Potential of Anti-CMV Immunoglobulin Cytotect CP® In Vitro and Ex Vivo in a First-Trimester Placenta Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell and Viruses
2.2. Antiviral Compounds
2.3. In Vitro Assays
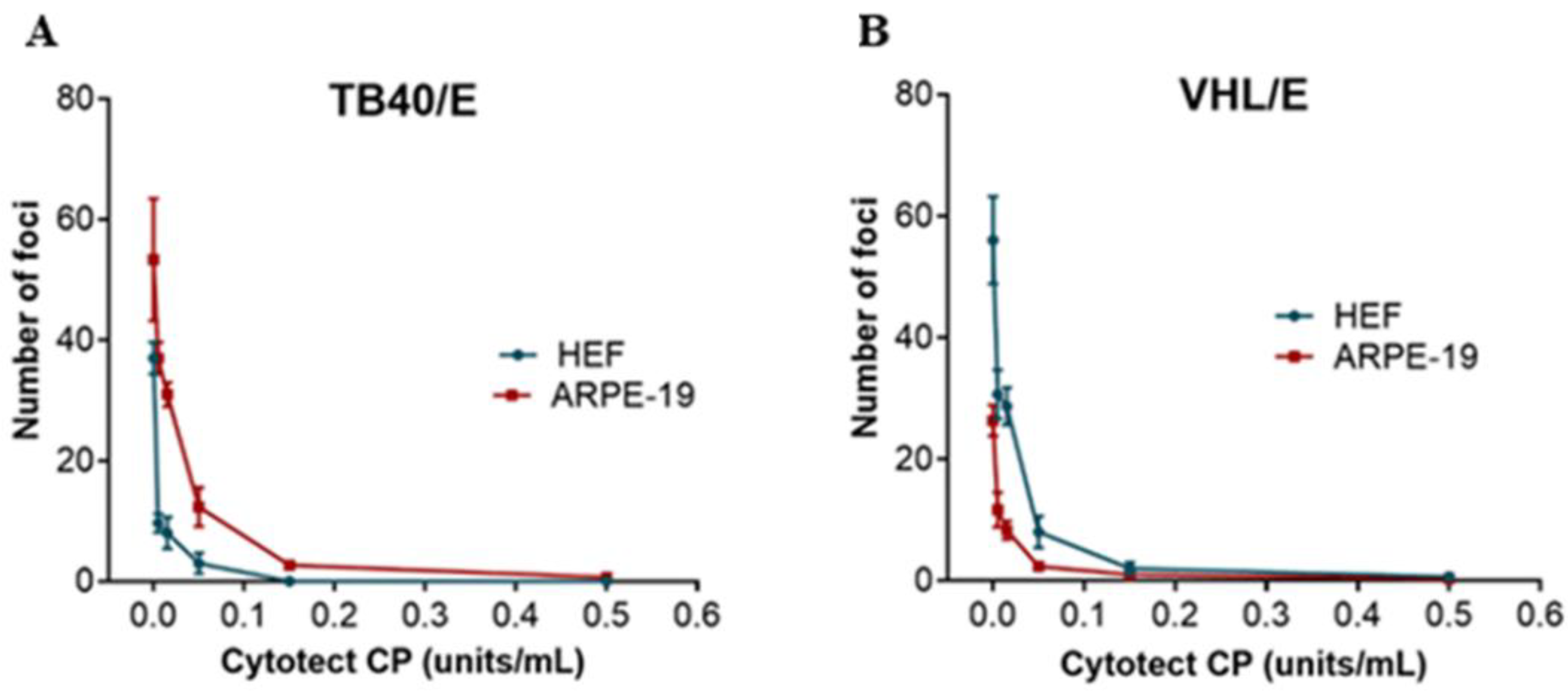
2.3.1. Neutralization Assays
2.3.2. Cytotoxicity Assays
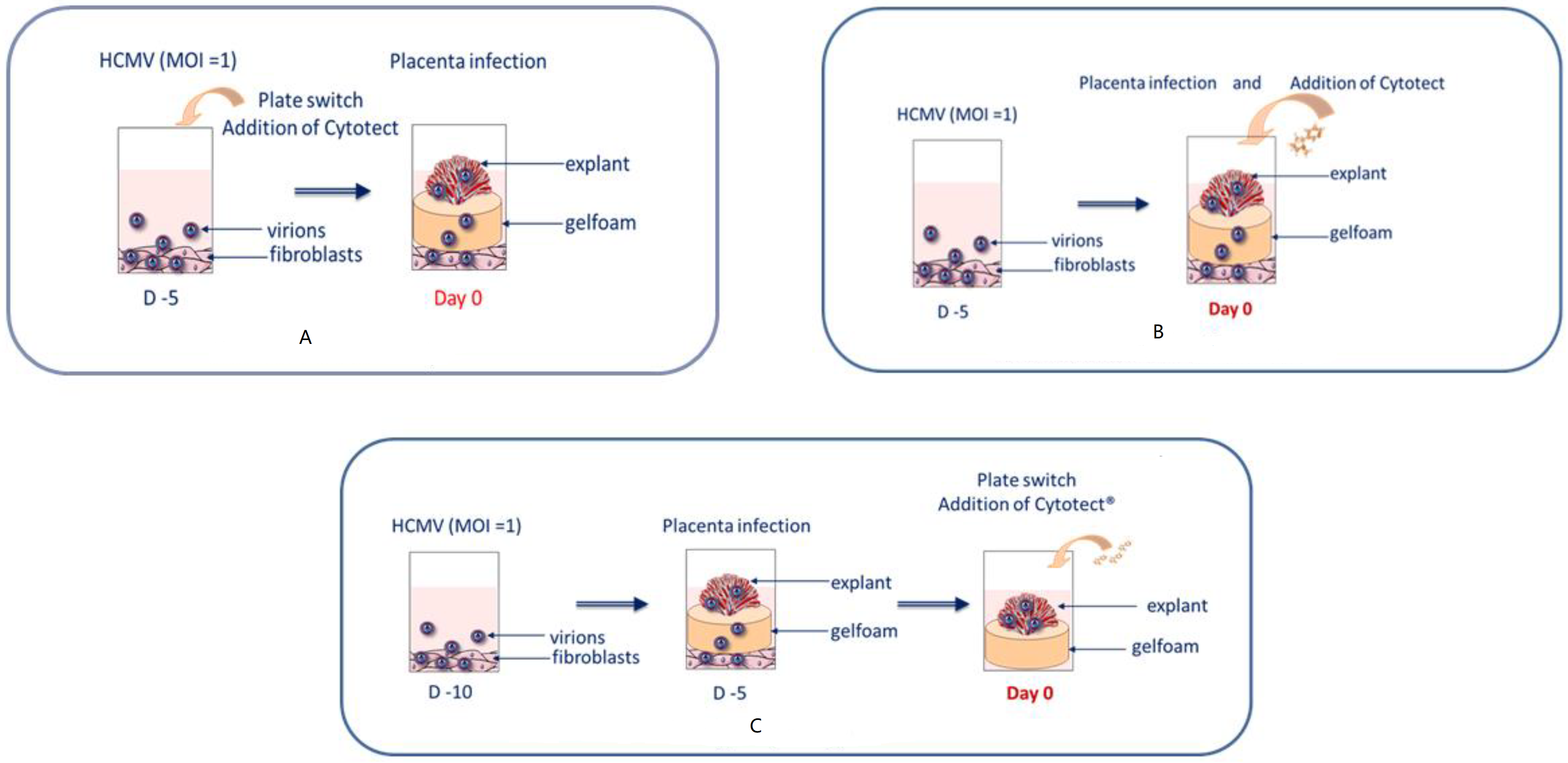
2.4. Placental Villi Explants
2.5. Ex Vivo Assays
2.5.1. Viral Load in Villi Explants
2.5.2. β-hCG Dosage for Cytotoxicity in Villi Explants
2.6. Statistical Analyses
3. Results
3.1. In Vitro Assays
3.2. Ex Vivo Assays
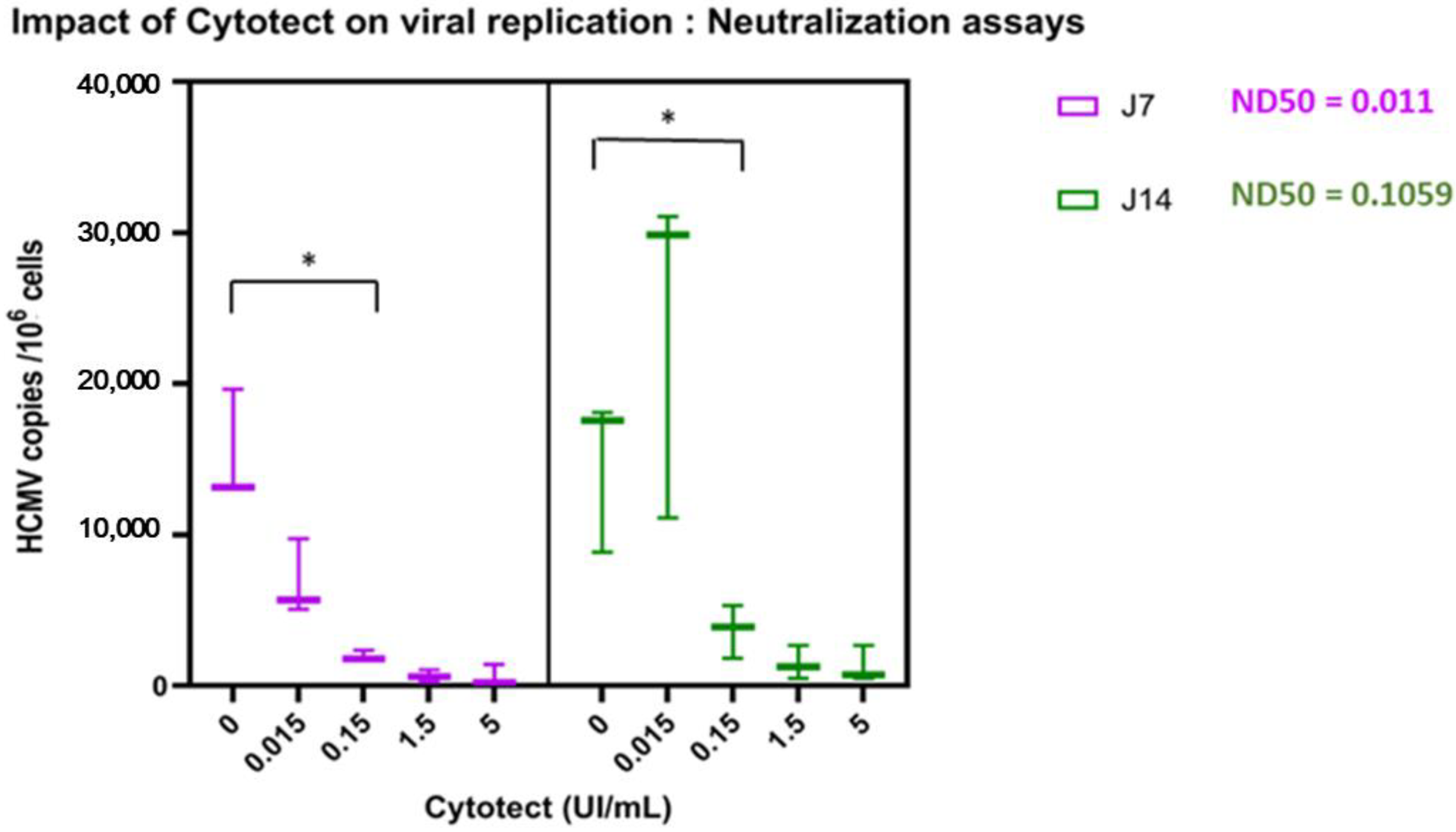
3.2.1. Replication of TB40/E in Villi Explants
3.2.2. Impact of Cytotect CP® on Viral Replication
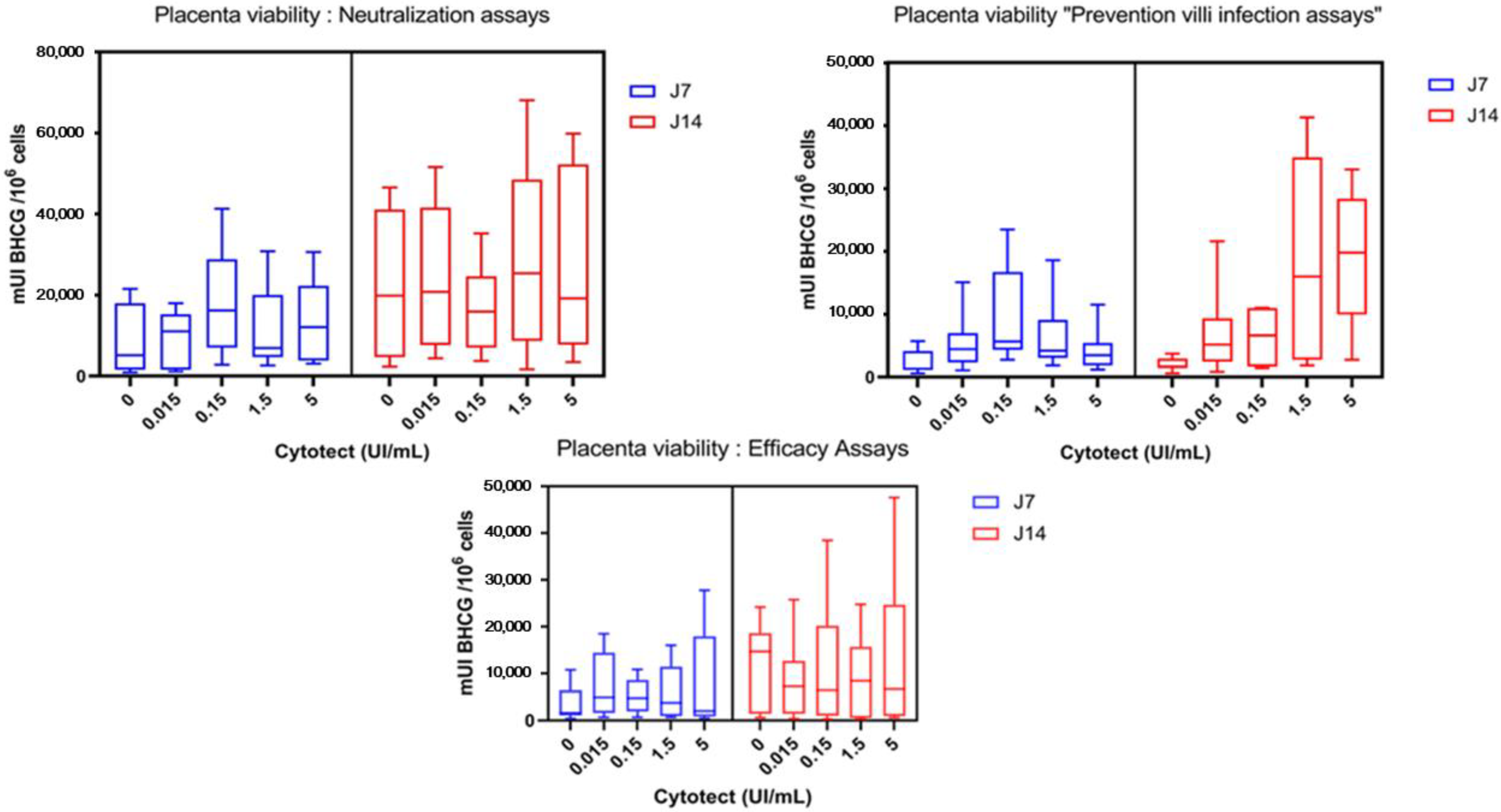
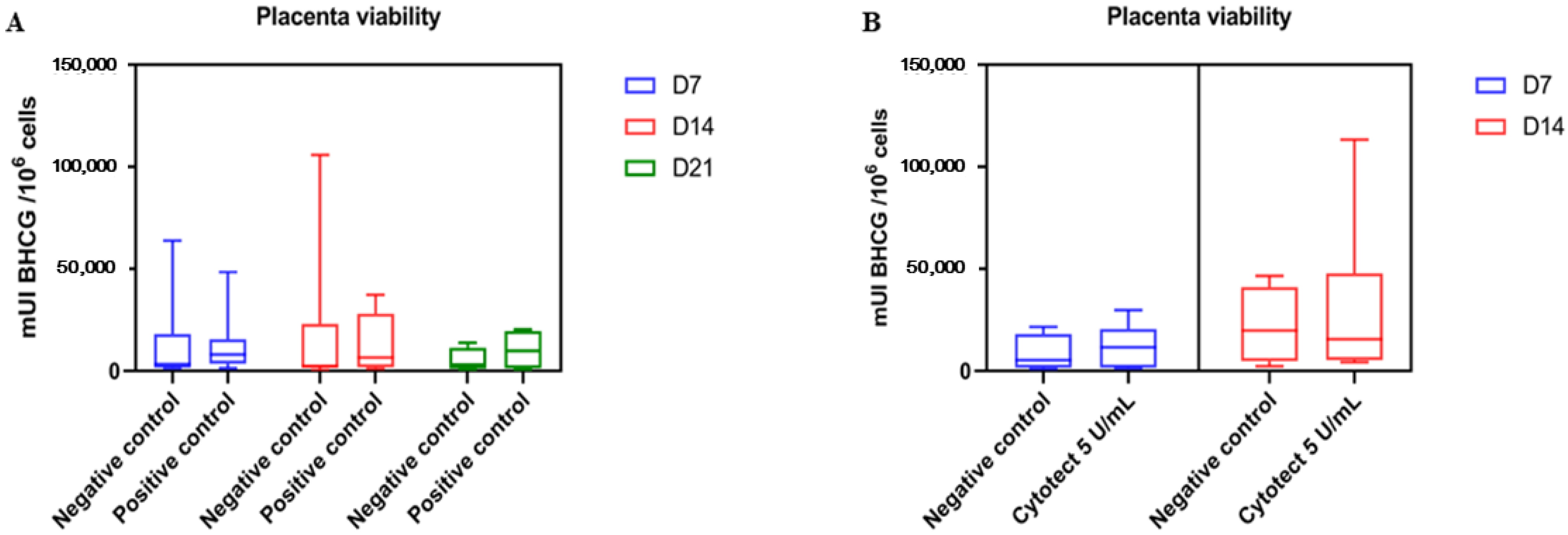
3.2.3. Placenta Viability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumazaki, K.; Ozono, K.; Yahara, T.; Wada, Y.; Suehara, N.; Takeuchi, M.; Nakayama, M. Detection of cytomegalovirus DNA in human placenta. J. Med. Virol. 2002, 68, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Tabata, T.; Petitt, M.; Fang-Hoover, J.; Zydek, M.; Pereira, L. Persistent Cytomegalovirus Infection in Amniotic Membranes of the Human Placenta. Am. J. Pathol. 2016, 186, 2970–2986. [Google Scholar] [CrossRef] [Green Version]
- Sinzger, C.; Müntefering, H.; Loning, T.; Stöss, H.; Plachter, B.; Jahn, G. Cell types infected in human cytomegalovirus placentitis identified by immunohistochemical double staining. Virchows Arch. 1993, 423, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, S.T.; Scott, G.; Naing, Z.; Iwasenko, J.; Hall, B.; Graf, N.; Arbuckle, S.; Craig, M.E.; Rawlinson, W.D. Human Cytomegalovirus-Induces Cytokine Changes in the Placenta with Implications for Adverse Pregnancy Outcomes. PLoS ONE 2012, 7, e52899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becroft, D.M. Prenatal cytomegalovirus infection: Epidemiology, pathology and pathogenesis. Perspect. Pediatr. Pathol. 1981, 6, 41. [Google Scholar]
