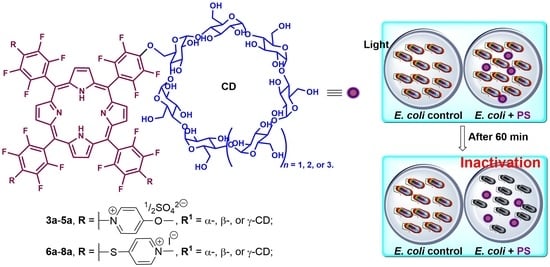
The Antimicrobial Photoinactivation Effect on Escherichia coli through the Action of Inverted Cationic Porphyrin–Cyclodextrin Conjugates
Abstract
:1. Introduction
2. Experimental Procedure
2.1. General
2.2. Synthesis of 5-(pentafluorophenyl)-10,15,20-tris[2,3,5,6-tetrafluoro-4-(4-oxopyridin-1(4H)-yl)phenyl]porphyrin, Por 1
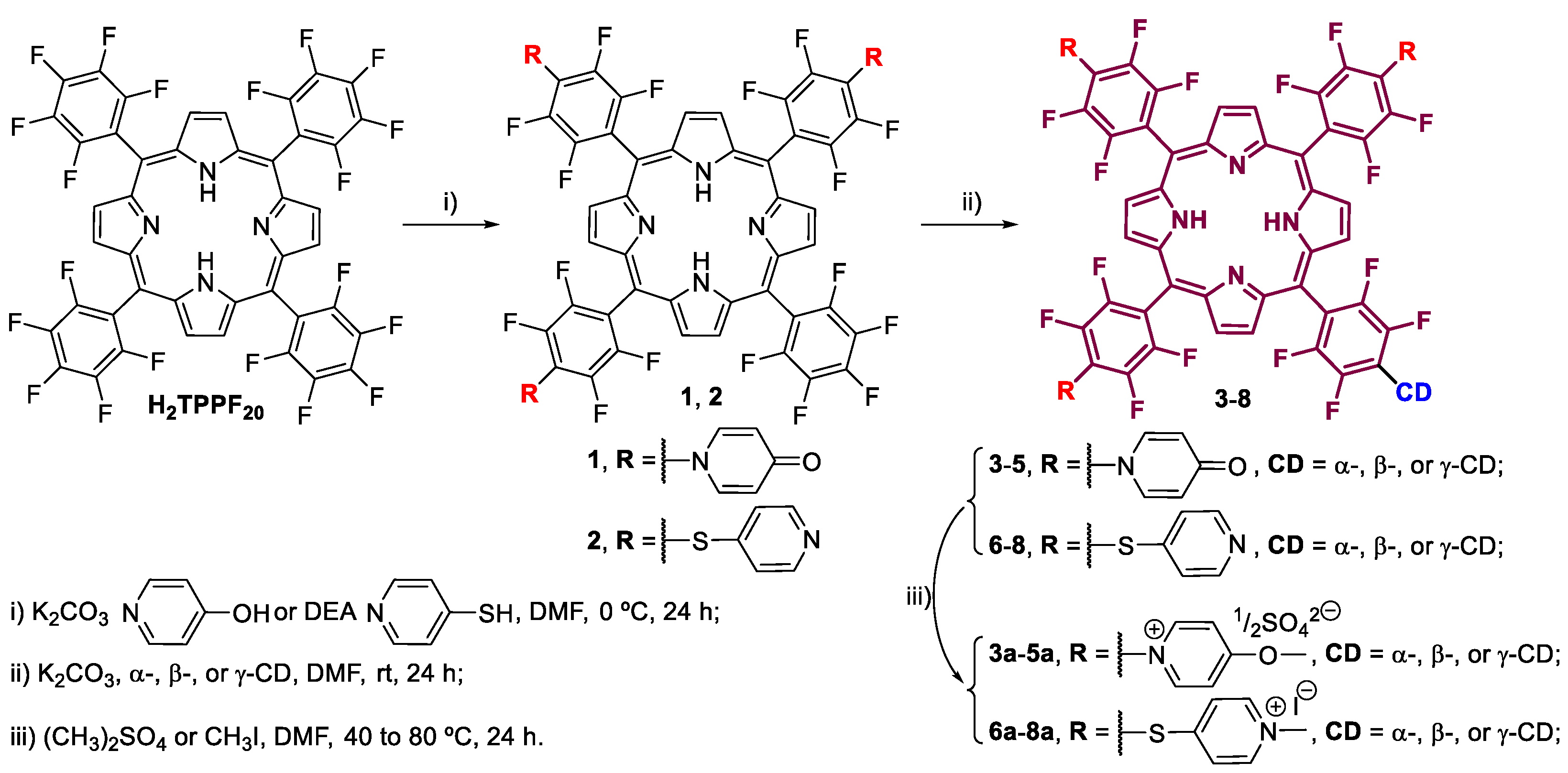
2.3. Synthesis of Por–CD Conjugates, Pors 3–5
2.4. Synthesis of Cationic Por–CD Conjugates, Pors 3a–5a
2.5. Synthesis of Por–CD Conjugate, Por 7
2.6. Synthesis of Cationic Por–CD Conjugate, Por 7a
2.7. Photostability Assays
Light Conditions
2.8. Singlet Oxygen Generation
2.9. Bacterial Strain and Growth Conditions
2.10. Photodynamic Inactivation Studies
2.11. Statistical Analysis
3. Results and Discussion
3.1. Synthesis, Photophysical, and Photochemical Characterization of the Porphyrin Derivatives
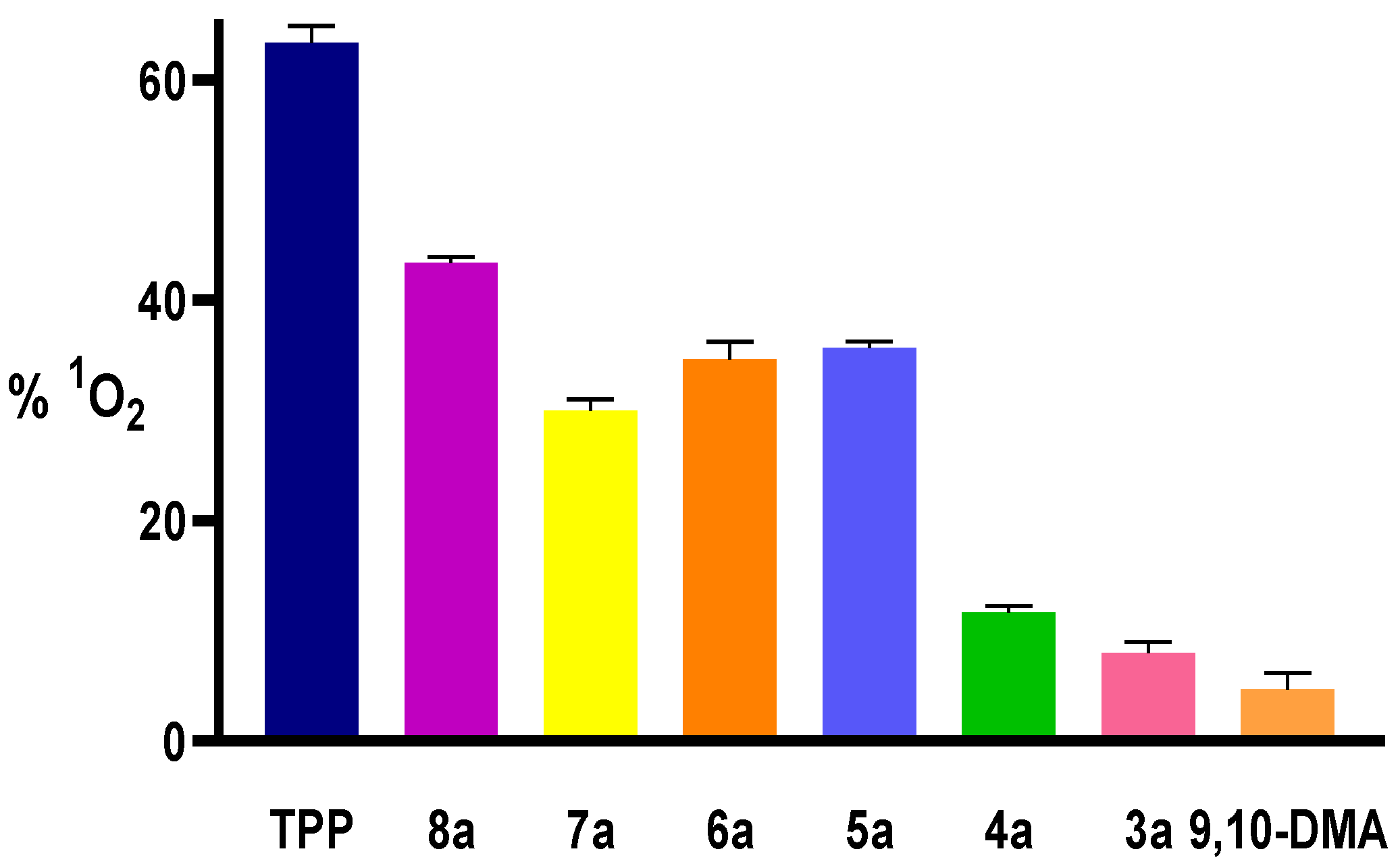
3.1.1. Photophysical and Photochemical Studies
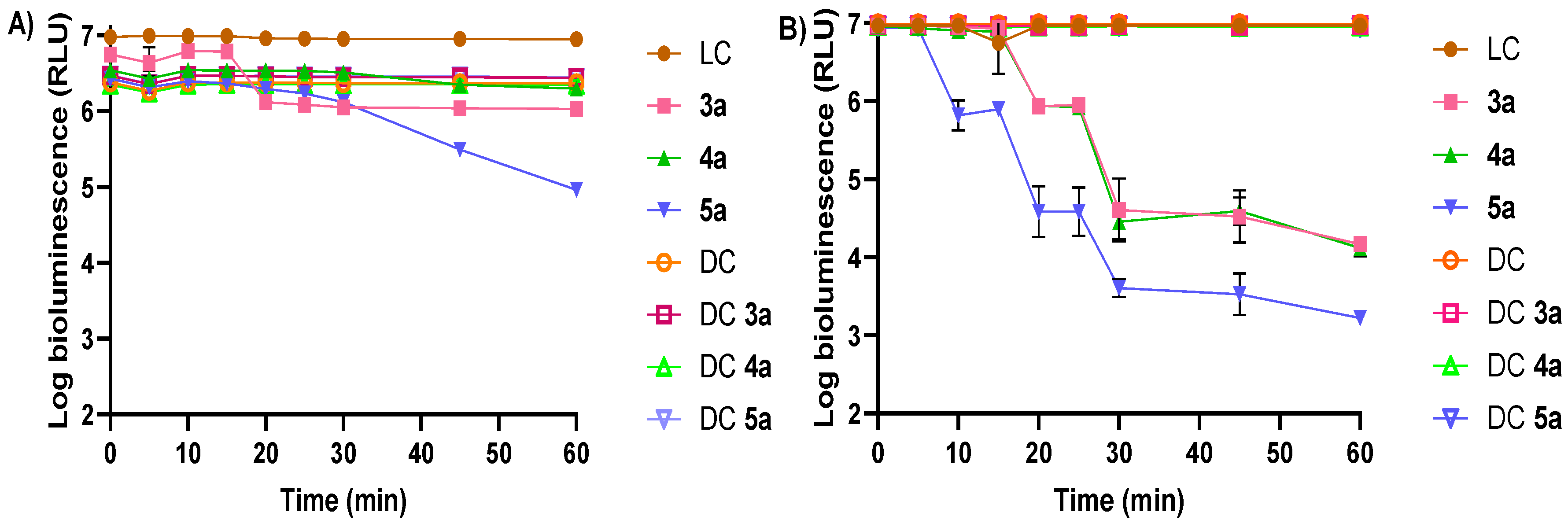
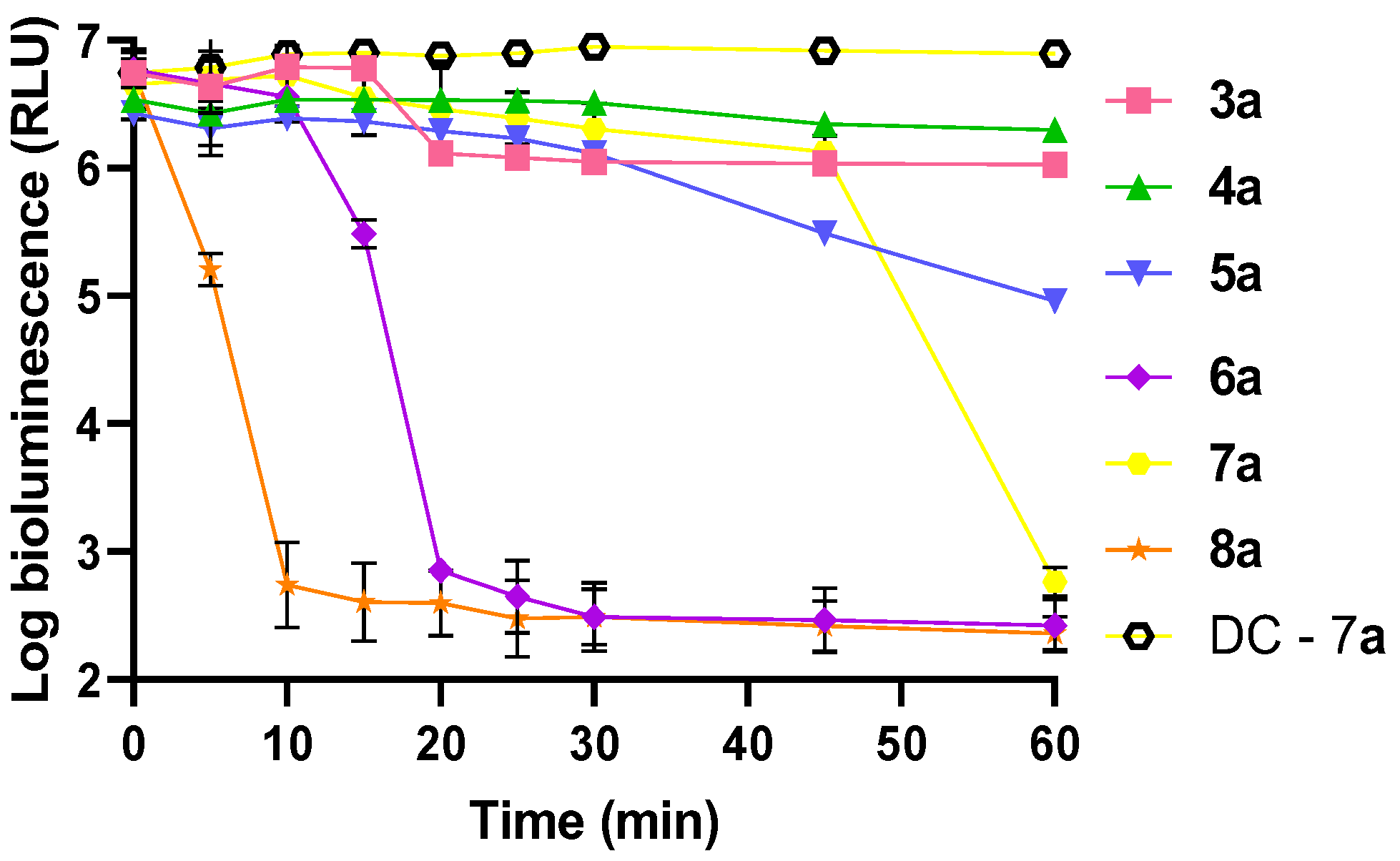
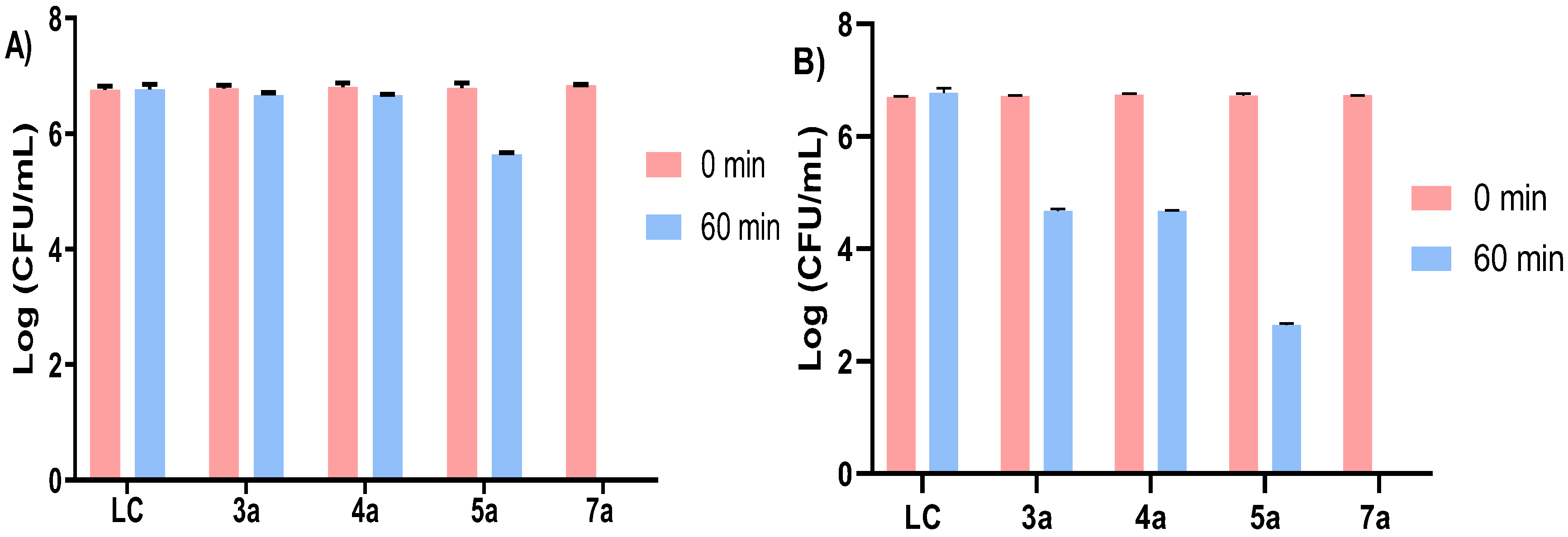
3.1.2. Photodynamic Inactivation of Escherichia coli
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lloyd-Price, J.; Abu-Ali, G.; Huttenhower, C. The healthy human microbiome. Genome Med. 2016, 8, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Percival, S.L.; Williams, D.W. Chapter Six—Escherichia coli. In Microbiology of Waterborne Diseases, 2nd ed.; Percival, S.L., Yates, M.V., Williams, D.W., Chalmers, R.M., Gray, N.F., Eds.; Academic Press: London, UK, 2014; pp. 89–117. [Google Scholar]
- Wainwright, M.; Maisch, T.; Nonell, S.; Plaetzer, K.; Almeida, A.; Tegos, G.P.; Hamblin, M.R. Photoantimicrobials—Are we afraid of the light? Lancet Infect. Dis. 2017, 17, e49–e55. [Google Scholar] [CrossRef]
- Calmeiro, J.M.D.; Dias, C.J.; Ramos, C.I.V.; Almeida, A.; Tome, J.P.C.; Faustino, M.A.F.; Lourenco, L.M.O. Comparative photodynamic inactivation of bioluminescent E. coli by pyridinium and inverted pyridinium chlorins. Dye. Pigm. 2020, 173, 107410. [Google Scholar] [CrossRef]
