Effects of Menstrual Cycle on the Accumulation of Human Papillomavirus-Infected Cells Exfoliated from the Cervix That Drift into the Vagina
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Specimen Collection
2.2. HPV Genotyping using LBC Samples
2.3. Data Analysis
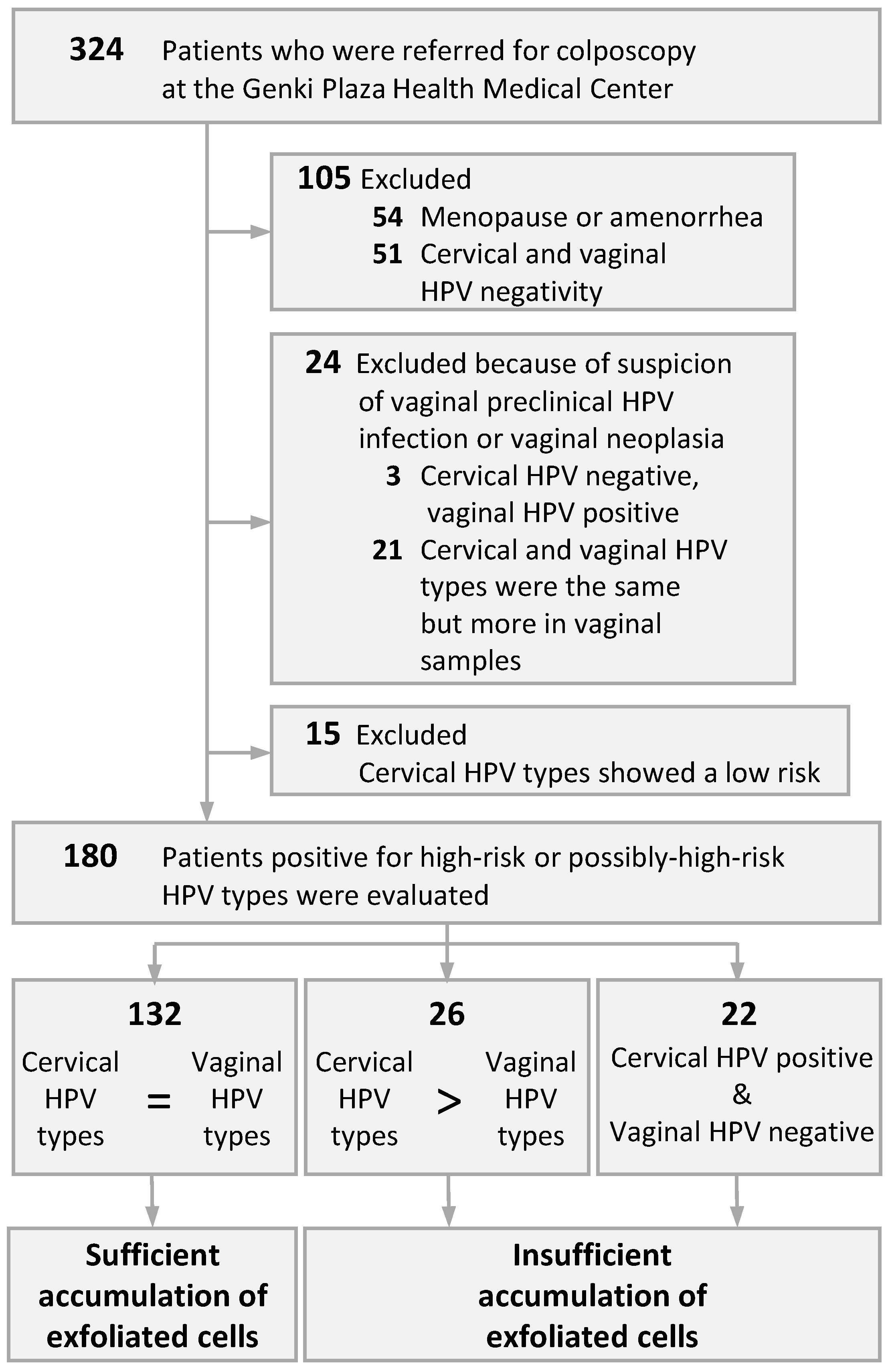
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saslow, D.; Solomon, D.; Lawson, H.W.; Killackey, M.; Kulasingam, S.L.; Cain, J.; Garcia, F.A.; Moriarty, A.T.; Waxman, A.G.; Wilbur, D.C. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J. Clin. 2012, 62, 147–172. [Google Scholar] [CrossRef] [PubMed]
- Wright, T.C.; Stoler, M.H.; Behrens, C.M.; Sharma, A.; Zhang, G.; Wright, T.L. Primary cervical cancer screening with human papillomavirus: End of study results from the ATHENA study using HPV as the first-line screening test. Gynecol. Oncol. 2015, 136, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Aoki, E.S.; Yin, R.; Li, K.; Bhatla, N.; Singhal, S.; Ocviyanti, D.; Saika, K.; Suh, M.; Kim, M.; Termrungruanglert, W. National screening programs for cervical cancer in Asian countries. J. Gynecol. Oncol. 2020, 31, e55. [Google Scholar] [CrossRef] [PubMed]
- Center for Cancer Control and Information Services. Available online: https://ganjoho.jp/en/index.html (accessed on 31 January 2022).
- Waller, J.; Bartoszek, M.; Marlow, L.; Wardle, J. Barriers to cervical cancer screening attendance in England: A population-based survey. J. Med. Screen. 2009, 16, 199–204. [Google Scholar] [CrossRef]
- Arbyn, M.; Verdoodt, F.; Snijders, P.J.; Verhoef, V.M.; Suonio, E.; Dillner, L.; Minozzi, S.; Bellisario, C.; Banzi, R.; Zhao, F.-H. Accuracy of human papillomavirus testing on self-collected versus clinician-collected samples: A meta-analysis. Lancet Oncol. 2014, 15, 172–183. [Google Scholar] [CrossRef]
- Verdoodt, F.; Jentschke, M.; Hillemanns, P.; Racey, C.; Snijders, P.; Arbyn, M. Reaching women who do not participate in the regular cervical cancer screening programme by offering self-sampling kits: A systematic review and meta-analysis of randomised trials. Eur. J. Cancer 2015, 51, 2375–2385. [Google Scholar] [CrossRef]
- Sultana, F.; Mullins, R.; English, D.R.; Simpson, J.A.; Drennan, K.T.; Heley, S.; Wrede, C.D.; Brotherton, J.M.; Saville, M.; Gertig, D.M. Women’s experience with home-based self-sampling for human papillomavirus testing. BMC Cancer 2015, 15, 849. [Google Scholar] [CrossRef]