- Chatzakis, C.; Ville, Y.; Makrydimas, G.; Dinas, K.; Zavlanos, A.; Sotiriadis, A. Timing of primary maternal cytomegalovirus infection and rates of vertical transmission and fetal consequences. Am. J. Obstet. Gynecol. 2020, 223, 870–883.e11. [Google Scholar] [CrossRef]
- Leruez-Ville, M.; Foulon, I.; Pass, R.; Ville, Y. Cytomegalovirus infection during pregnancy: State of the science. Am. J. Obstet. Gynecol. 2020, 223, 330–349. [Google Scholar] [CrossRef]
- Hyde, T.B.; Schmid, D.S.; Cannon, M.J. Cytomegalovirus seroconversion rates and risk factors: Implications for congenital CMV. Rev. Med. Virol. 2010, 20, 311–326. [Google Scholar] [CrossRef]
- Hughes, B.L.; Clifton, R.G.; Rouse, D.J.; Saade, G.R.; Dinsmoor, M.J.; Reddy, U.M.; Pass, R.; Allard, D.; Mallett, G.; Fette, L.M.; et al. A Trial of Hyperimmune Globulin to Prevent Congenital Cytomegalovirus Infection. N. Engl. J. Med. 2021, 385, 436–444. [Google Scholar] [CrossRef]
- Kagan, K.O.; Enders, M.; Schampera, M.S.; Baeumel, E.; Hoopmann, M.; Geipel, A.; Berg, C.; Goelz, R.; De Catte, L.; Wallwiener, D.; et al. Prevention of maternal–fetal transmission of cytomegalovirus after primary maternal infection in the first trimester by biweekly hyperimmunoglobulin administration. Ultrasound Obstetr. Gynecol. 2019, 53, 383–389. [Google Scholar] [CrossRef]
- Nigro, G.; Adler, S.P.; La Torre, R.; Best, A.M.; Congenital Cytomegalovirus Collaborating Group. Passive Immunization during Pregnancy for Congenital Cytomegalovirus Infection. N. Engl. J. Med. 2005, 353, 1350–1362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartlett, A.W.; Hamilton, S.T.; Shand, A.W.; Rawlinson, W.D. Fetal therapies for cytomegalovirus: What we tell prospective parents. Prenat. Diagn. 2020, 40, 1681–1692. [Google Scholar] [CrossRef] [PubMed]