- Lourenco, L.M.O.; Rocha, D.M.G.C.; Ramos, C.I.V.; Gomes, M.C.; Almeida, A.; Faustino, M.A.F.; Almeida Paz, F.A.; Neves, M.G.P.M.S.; Cunha, Â.; Tomé, J.P.C. Photoinactivation of planktonic and biofilm forms of Escherichia coli through the Eaction of cationic zinc (II) phthalocyanines. ChemPhotoChem 2019, 3, 251–260. [Google Scholar] [CrossRef]
- Calmeiro, J.M.D.; Gamelas, S.R.D.; Gomes, A.T.P.C.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Almeida, A.; Tome, J.P.C.; Lourenco, L.M.O. Versatile thiopyridyl/pyridinone porphyrins combined with potassium iodide and thiopyridinium/methoxypyridinium porphyrins on E. coli Photoinactivation. Dye. Pigm. 2020, 181, 108476. [Google Scholar] [CrossRef]
- Heffron, J.; Bork, M.; Mayer, B.K.; Skwor, T. A Comparison of Porphyrin Photosensitizers in Photodynamic Inactivation of RNA and DNA Bacteriophages. Viruses 2021, 13, 530. [Google Scholar] [CrossRef]
- Garcia, M.; David, B.; Sierra-Garcia, I.N.; Faustino, M.A.F.; Alves, A.; Esteves, A.C.; Cunha, A. Photodynamic inactivation of Lasiodiplodia theobromae: Lighting the way towards an environmentally friendly phytosanitary treatment. Biol. Lett. 2021, 17, 20200820. [Google Scholar] [CrossRef]
- Souza, T.H.; Andrade, C.G.; Cabral, F.V.; Sarmento-Neto, J.F.; Rebouças, J.S.; Santos, B.S.; Ribeiro, M.S.; Figueiredo, R.C.; Fontes, A. Efficient photodynamic inactivation of Leishmania parasites mediated by lipophilic water-soluble Zn (II) porphyrin ZnTnHex-2-PyP4+. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2021, 1865, 129897. [Google Scholar] [CrossRef]
- Foggiato, A.A.; Silva, D.F.; Castro, R.C.F.R. Effect of photodynamic therapy on surface decontamination in clinical orthodontic instruments. Photodiagnosis Photodyn. Ther. 2018, 24, 123–128. [Google Scholar] [CrossRef]
- Bartolomeu, M.; Reis, S.; Fontes, M.; Neves, M.G.P.M.S.P.; Faustino, M.A.F.; Almeida, A. Photodynamic action against wastewater microorganisms and chemical pollutants: An effective approach with low environmental impact. Water 2017, 9, 630. [Google Scholar] [CrossRef] [Green Version]