- Zhao, F.-H.; Lewkowitz, A.K.; Chen, F.; Lin, M.J.; Hu, S.-Y.; Zhang, X.; Pan, Q.-J.; Ma, J.-F.; Niyazi, M.; Li, C.-Q. Pooled analysis of a self-sampling HPV DNA test as a cervical cancer primary screening method. J. Natl. Cancer Inst. 2012, 104, 178–188. [Google Scholar] [CrossRef]
- Polman, N.J.; Ebisch, R.M.; Heideman, D.A.; Melchers, W.J.; Bekkers, R.L.; Molijn, A.C.; Meijer, C.J.; Quint, W.G.; Snijders, P.J.; Massuger, L.F. Performance of human papillomavirus testing on self-collected versus clinician-collected samples for the detection of cervical intraepithelial neoplasia of grade 2 or worse: A randomised, paired screen-positive, non-inferiority trial. Lancet Oncol. 2019, 20, 229–238. [Google Scholar] [CrossRef]
- Sargent, A.; Fletcher, S.; Bray, K.; Kitchener, H.C.; Crosbie, E.J. Cross-sectional study of HPV testing in self-sampled urine and comparison with matched vaginal and cervical samples in women attending colposcopy for the management of abnormal cervical screening. BMJ Open 2019, 9, e025388. [Google Scholar] [CrossRef]
- Inturrisi, F.; Aitken, C.A.; Melchers, W.J.; van den Brule, A.J.; Molijn, A.; Hinrichs, J.W.; Niesters, H.G.; Siebers, A.G.; Schuurman, R.; Heideman, D.A. Clinical performance of high-risk HPV testing on self-samples versus clinician samples in routine primary HPV screening in the Netherlands: An observational study. Lancet Regional Health-Eur. 2021, 11, 100235. [Google Scholar] [CrossRef] [PubMed]
- Flores, C.A.; Gutierrez, G.G.; Leon, J.O.; Rodriguez, D.C.; Sørbye, S. Self-collected versus clinician-collected cervical samples for the detection of HPV infections by 14-type DNA and 7-type mRNA tests. BMC Infect. Dis. 2021, 21, 504. [Google Scholar] [CrossRef]
- Belinson, J.L.; Hu, S.; Niyazi, M.; Pretorius, R.G.; Wang, H.; Wen, C.; Smith, J.S.; Li, J.; Taddeo, F.J.; Burchette, R.J. Prevalence of type-specific human papillomavirus in endocervical, upper and lower vaginal, perineal and vaginal self-collected specimens: Implications for vaginal self-collection. Int. J. Cancer 2010, 127, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Castle, P.E.; Schiffman, M.; Bratti, M.C.; Hildesheim, A.; Herrero, R.; Hutchinson, M.L.; Rodriguez, A.C.; Wacholder, S.; Sherman, M.E.; Kendall, H. A population-based study of vaginal human papillomavirus infection in hysterectomized women. J. Infect. Dis. 2004, 190, 458–467. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Truett, G.; Heeger, P.; Mynatt, R.; Truett, A.; Walker, J.; Warman, M. Preparation of PCR-quality mouse genomic DNA with hot sodium hydroxide and tris (HotSHOT). Biotechniques 2000, 29, 52–54. [Google Scholar] [CrossRef] [PubMed]
- Okodo, M.; Okayama, K.; Teruya, K.; Sasagawa, T. Uniplex E6/E7 PCR method detecting E6 or E7 genes in 39 human papillomavirus types. J. Med. Virol. 2018, 90, 981–988. [Google Scholar] [CrossRef]
- Guan, Y.; Castle, P.E.; Wang, S.; Li, B.; Feng, C.; Ci, P.; Li, X.; Gravitt, P.; Qiao, Y.-L. A cross-sectional study on the acceptability of self-collection for HPV testing among women in rural China. Sex. Transm. Infect. 2012, 88, 490–494. [Google Scholar] [CrossRef]
- Koss, L.G.; Melamed, M.R. Koss’ Diagnostic Cytology and Its Histopathologic Bases; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006; Volume 1. [Google Scholar]
- Gorodeski, G.I. Estrogen modulation of epithelial permeability in cervical-vaginal cells of premenopausal and postmenopausal women. Menopause 2007, 14, 1012. [Google Scholar] [CrossRef][Green Version]
- Fairley, C.K.; Robinson, P.M.; Chen, S.; Tabrizi, S.N.; Garland, S.M. The detection of HPV DNA, the size of tampon specimens and the menstrual cycle. Sex. Transm. Infect. 1994, 70, 171–174. [Google Scholar] [CrossRef][Green Version]
- Sanner, K.; Wikström, I.; Gustavsson, I.; Wilander, E.; Lindberg, J.H.; Gyllensten, U.; Olovsson, M. Daily self-sampling for high-risk human papillomavirus (HR-HPV) testing. J. Clin. Virol. 2015, 73, 1–7. [Google Scholar] [CrossRef]
- Sherman, M.; Carreon, J.; Schiffman, M. Performance of cytology and human papillomavirus testing in relation to the menstrual cycle. Br. J. Cancer 2006, 94, 1690–1696. [Google Scholar] [CrossRef] [PubMed]
- Gustavsson, I.; Aarnio, R.; Berggrund, M.; Hedlund-Lindberg, J.; Sanner, K.; Wikström, I.; Enroth, S.; Olovsson, M.; Gyllensten, U. Randomised study of HPV prevalence and detection of CIN2+ in vaginal self-sampling compared to cervical specimens collected by medical personnel. Int. J. Cancer 2019, 144, 89–97. [Google Scholar] [CrossRef] [PubMed]