- Revello, M.G.; Lazzarotto, T.; Guerra, B.; Spinillo, A.; Ferrazzi, E.; Kustermann, A.; Guaschino, S.; Vergani, P.; Todros, T.; Frusca, T.; et al. A randomized trial of hyperimmune globulin to prevent congenital cytomegalovirus. N. Engl. J. Med. 2014, 370, 1316–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nigro, G. Hyperimmune globulin in pregnancy for the prevention of congenital cytomegalovirus disease. Expert Rev. Anti-Infect. Ther. 2017, 15, 977–986. [Google Scholar] [CrossRef]
- Seidel, V.; Hackelöer, M.; Rancourt, R.C.; Henrich, W.; Siedentopf, J.-P. Fetal and maternal outcome after hyperimmunoglobulin administration for prevention of maternal–fetal transmission of cytomegalovirus during pregnancy: Retrospective cohort analysis. Arch. Gynecol. Obstet. 2020, 302, 1353–1359. [Google Scholar] [CrossRef]
- Devlieger, R.; Buxmann, H.; Nigro, G.; Enders, M.; Jückstock, J.; Siklós, P.; Wartenberg-Demand, A.; Schüttrumpf, J.; Schütze, J.; Rippel, N.; et al. Serial Monitoring and Hyperimmunoglobulin versus Standard of Care to Prevent Congenital Cytomegalovirus Infection: A Phase III Randomized Trial. Fetal Diagn. Ther. 2021, 48, 611–623. [Google Scholar] [CrossRef]
- Carbone, J. The Immunology of Posttransplant CMV Infection: Potential Effect of CMV Immunoglobulins on Distinct Components of the Immune Response to CMV. Transplantation 2016, 100, S11–S18. [Google Scholar] [CrossRef] [Green Version]
- Bonaros, N.; Mayer, B.; Schachner, T.; Laufer, G.; Kocher, A. CMV-hyperimmune globulin for preventing cytomegalovirus infection and disease in solid organ transplant recipients: A meta-analysis. Clin. Transpl. 2008, 22, 89–97. [Google Scholar] [CrossRef]