- Cossu, M.; Ledda, L.; Cossu, A. Emerging trends in the photodynamic inactivation (PDI) applied to the food decontamination. Food Res. Int. 2021, 144, 110358. [Google Scholar] [CrossRef] [PubMed]
- Sawada, T.; Yasukawa, T.; Imaizumi, H.; Matsubara, H.; Kimura, K.; Terasaki, H.; Ishikawa, H.; Murakami, T.; Takeuchi, M.; Mitamura, Y. Ten-year changes in visual acuity at baseline and at 2 years after treatment in a Japanese population with age-related macular degeneration. Graefe’s Arch. Clin. Exp. Ophthalmol. 2021, 259, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Babu, B.; Sindelo, A.; Mack, J.; Nyokong, T. Thien-2-yl substituted chlorins as photosensitizers for photodynamic therapy and photodynamic antimicrobial chemotherapy. Dye. Pigm. 2021, 185, 108886. [Google Scholar] [CrossRef]
- Silva, J.N.; Silva, A.M.G.; Tomé, J.P.C.; Ribeiro, A.O.; Domingues, M.R.M.; Cavaleiro, J.A.S.; Silva, A.M.S.; Neves, M.G.P.M.S.P.; Tomé, A.C.; Serra, O.A.; et al. Photophysical properties of a photocytotoxic fluorinated chlorin conjugated to four β-cyclodextrins. Photochem. Photobiol. Sci. 2008, 7, 834–843. [Google Scholar] [CrossRef]
- Reynoso, E.; Ferreyra, D.D.; Durantini, E.N.; Spesia, M.B. Photodynamic inactivation to prevent and disrupt Staphylococcus aureus biofilm under different media conditions. Photodermatol. Photoimmunol. Photomed. 2019, 35, 322–331. [Google Scholar] [CrossRef]
- Ribeiro, C.P.S.; Gamelas, S.R.D.; Faustino, M.A.F.; Gomes, A.T.P.C.; Tomé, J.P.C.; Almeida, A.; Lourenco, L.M.O. Unsymmetrical cationic porphyrin-cyclodextrin bioconjugates for photoinactivation of Escherichia coli. Photodiagnosis Photodyn. Ther. 2020, 31, 101788. [Google Scholar] [CrossRef]
- Gonçalves, P.J.; Bezzerra, F.C.; Teles, A.V.; Menezes, L.B.; Alves, K.M.; Alonso, L.; Alonso, A.; Andrade, M.A.; Borissevitch, I.E.; Souza, G.R. Photoinactivation of Salmonella enterica (serovar Typhimurium) by tetra-cationic porphyrins containing peripheral [Ru (bpy) 2Cl]+ units. J. Photochem. Photobiol. A Chem. 2020, 391, 112375. [Google Scholar] [CrossRef]