| Case | Cervical HPV Types | Vaginal HPV Types | Evaluation | Cycles | Case | Cervical HPV Types | Vaginal HPV Types | Evaluation | Cycles |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 16,52,58,66 | 16,52,58,66 | C = V | L-Pro | 91 | 66 | 66 | C = V | L-Sec |
| 2 | 52,6b | 52,6b | C = V | L-Sec | 92 | 52,42,74 | 52,42,74 | C = V | Mens |
| 3 | 51,58 | 51,58 | C = V | E-Pro | 93 | 52 | 52 | C = V | Mens |
| 4 | 31,81,82 | 81,82 | C > V | E-Pro | 94 | 31,58,74 | 58,74 | C > V | E-Pro |
| 5 | 18 | 18 | C = V | E-Sec | 95 | 16 | 16 | C = V | E-Sec |
| 6 | 67 | Negative | N | L-Sec | 96 | 39,58,53 | 39,58,53 | C = V | E-Sec |
| 7 | 16 | Negative | N | L-Sec | 97 | 51 | 51 | C = V | L-Sec |
| 8 | 52 | 52 | C = V | L-Pro | 98 | 16 | 16 | C = V | L-Pro |
| 9 | 82,90 | 82,90 | C = V | E-Pro | 99 | 59,90 | 59,90 | C = V | E-Sec |
| 10 | 33,52 | 33,52 | C = V | L-Pro | 100 | 52 | Negative | N | L-Sec |
| 11 | 52 | 52 | C = V | L-Pro | 101 | 31,52,69,74 | 31,52,69,74 | C = V | E-Pro |
| 12 | 52,66 | 52,66 | C = V | L-Sec | 102 | 52,6b | 52,6b | C = V | L-Sec |
| 13 | 56 | 56 | C = V | E-Sec | 103 | 16 | 16 | C = V | L-Sec |
| 14 | 16,45,52,40 | 16,45,40 | C > V | E-Pro | 104 | 52 | 52 | C = V | L-Pro |
| 15 | 58 | 58 | C = V | Mens | 105 | 56 | 56 | C = V | E-Sec |
| 16 | 16 | 16 | C = V | L-Pro | 106 | 66 | 66 | C = V | L-Sec |
| 17 | 56,62 | 62 | C > V | L-Sec | 107 | 31,52,58,70 | 31,58,70 | C > V | L-Pro |
| 18 | 52 | 52 | C = V | E-Sec | 108 | 52 | Negative | N | E-Pro |
| 19 | 39,58,53 | 39,58,53 | C = V | E-Sec | 109 | 51,58,82 | 51,58 | C > V | Mens |
| 20 | 16,66 | 16,66 | C = V | L-Sec | 110 | 58 | 58 | C = V | L-Sec |
| 21 | 33 | 33 | C = V | L-Sec | 111 | 16,51,52,56 | 16,51,52,56 | C = V | L-Sec |
| 22 | 52 | 52 | C = V | E-Sec | 112 | 58 | 58 | C = V | L-Pro |
| 23 | 52,53,74 | 53,74 | C > V | L-Sec | 113 | 31,71 | 31,71 | C = V | E-Sec |
| 24 | 52,84 | 52,84 | C = V | E-Sec | 114 | 51,82 | 51,82 | C = V | L-Sec |
| 25 | 56 | Negative | N | L-Sec | 115 | 51,53,54 | 51,53,54 | C = V | E-Pro |
| 26 | 51,58 | 58 | C > V | E-Sec | 116 | 39,59,40 | 39,59,40 | C = V | L-Sec |
| 27 | 52,58 | 52 | C > V | L-Sec | 117 | 56,66,74 | 56,66,74 | C = V | L-Pro |
| 28 | 51,58,82 | 51,58,82 | C = V | E-Sec | 118 | 53,66 | 53 | C > V | L-Pro |
