Multidisciplinary Management of Suspected Lyme Borreliosis: Clinical Features of 569 Patients, and Factors Associated with Recovery at 3 and 12 Months, a Prospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
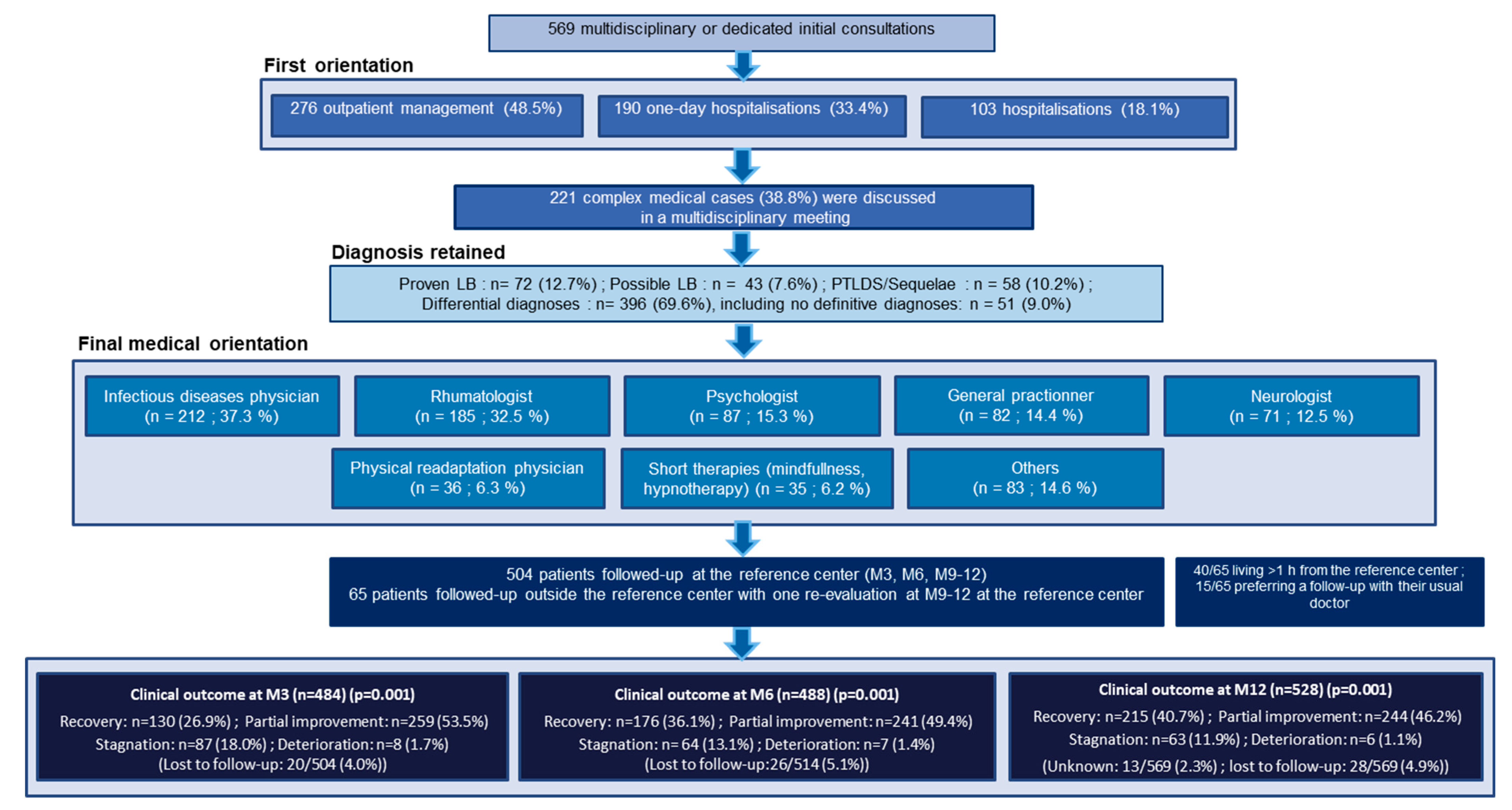
2.1. Population, Setting, and Intervention
2.2. Patient Data
2.3. Statistical Analysis
2.4. Approval of the Ethics Committee
3. Results
3.1. Comparison of the Clinical and Epidemiological Characteristics of the Patients
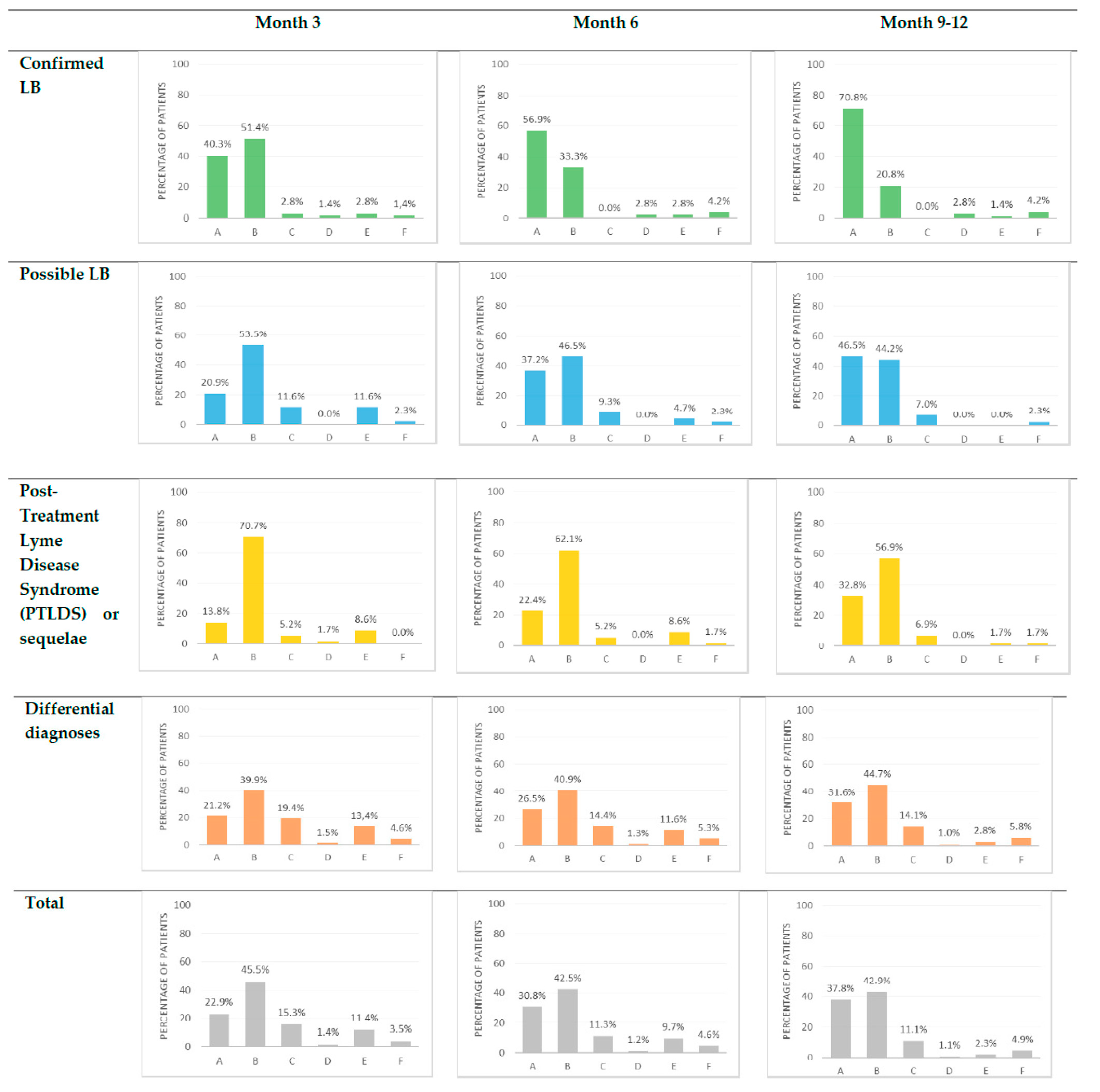
3.2. Factors Associated with Rapid Recovery
3.3. Factors Associated with Recovery at a Later Point in Time
3.4. Description of Patients with Stagnation or Deterioration in the Groups with a Primary Diagnosis of LB at 3 Months
4. Discussion
4.1. Summary of the Principal Findings
4.2. Similar Multidisciplinary Experiences in France and Europe
4.3. Meaning of the Study and Implication for Practice
4.3.1. Serology Does Not Rule the Diagnosis of LB
4.3.2. Non-Recommended Antibiotic Therapies Are Associated with a Poorer Clinical Evolution
4.3.3. A Longer Delay between the Onset of Symptoms and the First Consultation at the TBD-RC Is Associated with a Poorer Evolution
4.4. Strengths and Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schwartz, A.M.; Hinckley, A.F.; Mead, P.S.; Hook, S.A.; Kugeler, K.J. Surveillance for Lyme Disease-United States, 2008–2015. Morb. Mortal. Wkly. Rep. Surveill. Summ. 2017, 66, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sykes, R.A.; Makiello, P. An estimate of Lyme borreliosis incidence in Western Europe. J. Public Health 2017, 39, 74–81. [Google Scholar] [CrossRef] [Green Version]
- Santé Publique France: Borréliose de Lyme. Available online: https://www.santepubliquefrance.fr/maladies-et-traumatismes/maladies-a-transmission-vectorielle/borreliose-de-lyme/donnees/#tabs (accessed on 11 March 2022).