- Sagrillo, F.S.; Dias, C.; Gomes, A.T.P.C.; Faustino, M.A.F.; Almeida, A.; de Souza, A.G.; Costa, A.R.P.; Boechat, F.d.C.S.; de Souza, M.C.B.V.; Neves, M.G.P.M.S.; et al. Synthesis and photodynamic effects of new porphyrin/4-oxoquinoline derivatives in the inactivation of S. aureus. Photochem. Photobiol. Sci. 2019, 18, 1910–1922. [Google Scholar] [CrossRef]
- Revuelta-Maza, M.Á.; Gonzalez-Jimenez, P.; Hally, C.; Agut, M.; Nonell, S.; de la Torre, G.; Torres, T. Fluorine-substituted tetracationic ABAB-phthalocyanines for efficient photodynamic inactivation of Gram-positive and Gram-negative bacteria. Eur. J. Med. Chem. 2020, 187, 111957. [Google Scholar] [CrossRef]
- Ribeiro, C.P.S.; Lourenco, L.M.O. Overview of cationic phthalocyanines for effective photoinactivation of pathogenic microorganisms. J. Photochem. Photobiol. C Photochem. Rev. 2021, 48, 100422. [Google Scholar] [CrossRef]
- Güzel, E.; Medina, D.-P.; Medel, M.; Kandaz, M.; Torres, T.; Morgade, M.S.R. A versatile, divergent route for the synthesis of ABAC tetraazaporphyrins, molecularly engineered, push-pull phthalocyanine-type dyes. J. Mater. Chem. C 2021, 9, 10802–10810. [Google Scholar] [CrossRef]
- Gamelas, S.R.D.; Gomes, A.T.P.C.; Faustino, M.A.F.; Tomé, A.C.; Tomé, J.C.P.; Almeida, A.; Lourenco, L.M.O. Photoinactivation of Escherichia coli with Water-Soluble Ammonium-Substituted Phthalocyanines. ACS Appl. Bio Mater. 2020, 3, 4044–4051. [Google Scholar] [CrossRef] [PubMed]
- Marciel, L.; Teles, L.; Moreira, B.; Pacheco, M.; Lourenco, L.M.O.; Neves, M.G.P.M.S.; Tomé, J.P.C.; Faustino, M.A.F.; Almeida, A. An effective and potentially safe blood disinfection protocol using tetrapyrrolic photosensitizers. Future Med. Chem. 2017, 9, 365–379. [Google Scholar] [CrossRef]
- Ramos, C.I.V.; Almeida, S.P.; Lourenco, L.M.O.; Pereira, P.M.R.; Fernandes, R.; Faustino, M.A.F.; Tomé, J.P.C.; Carvalho, J.; Cruz, C.; Neves, M.G.P.M.S. Multicharged phthalocyanines as selective ligands for G-quadruplex DNA structures. Molecules 2019, 24, 733. [Google Scholar] [CrossRef] [Green Version]