- Kagan, K.O.; Enders, M.; Hoopmann, M.; Geipel, A.; Simonini, C.; Berg, C.; Gottschalk, I.; Faschingbauer, F.; Schneider, M.O.; Ganzenmueller, T.; et al. Outcome of pregnancies with recent primary cytomegalovirus infection in first trimester treated with hyperimmunoglobulin: Observational study. Ultrasound Obstet. Gynecol. 2021, 57, 560–567. [Google Scholar] [CrossRef]
- Tabata, T.; Petitt, M.; Fang-Hoover, J.; Freed, D.C.; Li, F.; An, Z.; Wang, D.; Fu, T.M.; Pereira, L. Neutralizing Monoclonal Antibodies Reduce Human Cytomegalovirus Infection and Spread in Developing Placentas. Vaccines 2019, 7, 135. [Google Scholar] [CrossRef] [Green Version]
- Paul-Ehrlich-Institut—Préparations d’Immunoglobulines. Available online: https://www.pei.de/DE/arzneimittel/antikoerper/immunglobulinpraeparate/immunglobulinpraeparate-node.html;jsessionid=29DBBB9C2CE956CF6F9031BC2C19E0B3.intranet231 (accessed on 25 February 2022).
- Germer, M.; Herbener, P.; Schüttrumpf, J. Functional Properties of Human Cytomegalovirus Hyperimmunoglobulin and Standard Immunoglobulin Preparations. Ann. Transpl. 2016, 21, 558–564. [Google Scholar] [CrossRef] [PubMed]
- van Gent, R.; Jaadar, H.; Tjon, A.S.W.; Mancham, S.; Kwekkeboom, J. T-cell inhibitory capacity of hyperimmunoglobulins is influenced by the production process. Int. Immunopharmacol. 2014, 19, 142–144. [Google Scholar] [CrossRef] [PubMed]
- Morere, L.; Andouard, D.; Labrousse, F.; Saade, F.; Calliste, C.A.; Cotin, S.; Aubard, Y.; Rawlinson, W.D.; Esclaire, F.; Hantz, S.; et al. Ex vivo model of congenital cytomegalovirus infection and new combination therapies. Placenta 2015, 36, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, C.; Marschall, M.; Andouard, D.; El Hamel, C.; Chianea, T.; Tsogoeva, S.B.; Hantz, S.; Alain, S. A highly potent trimeric derivative of artesunate shows promising treatment profiles in experimental models for congenital HCMV infection in vitro and ex vivo. Antiviral Res. 2020, 175, 104700. [Google Scholar] [CrossRef] [PubMed]
