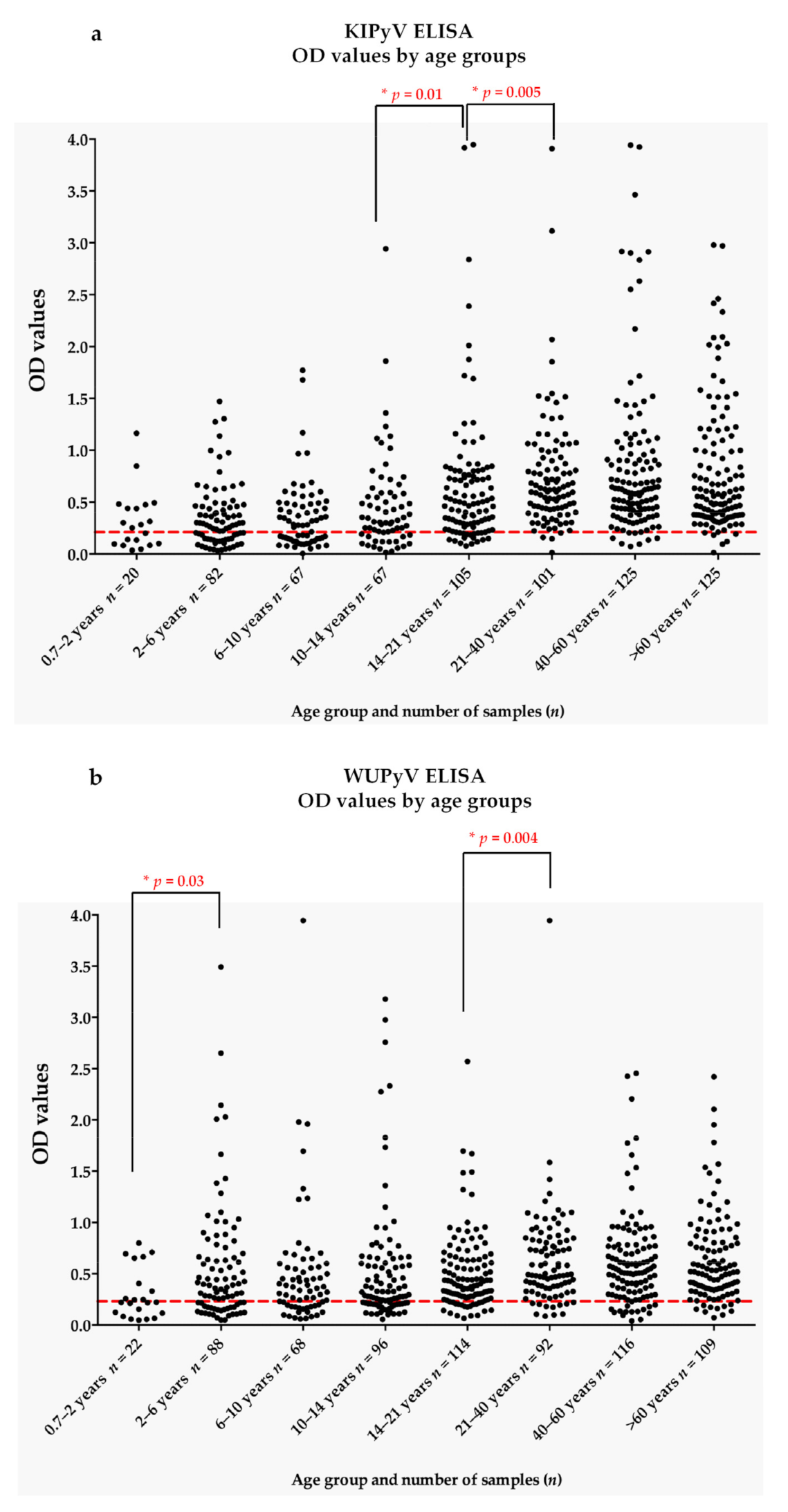
3.1. Seroprevalence of KIPyV and WUPyV
3.2. DNA Prevalence of KIPyV and WUPyV
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allander, T.; Andreasson, K.; Gupta, S.; Bjerkner, A.; Bogdanovic, G.; Persson, M.A.; Dalianis, T.; Ramqvist, T.; Andersson, B. Identification of a third human polyomavirus. J. Virol. 2007, 81, 4130–4136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaynor, A.M.; Nissen, M.D.; Whiley, D.M.; Mackay, I.M.; Lambert, S.B.; Wu, G.; Brennan, D.C.; Storch, G.A.; Sloots, T.P.; Wang, D. Identification of a novel polyomavirus from patients with acute respiratory tract infections. PLoS Pathog. 2007, 3, e64. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.L.; Le, B.M.; Wang, D. Serologic evidence of frequent human infection with WU and KI polyomaviruses. Emerg. Infect. Dis. 2009, 15, 1199–1205. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.J.; Paulson, K.G.; Wipf, G.C.; Miranda, D.; Madeleine, M.M.; Johnson, L.G.; Lemos, B.D.; Lee, S.; Warcola, A.H.; Iyer, J.G.; et al. Association of Merkel Cell Polyomavirus-Specific Antibodies With Merkel Cell Carcinoma. J. Natl. Cancer Inst. 2009, 101, 1510–1522. [Google Scholar] [CrossRef]
- Neske, F.; Prifert, C.; Scheiner, B.; Ewald, M.; Schubert, J.; Opitz, A.; Weissbrich, B. High prevalence of antibodies against polyomavirus WU, polyomavirus KI, and human bocavirus in German blood donors. BMC Infect. Dis. 2010, 10, 215. [Google Scholar] [CrossRef] [Green Version]
- Gossai, A.; Waterboer, T.; Nelson, H.H.; Michel, A.; Willhauck-Fleckenstein, M.; Farzan, S.F.; Hoen, A.G.; Christensen, B.C.; Kelsey, K.T.; Marsit, C.J.; et al. Seroepidemiology of Human Polyomaviruses in a US Population. Am. J. Epidemiol. 2016, 183, 61–69. [Google Scholar] [CrossRef]
- Kamminga, S.; van der Meijden, E.; Feltkamp, M.C.W.; Zaaijer, H.L. Seroprevalence of fourteen human polyomaviruses determined in blood donors. PLoS ONE 2018, 13, e0206273. [Google Scholar] [CrossRef] [Green Version]
- Sroller, V.; Hamsikova, E.; Ludvikova, V.; Musil, J.; Nemeckova, S.; Salakova, M. Seroprevalence rates of HPyV6, HPyV7, TSPyV, HPyV9, MWPyV and KIPyV polyomaviruses among the healthy blood donors. J. Med. Virol. 2016, 88, 1254–1261. [Google Scholar] [CrossRef]
- Babakir-Mina, M.; Ciccozzi, M.; Perno, C.F.; Ciotti, M. The human polyomaviruses KI and WU: Virological background and clinical implications. Apmis 2013, 121, 746–754. [Google Scholar] [CrossRef]