- Sridhar, K.; Inbaraj, B.S.; Chen, B.-H. Recent developments on production, purification and biological activity of marine peptides. Food Res. Int. 2021, 147, 110468. [Google Scholar] [CrossRef] [PubMed]
- Alves, E.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Cunha, Â.; Nadais, H.; Almeida, A. Potential applications of porphyrins in photodynamic inactivation beyond the medical scope. J. Photochem. Photobiol. C: Photochem. Rev. 2015, 22, 34–57. [Google Scholar] [CrossRef] [Green Version]
- Pereira, M.A.; Faustino, M.A.F.; Tomé, J.P.C.; Neves, M.G.P.M.S.; Tome, A.C.; Cavaleiro, J.A.S.; Cunha, A.; Almeida, A. Influence of external bacterial structures on the efficiency of photodynamic inactivation by a cationic porphyrin. Photochem. Photobiol. Sci. 2014, 13, 680–690. [Google Scholar] [CrossRef]
- Hamblin, M.R. Antimicrobial photodynamic inactivation: A bright new technique to kill resistant microbes. Curr. Opin. Microbiol. 2016, 33, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Simões, C.; Gomes, M.C.; Neves, M.G.P.M.S.; Cunha, Â.; Tomé, J.P.C.; Tomé, A.C.; Cavaleiro, J.A.S.; Almeida, A.; Faustino, M.A.F. Photodynamic inactivation of Escherichia coli with cationic meso-tetraarylporphyrins—The charge number and charge distribution effects. Catal. Today 2016, 266, 197–204. [Google Scholar] [CrossRef]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef] [Green Version]
- Mesquita, M.Q.; Dias, C.J.; Neves, M.G.P.M.S.; Almeida, A.; Faustino, M.A.F. Revisiting current photoactive materials for antimicrobial photodynamic therapy. Molecules 2018, 23, 2424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, C.I.M.; Rodríguez-Pérez, L.; Gonçalves, G.; Pinto, S.N.; Melle-Franco, M.; Marques, P.A.A.P.; Faustino, M.A.F.; Herranz, M.Á.; Martin, N.; Neves, M.G.P.M.S.P.; et al. Novel hybrids based on graphene quantum dots covalently linked to glycol corroles for multiphoton bioimaging. Carbon 2020, 166, 164–174. [Google Scholar] [CrossRef]
- Gomes, A.T.P.C.; Faustino, M.A.F.; Neves, M.G.P.M.S.P.; Ferreira, V.F.; Juarranz, A.; Cavaleiro, J.A.S.; Sanz-Rodríguez, F. Photodynamic effect of glycochlorin conjugates in human cancer epithelial cells. RSC Adv. 2015, 5, 33496–33502. [Google Scholar] [CrossRef]