| 29 | 56,61,74 | 56,61,74 | C = V | E-Sec | 119 | 66 | 66 | C = V | Mens |
| 30 | 39,71 | 39,71 | C = V | L-Sec | 120 | 58 | 58 | C = V | E-Sec |
| 31 | 16 | 16 | C = V | L-Pro | 121 | 16 | 16 | C = V | L-Pro |
| 32 | 52,54,62,70,90 | 52,54,62,70,90 | C = V | L-Sec | 122 | 58 | 58 | C = V | L-Pro |
| 33 | 39,58 | 58 | C > V | E-Sec | 123 | 16 | 16 | C = V | L-Pro |
| 34 | 53,81,90 | 53,81,90 | C = V | L-Sec | 124 | 58 | 58 | C = V | E-Sec |
| 35 | 51 | Negative | N | E-Pro | 125 | 52 | Negative | N | L-Sec |
| 36 | 52 | Negative | N | E-Pro | 126 | 58 | 58 | C = V | E-Pro |
| 37 | 16 | Negative | N | L-Pro | 127 | 39,51,53,42 | 39,51,53,42 | C = V | E-Sec |
| 38 | 52,56 | 52,56 | C = V | E-Pro | 128 | 68 | 68 | C = V | E-Sec |
| 39 | 31 | 31 | C = V | L-Sec | 129 | 31 | Negative | N | E-Sec |
| 40 | 51,71 | 51,71 | C = V | E-Pro | 130 | 51,71 | 51,71 | C = V | E-Pro |
| 41 | 59,74 | 59,74 | C = V | E-Pro | 131 | 34 | 34 | C = V | L-Sec |
| 42 | 52 | 52 | C = V | E-Sec | 132 | 66,62,81 | 66,62,81 | C = V | L-Sec |
| 43 | 51,82 | 51,82 | C = V | L-Sec | 133 | 82,90 | 82,90 | C = V | Mens |
| 44 | 51 | Negative | N | E-Sec | 134 | 53 | 53 | C = V | L-Sec |
| 45 | 31,74 | 74 | C > V | E-Sec | 135 | 52 | 52 | C = V | L-Sec |
| 46 | 52 | 52 | C = V | E-Sec | 136 | 31 | 31 | C = V | L-Sec |
| 47 | 16 | 16 | C = V | L-Pro | 137 | 62,81,90 | 62,81,90 | C = V | E-Sec |
| 48 | 52 | 52 | C = V | L-Sec | 138 | 18 | Negative | N | L-Sec |
| 49 | 16,52,56 | 52,56 | C > V | E-Sec | 139 | 51 | 51 | C = V | E-Sec |
| 50 | 52,58 | 52 | C > V | E-Sec | 140 | 52 | 52 | C = V | L-Sec |
| 51 | 58,67 | 58,67 | C = V | E-Sec | 141 | 58 | 58 | C = V | E-Pro |
| 52 | 16,31,51,52,58,82,54,70 | 16,31,51,52,58, 82,54,70 | C = V | L-Pro | 142 | 51,82,62 | 51,82,62 | C = V | E-Sec |
| 53 | 52,6b | 52,6b | C = V | L-Pro | 143 | 51,74 | 74 | C > V | L-Pro |
| 54 | 58 | 58 | C = V | E-Sec | 144 | 53,40,62,81 | 53,40,62,81 | C = V | L-Sec |
| 55 | 53 | 53 | C = V | E-Sec | 145 | 16,39 | 16,39 | C = V | E-Sec |
| 56 | 52,40,81 | 52,40,81 | C = V | Mens | 146 | 67 | Negative | N | E-Sec |
| 57 | 52 | 52 | C = V | L-Sec | 147 | 52 | 52 | C = V | E-Sec |
| 58 | 56,66 | 56,66 | C = V | E-Sec | 148 | 56 | Negative | N | E-Pro |
| 59 | 52,62 | 52,62 | C = V | Mens | 149 | 51,82 | 51,82 | C = V | E-Sec |