- Moens, U.; Krumbholz, A.; Ehlers, B.; Zell, R.; Johne, R.; Calvignac-Spencer, S.; Lauber, C. Biology, evolution, and medical importance of polyomaviruses: An update. Infect. Genet. Evol. 2017, 54, 18–38. [Google Scholar] [CrossRef]
- Shoji, K.; Koyama-Wakai, C.; Uda, K.; Fukuda, A.; Sakamoto, S.; Kasahara, M.; Miyairi, I. WU polyomavirus detection in a pediatric liver transplant recipient with interstitial pneumonitis. J. Infect. Chemother. 2021, 27, 530–532. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wei, T.L.; Huang, Y.M.; Guo, Q.; Xie, Z.P.; Song, J.D.; Chen, A.J.; Zheng, L.S. Isolation and characterization of WUPyV in polarized human airway epithelial cells. BMC Infect. Dis. 2020, 20, 488. [Google Scholar] [CrossRef] [PubMed]
- Caldeira, D.B.; Luna, L.K.D.; Watanabe, A.; Perosa, A.H.; Granato, C.; Bellei, N. The occurrence of polyomaviruses WUPyV and KIPyV among patients with severe respiratory infections. Braz. J. Microbiol. 2019, 50, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Siebrasse, E.A.; Pastrana, D.V.; Nguyen, N.L.; Wang, A.; Roth, M.J.; Holland, S.M.; Freeman, A.F.; McDyer, J.; Buck, C.B.; Wang, D. WU Polyomavirus in Respiratory Epithelial Cells from Lung Transplant Patient with Job Syndrome. Emerg. Infect. Dis. 2015, 21, 103–106. [Google Scholar] [CrossRef] [Green Version]
- Siebrasse, E.A.; Nguyen, N.L.; Willby, M.J.; Erdman, D.D.; Menegus, M.A.; Wang, D. Multiorgan WU Polyomavirus Infection in Bone Marrow Transplant Recipient. Emerg. Infect. Dis. 2016, 22, 24–31. [Google Scholar] [CrossRef] [Green Version]
- Siebrasse, E.A.; Nguyen, N.L.; Smith, C.; Simmonds, P.; Wang, D. Immunohistochemical detection of KI polyomavirus in lung and spleen. Virology 2014, 468, 178–184. [Google Scholar] [CrossRef] [Green Version]
- Zhu, T.; Lu, Q.B.; Zhang, S.Y.; Wo, Y.; Zhuang, L.; Zhang, P.H.; Zhang, X.A.; Wei, W.; Liu, W. Molecular epidemiology of WU polyomavirus in hospitalized children with acute respiratory tract infection in China. Future Microbiol. 2017, 12, 481–489. [Google Scholar] [CrossRef]
- Dehority, W.N.; Eickman, M.M.; Schwalm, K.C.; Gross, S.M.; Schroth, G.P.; Young, S.A.; Dinwiddie, D.L. Complete Genome Sequence of a KI Polyomavirus Isolated From an Otherwise Healthy Child With Severe Lower Respiratory Tract Infection. J. Med. Virol. 2017, 89, 926–930. [Google Scholar] [CrossRef]