- Shahar-Nissan, K.; Pardo, J.; Peled, O.; Krause, I.; Bilavsky, E.; Wiznitzer, A.; Hadar, E.; Amir, J. Valaciclovir to prevent vertical transmission of cytomegalovirus after maternal primary infection during pregnancy: A randomised, double-blind, placebo-controlled trial. Lancet 2020, 396, 779–785. [Google Scholar] [CrossRef]
- Buxmann, H.; Stackelberg, O.M.; Schlößer, R.L.; Enders, G.; Gonser, M.; Meyer-Wittkopf, M.; Hamprecht, K.; Enders, M. Use of cytomegalovirus hyperimmunoglobulin for prevention of congenital cytomegalovirus disease: A retrospective analysis. J. Perinat. Med. 2012, 40, 439–446. [Google Scholar] [CrossRef]
- Gabrielli, L.; Bonasoni, M.P.; Foschini, M.P.; Silini, E.M.; Spinillo, A.; Revello, M.G.; Chiereghin, A.; Piccirilli, G.; Petrisli, E.; Turello, G.; et al. Histological Analysis of Term Placentas from Hyperimmune Globulin-Treated and Untreated Mothers with Primary Cytomegalovirus Infection. Fetal Diagn. Ther. 2018, 45, 111–117. [Google Scholar] [CrossRef]
- Hamprecht, K.; Kagan, K.O.; Goelz, R. Hyperimmune Globulin to Prevent Congenital CMV Infection. N. Engl. J. Med. 2014, 370, 2543–2545. [Google Scholar] [CrossRef]
- Feldman, B.; Yinon, Y.; Tepperberg Oikawa, M.; Yoeli, R.; Schiff, E.; Lipitz, S. Pregestational, periconceptional, and gestational primary maternal cytomegalovirus infection: Prenatal diagnosis in 508 pregnancies. Am. J. Obstet. Gynecol. 2011, 205, 342.e1–342.e6. [Google Scholar] [CrossRef]
- Visentin, S.; Manara, R.; Milanese, L.; Da Roit, A.; Forner, G.; Salviato, E.; Citton, V.; Magno, F.M.; Orzan, E.; Morando, C.; et al. Early primary cytomegalovirus infection in pregnancy: Maternal hyperimmunoglobulin therapy improves outcomes among infants at 1 year of age. Clin. Infect. Dis. 2012, 55, 497–503. [Google Scholar] [CrossRef] [Green Version]