| 60 | 51,53,54 | 51,53,54 | C = V | E-Pro | 150 | 31 | 31 | C = V | L-Pro |
| 61 | 56,34 | 34 | C > V | L-Pro | 151 | 51 | Negative | N | L-Sec |
| 62 | 16 | Negative | N | L-Sec | 152 | 31,51,52,58 | 31,58 | C > V | E-Pro |
| 63 | 16,66 | 16,66 | C = V | E-Sec | 153 | 53,74 | 53,74 | C = V | L-Sec |
| 64 | 52,58 | 52,58 | C = V | E-Pro | 154 | 66,40 | 40 | C > V | E-Sec |
| 65 | 52 | Negative | N | E-Pro | 155 | 16,66 | 16,66 | C = V | L-Sec |
| 66 | 51,82 | Negative | N | Mens | 156 | 31 | 31 | C = V | L-Pro |
| 67 | 82 | 82 | C = V | E-Sec | 157 | 58 | 58 | C = V | L-Pro |
| 68 | 68 | 68 | C = V | Mens | 158 | 31,90 | 31,90 | C = V | E-Sec |
| 69 | 45,59,53,62,66,67,81 | 45,59,53,62,66, 67,81 | C = V | L-Sec | 159 | 58 | 58 | C = V | E-Sec |
| 70 | 51 | 51 | C = V | L-Pro | 160 | 31,33,53,68 | 31,33,53,68 | C = V | E-Pro |
| 71 | 53 | 53 | C = V | L-Pro | 161 | 31,68,67 | 31,68,67 | C = V | E-Sec |
| 72 | 59,74 | 59,74 | C = V | E-Sec | 162 | 56 | 56 | C = V | L-Sec |
| 73 | 51,82 | 51 | C > V | L-Pro | 163 | 52 | 52 | C = V | L-Pro |
| 74 | 52 | Negative | N | E-Pro | 164 | 53 | 53 | C = V | L-Pro |
| 75 | 33,52 | 33 | C > V | E-Pro | 165 | 26,90 | 90 | C > V | L-Sec |
| 76 | 52 | 52 | C = V | E-Sec | 166 | 16 | 16 | C = V | E-Sec |
| 77 | 51,58 | 51 | C > V | L-Pro | 167 | 52,67,74,89 | 52,67,74,89 | C = V | L-Pro |
| 78 | 33 | 33 | C = V | L-Sec | 168 | 66,30 | 66,30 | C = V | E-Sec |
| 79 | 31,90 | 31,90 | C = V | L-Sec | 169 | 16 | 16 | C = V | E-Pro |
| 80 | 39,59,40 | 39,59,40 | C = V | E-Sec | 170 | 52,59 | 59 | C > V | E-Pro |
| 81 | 58 | 58 | C = V | E-Sec | 171 | 16,51,52,61 | 16,51,52,61 | C = V | E-Sec |
| 82 | 51,61,74 | 61,74 | C > V | E-Sec | 172 | 16 | 16 | C = V | E-Pro |
| 83 | 66 | 66 | C = V | E-Sec | 173 | 52,82 | 52,82 | C = V | Mens |
| 84 | 66,40 | 40 | C > V | E-Pro | 174 | 33,52 | Negative | N | E-Pro |
| 85 | 56 | Negative | N | L-Sec | 175 | 58 | 58 | C = V | L-Pro |
| 86 | 59 | 59 | C = V | E-Sec | 176 | 51 | 51 | C = V | E-Pro |
| 87 | 31,52,69,42,55,74 | 31,52,69,42,55, 74 | C = V | E-Sec | 177 | 82 | 82 | C = V | E-Sec |
| 88 | 52,67,74 | 52,67,74 | C = V | L-Sec | 178 | 18 | 18 | C = V | L-Pro |
| 89 | 16,52 | 16,52 | C = V | L-Pro | 179 | 66 | 66 | C = V | L-Sec |
| 90 | 52 | Negative | N | E-Pro | 180 | 18,31,52,59,67,55,74,90 | 31,52,67,55,74,90 | C > V | L-Sec |