- Musuuza, J.S.; Watson, L.; Parmasad, V.; Putman-Buehler, N.; Christensen, L.; Safdar, N. Prevalence and outcomes of co-infection and superinfection with SARS-CoV-2 and other pathogens: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0251170. [Google Scholar] [CrossRef]
- Prezioso, C.; Moens, U.; Oliveto, G.; Brazzini, G.; Piacentini, F.; Frasca, F.; Viscido, A.; Scordio, M.; Guerrizio, G.; Rodio, D.M.; et al. KI and WU Polyomavirus in Respiratory Samples of SARS-CoV-2 Infected Patients. Microorganisms 2021, 9, 1259. [Google Scholar] [CrossRef]
- Kean, J.M.; Rao, S.; Wang, M.; Garcea, R.L. Seroepidemiology of human polyomaviruses. PLoS Pathog. 2009, 5, e1000363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Touze, A.; Gaitan, J.; Arnold, F.; Cazal, R.; Fleury, M.J.; Combelas, N.; Sizaret, P.Y.; Guyetant, S.; Maruani, A.; Baay, M.; et al. Generation of Merkel Cell Polyomavirus (MCV)-Like Particles and Their Application to Detection of MCV Antibodies. J. Clin. Microbiol. 2010, 48, 1767–1770. [Google Scholar] [CrossRef] [Green Version]
- Csoma, E.; Lengyel, G.; Banyai, K.; Takacs, P.; Anosi, N.; Marton, S.; Matyus, M.; Paszti, E.; Gergely, L.; Szucs, A. Study of Karolinska Institutet and Washington University polyomaviruses in tonsil, adenoid, throat swab and middle ear fluid samples. Future Microbiol. 2018, 13, 1719–1730. [Google Scholar] [CrossRef] [PubMed]
- Kuypers, J.; Campbell, A.P.; Guthrie, K.A.; Wright, N.L.; Englund, J.A.; Corey, L.; Boeckh, M. WU and KI polyomaviruses in respiratory samples from allogeneic hematopoietic cell transplant recipients. Emerg. Infect. Dis. 2012, 18, 1580–1588. [Google Scholar] [CrossRef] [PubMed]
- Cason, C.; Monasta, L.; Zanotta, N.; Campisciano, G.; Maestri, I.; Tommasino, M.; Pawlita, M.; Villani, S.; Comar, M.; Delbue, S. Antibody response to polyomavirus primary infection: High seroprevalence of Merkel cell polyomavirus and lymphoid tissue involvement. J. Neurovirol. 2018, 24, 314–322. [Google Scholar] [CrossRef]
- Miller, M.A.; Weibel, C.; Kahn, J.S.; Andiman, W.A. Seroepidemiology of WU polyomavirus among children exposed perinatally to HIV-1. J. Med. Virol. 2012, 84, 188–193. [Google Scholar] [CrossRef]
- Debiaggi, M.; Canducci, F.; Brerra, R.; Sampaolo, M.; Marinozzi, M.C.; Parea, M.; Arghittu, M.; Alessandrino, E.P.; Nava, S.; Nucleo, E.; et al. Molecular epidemiology of KI and WU polyomaviruses in infants with acute respiratory disease and in adult hematopoietic stem cell transplant recipients. J. Med. Virol. 2010, 82, 153–156. [Google Scholar] [CrossRef]