- Tanimura, K.; Shi, Y.; Uchida, A.; Uenaka, M.; Imafuku, H.; Ikuta, T.; Fujioka, K.; Morioka, I.; Deguchi, M.; Minematsu, T.; et al. Immunoglobulin fetal therapy and neonatal therapy with antiviral drugs improve neurological outcome of infants with symptomatic congenital cytomegalovirus infection. J. Reprod. Immunol. 2020, 143, 103263. [Google Scholar] [CrossRef] [PubMed]
- Chiaie, L.D.; Neuberger, P.; Vochem, M.; Lihs, A.; Karck, U.; Enders, M. No evidence of obstetrical adverse events after hyperimmune globulin application for primary cytomegalovirus infection in pregnancy: Experience from a single centre. Arch. Gynecol. Obstet. 2018, 297, 1389–1395. [Google Scholar] [CrossRef] [PubMed]
- Hemmings, D.G.; Kilani, R.; Nykiforuk, C.; Preiksaitis, J.; Guilbert, L.J. Permissive Cytomegalovirus Infection of Primary Villous Term and First Trimester Trophoblasts. J. Virol. 1998, 72, 4970–4979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halwachs-Baumann, G.; Wilders-Truschnig, M.; Desoye, G.; Hahn, T.; Kiesel, L.; Klingel, K.; Rieger, P.; Jahn, G.; Sinzger, C. Human trophoblast cells are per-missive to the complete replicative cycle of human cytomegalovirus. J. Virol. 1998, 72, 7598–7602. [Google Scholar] [CrossRef] [Green Version]
- Bardon, V.F.; Magny, J.-F.; Parodi, M.; Couderc, S.; Garcia, P.; Maillotte, A.-M.; Benard, M.; Pinquier, D.; Astruc, D.; Patural, H.; et al. Sequelae of Congenital Cytomegalovirus Following Maternal Primary Infections Are Limited to Those Acquired in the First Trimester of Pregnancy. Clin. Infect. Dis. 2019, 69, 1526–1532. [Google Scholar] [CrossRef]
- Kalser, J.; Adler, B.; Mach, M.; Kropff, B.; Puchhammer-Stöckl, E.; Görzer, I. Differences in Growth Properties among Two Human Cytomegalovirus Glycoprotein O Genotypes. Front. Microbiol. 2017, 8, 1609. [Google Scholar] [CrossRef]
- Wussow, F.; Chiuppesi, F.; Contreras, H.; Diamond, D.J. Neutralization of Human Cytomegalovirus Entry into Fibroblasts and Epithelial Cells. Vaccines 2017, 5, 39. [Google Scholar] [CrossRef] [Green Version]