| Accumulation of Exfoliated Cells | |||
|---|---|---|---|
| Sufficient | Insufficient | ||
| Menstrual phases | Frequencies | 9 | 2 |
| Total percentage | 82% | 18% | |
| Expected frequencies | 8.1 | 2.9 | |
| Adjusted residual | 0.7 | −0.7 | |
| Early proliferative phase | Frequencies | 16 | 15 |
| Total percentage | 52% | 48% | |
| Expected frequencies | 22.7 | 8.3 | |
| Adjusted residual | −3.0 * | 3.0 * | |
| Late proliferative phase | Frequencies | 27 | 7 |
| Total percentage | 79% | 21% | |
| Expected frequencies | 24.9 | 9.1 | |
| Adjusted residual | 0.9 | −0.9 | |
| Early secretory phases | Frequencies | 45 | 10 |
| Total percentage | 82% | 18% | |
| Expected frequencies | 40.3 | 14.7 | |
| Adjusted residual | 1.7 | −1.7 | |
| Late secretory phases | Frequencies | 35 | 14 |
| Total percentage | 71% | 29% | |
| Expected frequencies | 35.9 | 13.1 | |
| Adjusted residual | −0.4 | 0.4 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okodo, M.; Okayama, K.; Teruya, K.; Tanabe, K.; Ito, C.; Ishii, Y.; Fujii, M.; Kimura, H.; Oda, M. Effects of Menstrual Cycle on the Accumulation of Human Papillomavirus-Infected Cells Exfoliated from the Cervix That Drift into the Vagina. Microorganisms 2022, 10, 693. https://doi.org/10.3390/microorganisms10040693
Okodo M, Okayama K, Teruya K, Tanabe K, Ito C, Ishii Y, Fujii M, Kimura H, Oda M. Effects of Menstrual Cycle on the Accumulation of Human Papillomavirus-Infected Cells Exfoliated from the Cervix That Drift into the Vagina. Microorganisms. 2022; 10(4):693. https://doi.org/10.3390/microorganisms10040693
Chicago/Turabian StyleOkodo, Mitsuaki, Kaori Okayama, Koji Teruya, Kazumasa Tanabe, Chieko Ito, Yasuyoshi Ishii, Masahiko Fujii, Hirokazu Kimura, and Mizue Oda. 2022. "Effects of Menstrual Cycle on the Accumulation of Human Papillomavirus-Infected Cells Exfoliated from the Cervix That Drift into the Vagina" Microorganisms 10, no. 4: 693. https://doi.org/10.3390/microorganisms10040693
APA StyleOkodo, M., Okayama, K., Teruya, K., Tanabe, K., Ito, C., Ishii, Y., Fujii, M., Kimura, H., & Oda, M. (2022). Effects of Menstrual Cycle on the Accumulation of Human Papillomavirus-Infected Cells Exfoliated from the Cervix That Drift into the Vagina. Microorganisms, 10(4), 693. https://doi.org/10.3390/microorganisms10040693