- Mourez, T.; Bergeron, A.; Ribaud, P.; Scieux, C.; de Latour, R.P.; Tazi, A.; Socie, G.; Simon, F.; LeGoff, J. Polyomaviruses KI and WU in immunocompromised patients with respiratory disease. Emerg. Infect. Dis. 2009, 15, 107–109. [Google Scholar] [CrossRef]
- Venter, M.; Visser, A.; Lassauniere, R. Human polyomaviruses, WU and KI in HIV exposed children with acute lower respiratory tract infections in hospitals in South Africa. J. Clin. Virol. 2009, 44, 230–234. [Google Scholar] [CrossRef] [Green Version]
- Sharp, C.P.; Norja, P.; Anthony, I.; Bell, J.E.; Simmonds, P. Reactivation and mutation of newly discovered WU, KI, and Merkel cell carcinoma polyomaviruses in immunosuppressed individuals. J. Infect. Dis. 2009, 199, 398–404. [Google Scholar] [CrossRef] [Green Version]
- Csoma, E.; Sapy, T.; Meszaros, B.; Gergely, L. Novel human polyomaviruses in pregnancy: Higher prevalence of BKPyV, but no WUPyV, KIPyV and HPyV9. J. Clin. Virol. 2012, 55, 262–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Csoma, E.; Meszaros, B.; Asztalos, L.; Gergely, L. WU and KI polyomaviruses in respiratory, blood and urine samples from renal transplant patients. J. Clin. Virol. 2015, 64, 28–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Csoma, E.; Meszaros, B.; Asztalos, L.; Konya, J.; Gergely, L. Prevalence of WU and KI polyomaviruses in plasma, urine, and respiratory samples from renal transplant patients. J. Med. Virol. 2011, 83, 1275–1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haapanen, M.; Renko, M.; Artama, M.; Kuitunen, I. The impact of the lockdown and the re-opening of schools and day cares on the epidemiology of SARS-CoV-2 and other respiratory infections in children—A nationwide register study in Finland. Eclinicalmedicine 2021, 34, 100807. [Google Scholar] [CrossRef]
| Number of Samples | Age in Years, Min–Max (Median) | Female/Male | |
|---|---|---|---|
| SARS-CoV-2-positive patients | 680 | 0.1–94.2 (35.7) | 353/327 |
| SARS-CoV-2-negative patients | 350 | 0–94.1 (48.9) | 174/176 |
| Total | 1030 | 0–94.2 (38.1) | 527/503 |
| Patient Group | Age in Years, Min–Max (Median); Mean | Female/Male | Seropositivity | |
|---|---|---|---|---|
| KIPyV ELISA | Total (n = 692) | 0.7–92 (21.9); 31.3 | 343/349 | 82.1% |
| P (n = 568) | 0.7–92 (30.8); 34.9 * p = 0.005 | 284/284 | ||
| N (n = 124) | 0.7–81 (8.80); 15.1 *** p = 0.0001 | 59/65 | ||
| Children (n = 325) | 0.7–17.9 (9.60); 9.37 | 156/169 | 68.9% | |