- Silva, S.; Pereira, P.M.R.; Silva, P.; Paz, F.A.A.; Faustino, M.A.F.; Cavaleiro, J.A.S.; Tomé, J.P.C. Porphyrin and phthalocyanine glycodendritic conjugates: Synthesis, photophysical and photochemical properties. Chem. Commun. 2012, 48, 3608–3610. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, E.M. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Carneiro, S.B.; Costa Duarte, F.Í.; Heimfarth, L.; Siqueira Quintans, J.d.S.; Quintans-Júnior, L.J.; Veiga Júnior, V.F.d.; Neves de Lima, Á.A. Cyclodextrin–drug inclusion complexes: In vivo and in vitro approaches. Int. J. Mol. Sci. 2019, 20, 642. [Google Scholar] [CrossRef] [Green Version]
- Barata, J.F.; Zamarron, A.; Neves, M.G.P.M.S.P.; Faustino, M.A.F.; Tomé, A.C.; Cavaleiro, J.A.S.; Röder, B.; Juarranz, Á.; Sanz-Rodríguez, F. Photodynamic effects induced by meso-tris (pentafluorophenyl) corrole and its cyclodextrin conjugates on cytoskeletal components of HeLa cells. Eur. J. Med. Chem. 2015, 92, 135–144. [Google Scholar] [CrossRef]
- Karginov, V.A. Cyclodextrin derivatives as anti-infectives. Curr. Opin. Pharmacol. 2013, 13, 717–725. [Google Scholar] [CrossRef] [Green Version]
- Agnes, M.; Thanassoulas, A.; Stavropoulos, P.; Nounesis, G.; Miliotis, G.; Miriagou, V.; Athanasiou, E.; Benkovics, G.; Malanga, M.; Yannakopoulou, K. Designed positively charged cyclodextrin hosts with enhanced binding of penicillins as carriers for the delivery of antibiotics: The case of oxacillin. Int. J. Pharm. 2017, 531, 480–491. [Google Scholar] [CrossRef]
- Castriciano, M.A.; Zagami, R.; Casaletto, M.P.; Martel, B.; Trapani, M.; Romeo, A.; Villari, V.; Sciortino, M.T.; Grasso, L.; Guglielmino, S. Poly (carboxylic acid)-cyclodextrin/anionic porphyrin finished fabrics as photosensitizer releasers for antimicrobial photodynamic therapy. Biomacromolecules 2017, 18, 1134–1144. [Google Scholar] [CrossRef]