- Bussel, J.B.; Haar, E.L.V.; Berkowitz, R.L. New developments in fetal and neonatal alloimmune thrombocytopenia. Am. J. Obstet. Gynecol. 2021, 225, 120–127. [Google Scholar] [CrossRef]
- Costa-Garcia, M.; Vera, A.; Moraru, M.; Vilches, C.; López-Botet, M.; Muntasell, A. Antibody-Mediated Response of NKG2CbrightNK Cells against Human Cytomegalovirus. J. Immunol. 2015, 194, 2715–2724. [Google Scholar] [CrossRef] [Green Version]
- Itell, H.L.; Nelson, C.; Martinez, D.R.; Permar, S.R. Maternal immune correlates of protection against placental transmission of cytomegalovirus. Placenta 2017, 60, S73–S79. [Google Scholar] [CrossRef]
- Maidji, E.; McDonagh, S.; Genbacev, O.; Tabata, T.; Pereira, L. Maternal Antibodies Enhance or Prevent Cytomegalovirus Infection in the Placenta by Neonatal Fc Receptor-Mediated Transcytosis. Am. J. Pathol. 2006, 168, 1210–1226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, L.; Maidji, E.; McDonagh, S.; Tabata, T. Insights into viral transmission at the uterine–placental interface. Trends Microbiol. 2005, 13, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L. Congenital Viral Infection: Traversing the Uterine-Placental Interface. Annu. Rev. Virol. 2018, 5, 273–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miescher, S.M.; Huber, T.M.; Kühne, M.; Lieby, P.; Snydman, D.; Vensak, J.L.; Berger, M. In vitro evaluation of cytomegalovirus-specific hyperimmune globulins vs. standard intravenous immunoglobulins. Vox Sang. 2015, 109, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Lazzarotto, T.; Blázquez-Gamero, D.; Delforge, M.-L.; Foulon, I.; Luck, S.; Modrow, S.; Leruez-Ville, M. Congenital Cytomegalovirus Infection: A Narrative Review of the Issues in Screening and Management From a Panel of European Experts. Front. Pediatr. 