| P (n = 224) | 0.7–17.9 (11.0); 10.1 | 111/113 | ||
| N (n = 101) | 0.7–19 (7.00); 7.74 ** p = 0.0002 | 45/56 | ||
| Adult (n = 367) | 18–92 (50.2); 50.8 | 187/180 | 93.7% | |
| P (n = 344) | 18–52 (50.4); 51.0 | 173/171 | ||
| N (n = 23) | 19–81 (43.8); 47.3 | 14/9 | ||
| WUPyV ELISA | Total (n = 705) | 0.7–92 (16.8); 28.7 | 353/352 | 79.1% |
| P (n = 558) | 0.7–92 (21.5); 31.5 * p = 0.02 | 298/260 | ||
| N (n = 147) | 0.7–81 (11.3); 18.0 *** p = 0.0001 | 55/92 | ||
| Children (n = 373) | 0.7–17.9 (10.4); 9.62 | 185/188 | 70.2% | |
| P (n = 262) | 0.7–17.9 (11.0); 10.1 | 143/119 | ||
| N (n = 111) | 0.7–17.9 (7.90); 8.45 *** p = 0.004 | 42/69 | ||
| Adult (n = 332) | 18–92 (50.1); 50.1 | 168/164 | 89.2% | |
| P (n = 296) | 18–92 (50.6); 50.4 | 155/141 | ||
| N (n = 36) | 21.7–81 (45.7); 47.5 | 13/23 |
| SARS-CoV-2-Positive Patients | SARS-CoV-2-Negative Patients | |||||
|---|---|---|---|---|---|---|
| Number of Samples | Age in Years, Min–Max (Median) | Female/Male | Number of Samples | Age in Years, Min–Max (Median) | Female/Male | |
| KIPyV DNA positive | 2 | 26.6 and 72.8 | 1/1 | 3 | 18.3; 50.1 and 72.8 | 3/0 |
| KIPyV DNA negative | 678 | 0.1–94.2 (35.7) | 352/326 | 347 | 0–94.1 (48.8) | 171/176 |
| WUPyV DNA positive | 1 | 36.2 | 1/0 | 1 | 3.7 | 1/0 |
| WUPyV DNA negative | 679 | 0.1–94.2 (35.6) | 352/327 | 349 | 0–94.1 (49) | 173/176 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katona, M.; Jeles, K.; Kovács, R.; Csoma, E. KI and WU Polyomaviruses: Seroprevalence Study and DNA Prevalence in SARS-CoV-2 RNA Positive and Negative Respiratory Samples. Microorganisms 2022, 10, 752. https://doi.org/10.3390/microorganisms10040752
Katona M, Jeles K, Kovács R, Csoma E. KI and WU Polyomaviruses: Seroprevalence Study and DNA Prevalence in SARS-CoV-2 RNA Positive and Negative Respiratory Samples. Microorganisms. 2022; 10(4):752. https://doi.org/10.3390/microorganisms10040752
Chicago/Turabian StyleKatona, Melinda, Krisztina Jeles, Renátó Kovács, and Eszter Csoma. 2022. "KI and WU Polyomaviruses: Seroprevalence Study and DNA Prevalence in SARS-CoV-2 RNA Positive and Negative Respiratory Samples" Microorganisms 10, no. 4: 752. https://doi.org/10.3390/microorganisms10040752
APA StyleKatona, M., Jeles, K., Kovács, R., & Csoma, E. (2022). KI and WU Polyomaviruses: Seroprevalence Study and DNA Prevalence in SARS-CoV-2 RNA Positive and Negative Respiratory Samples. Microorganisms, 10(4), 752. https://doi.org/10.3390/microorganisms10040752