- Armarego, W.L.; Chai, C.L.L. Purification of Laboratory Chemicals; Armarego, W.L.F., Chai, C.L.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Taniguchi, M.; Lindsey, J.S.; Bocian, D.F.; Holten, D. Comprehensive review of photophysical parameters (ε, Φf, τs) of tetraphenylporphyrin (H2TPP) and zinc tetraphenylporphyrin (ZnTPP)—Critical benchmark molecules in photochemistry and photosynthesis. J. Photochem. Photobiol. C Photochem. Rev. 2021, 46, 100401. [Google Scholar] [CrossRef]
- Ferreira, G.C.; Kadish, K.M.; Smith, K.M.; Guilard, R. The Handbook of Porphyrin Science; World Scientific: Singapore, 2013; Volume 27. [Google Scholar]
- Mahajan, P.G.; Dige, N.C.; Vanjare, B.D.; Kim, C.-H.; Seo, S.-Y.; Lee, K.H. Design and Synthesis of New Porphyrin Analogues as Potent Photosensitizers for Photodynamic Therapy: Spectroscopic Approach. J. Fluoresc. 2020, 30, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; Fernandes, E.; Lima, J.L.F.C. Fluorescence probes used for detection of reactive oxygen species. J. Biochem. Biophys. Methods 2005, 65, 45–80. [Google Scholar] [CrossRef] [PubMed]
- Castro-Olivares, R.; Günther, G.; Zanocco, A.L.; Lemp, E. Linear free energy relationship analysis of solvent effect on singlet oxygen reactions with mono and disubstituted anthracene derivatives. J. Photochem. Photobiol. A Chem. 2009, 207, 160–166. [Google Scholar] [CrossRef]
- Prostak, A.; Mark, H.B., Jr.; Hansen, W.N. Simultaneous electrochemical and internal-reflection spectrometric measurements using gold-film electrodes. J. Phys. Chem. 1968, 72, 2576–2582. [Google Scholar] [CrossRef]
- Vieira, C.; Gomes, A.T.P.C.; Mesquita, M.Q.; Moura, N.M.M.; Neves, M.G.P.M.S.P.; Faustino, M.A.F.; Almeida, A. An insight into the potentiation effect of potassium iodide on aPDT efficacy. Front. Microbiol. 2018, 9, 2665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, D.C.; Pais, V.F.; Silva, A.M.; Cavaleiro, J.A.; Pischel, U.; Tome, J.P.C. Cationic porphyrins with inverted pyridinium groups and their fluorescence properties. Tetrahedron Lett. 2014, 55, 4156–4159. [Google Scholar] [CrossRef]
- Zidovetzki, R.; Levitan, I. Use of cyclodextrins to manipulate plasma membrane cholesterol content: Evidence, misconceptions and control strategies. Biochim. Biophys. Acta (BBA)-Biomembr. 2007, 1768, 1311–1324. [Google Scholar] [CrossRef] [Green Version]