2020, 8, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rawlinson, W.D.; Boppana, S.B.; Fowler, K.B.; Kimberlin, D.W.; Lazzarotto, T.; Alain, S.; Daly, K.; Doutré, S.; Gibson, L.; Giles, M.; et al. Congenital cytomegalovirus infection in pregnancy and the neonate: Consensus recommendations for prevention, diagnosis, and therapy. Lancet Infect. Dis. 2017, 17, e177–e188. [Google Scholar] [CrossRef]



| Viral Strain | HEF | ARPE | ||
|---|---|---|---|---|
| ND50 ± SD (U/mL) | ND90 ± SD (U/mL) | ND50 ± SD (U/mL) | ND90 ± SD (U/mL) | |
| VHL/E | 0.014 ± 0.01 | 0.069 ± 0.02 | 0.011 ± 0.01 | 0.067 ± 0.02 |
| TB40/E | 0.033 ± 0.01 | 0.10 ± 0.01 | 0.032 ± 0.01 | 0.11 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coste Mazeau, P.; Jacquet, C.; Muller, C.; Courant, M.; El Hamel, C.; Chianea, T.; Hantz, S.; Alain, S. Potential of Anti-CMV Immunoglobulin Cytotect CP® In Vitro and Ex Vivo in a First-Trimester Placenta Model. Microorganisms 2022, 10, 694. https://doi.org/10.3390/microorganisms10040694
Coste Mazeau P, Jacquet C, Muller C, Courant M, El Hamel C, Chianea T, Hantz S, Alain S. Potential of Anti-CMV Immunoglobulin Cytotect CP® In Vitro and Ex Vivo in a First-Trimester Placenta Model. Microorganisms. 2022; 10(4):694. https://doi.org/10.3390/microorganisms10040694
Chicago/Turabian StyleCoste Mazeau, Perrine, Chloé Jacquet, Clotilde Muller, Mathis Courant, Chahrazed El Hamel, Thierry Chianea, Sébastien Hantz, and Sophie Alain. 2022. "Potential of Anti-CMV Immunoglobulin Cytotect CP® In Vitro and Ex Vivo in a First-Trimester Placenta Model" Microorganisms 10, no. 4: 694. https://doi.org/10.3390/microorganisms10040694
APA StyleCoste Mazeau, P., Jacquet, C., Muller, C., Courant, M., El Hamel, C., Chianea, T., Hantz, S., & Alain, S. (2022). Potential of Anti-CMV Immunoglobulin Cytotect CP® In Vitro and Ex Vivo in a First-Trimester Placenta Model. Microorganisms, 10(4), 694. https://doi.org/10.3390/microorganisms10040694