- Hedges, A. Cyclodextrins: Properties and applications. In Starch; Elsevier: Amsterdam, The Netherlands, 2009; pp. 833–851. [Google Scholar]
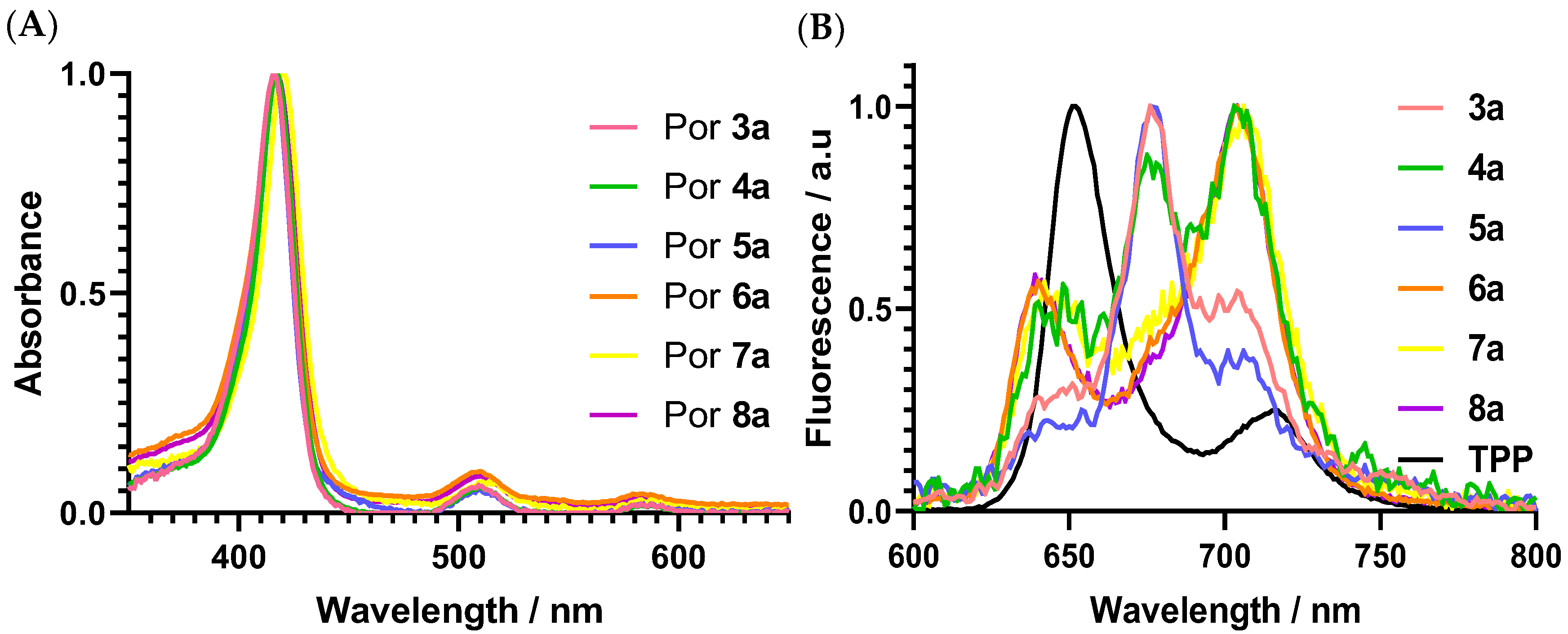
| Pors | Soret Band (nm) | log ε | Q Bands (nm) | log ε | λemiss. | ΦF a |
|---|---|---|---|---|---|---|
| 3a | 416 | 4.04 | 509 | 1.84 | 676 | 0.01 |
| 583 | 1.35 | |||||
| 4a | 417 | 3.37 | 509 | 2.14 | 704 | <0.01 |
| 583 | 1.61 | |||||
| 5a | 417 | 3.10 | 510 | 1.82 | 677 | <0.01 |
| 583 | 1.43 | |||||
| 6ab | 417 | 3.49 | 510 | 3.43 | 704 | <0.01 |
| 583 | 3.13 | |||||
| 7a | 420 | 3.09 | 514 | 1.95 | 706 | 0.01 |
| 588 | 1.62 | |||||
| 8ab | 420 | 3.78 | 510 | 2.70 | 704 | <0.01 |
| 583 | 2.30 |
| Pors | 3a | 4a | 5a | 6a | 7a | 8a |
|---|---|---|---|---|---|---|
| % Abs decay a | 6 | 8 | 6 | 14 | 11 | 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, C.P.S.; Faustino, M.A.F.; Almeida, A.; Lourenço, L.M.O. The Antimicrobial Photoinactivation Effect on Escherichia coli through the Action of Inverted Cationic Porphyrin–Cyclodextrin Conjugates. Microorganisms 2022, 10, 718. https://doi.org/10.3390/microorganisms10040718
Ribeiro CPS, Faustino MAF, Almeida A, Lourenço LMO. The Antimicrobial Photoinactivation Effect on Escherichia coli through the Action of Inverted Cationic Porphyrin–Cyclodextrin Conjugates. Microorganisms. 2022; 10(4):718. https://doi.org/10.3390/microorganisms10040718
Chicago/Turabian StyleRibeiro, Cláudia P. S., Maria A. F. Faustino, Adelaide Almeida, and Leandro M. O. Lourenço. 2022. "The Antimicrobial Photoinactivation Effect on Escherichia coli through the Action of Inverted Cationic Porphyrin–Cyclodextrin Conjugates" Microorganisms 10, no. 4: 718. https://doi.org/10.3390/microorganisms10040718
APA StyleRibeiro, C. P. S., Faustino, M. A. F., Almeida, A., & Lourenço, L. M. O. (2022). The Antimicrobial Photoinactivation Effect on Escherichia coli through the Action of Inverted Cationic Porphyrin–Cyclodextrin Conjugates. Microorganisms, 10(4), 718. https://doi.org/10.3390/microorganisms10040718