- Septfons, A.; Goronflot, T.; Jaulhac, B.; Roussel, V.; De Martino, S.; Guerreiro, S.; Launay, T.; Fournier, L.; de Valk, H.; Figoni, J.; et al. Epidemiology of Lyme borreliosis through two surveillance systems: The national Sentinelles GP network and the national hospital discharge database, France 2005 to 2016. Euro Surveill. 2019, 24, 1800134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanek, G.; Fingerle, V.; Hunfeld, K.-P.; Jaulhac, B.; Kaiser, R.; Krause, A.; Kristoferitsch, W.; O’Connell, S.; Ornstein, K.; Strle, F.; et al. Lyme borreliosis: Clinical case definitions for diagnosis and management in Europe. Clin. Microbiol. Infect. 2011, 17, 69–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rupprecht, T.A.; Birnbaum, T.; Pfister, H.-W. Pain and neuroborreliosis: Significance, diagnosis and treatment. Schmerz. Berl. Ger. 2008, 22, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Voitey, M.; Bouiller, K.; Chirouze, C.; Fournier, D.; Bozon, F.; Klopfenstein, T. Functional signs in patients consulting for presumed Lyme borreliosis. Med. Mal. Infect. 2019, 50, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, J.; Bernasconi, E.; Heininger, U.; Abbas, M.; Nadal, D.; Strahm, C.; Erb, S.; Zimmerli, S.; Furrer, H.; Delaloye, J.; et al. Update of the Swiss guidelines on post-treatment Lyme disease syndrome. Swiss Med. Wkly. 2016, 146, w14353. [Google Scholar] [CrossRef] [Green Version]
- Müllegger, R.R.; Glatz, M. Skin manifestations of Lyme borreliosis: Diagnosis and management. Am. J. Clin. Dermatol. 2008, 9, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Boyer, P.; Kieffer, P.; De Martino, S.; Zilliox, L.; Vogel, J.; Jaulhac, B.; Hansmann, Y. Borrelia burgdorferi sl and tick-borne encephalitis virus coinfection in Eastern France. Med. Mal. Infect. 2018, 48, 218–220. [Google Scholar] [CrossRef] [PubMed]
- Figoni, J.; Chirouze, C.; Hansmann, Y.; Lemogne, C.; Hentgen, V.; Saunier, A.; Bouiller, K.; Gehanno, J.; Rabaud, C.; Perrot, S.; et al. Lyme borreliosis and other tick-borne diseases. Guidelines from the French Scientific Societies (I): Prevention, epidemiology, diagnosis. Med. Mal. Infect. 2019, 49, 318–334. [Google Scholar] [CrossRef] [PubMed]
- Lohr, B.; Fingerle, V.; Norris, D.; Hunfeld, K.-P. Laboratory diagnosis of Lyme borreliosis: Current state of the art and future perspectives. Crit. Rev. Clin. Lab. Sci. 2018, 55, 219–245. [Google Scholar] [CrossRef]
- Leeflang, M.M.G.; Ang, C.W.; Berkhout, J.; Bijlmer, H.A.; Van Bortel, W.; Brandenburg, A.H.; Van Burgel, N.D.; Van Dam, A.P.; Dessau, R.B.; Fingerle, V.; et al. The diagnostic accuracy of serological tests for Lyme borreliosis in Europe: A systematic review and meta-analysis. BMC Infect. Dis. 2016, 16, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talagrand-Reboul, E.; Raffetin, A.; Zachary, P.; Jaulhac, B.; Eldin, C. Immunoserological Diagnosis of Human Borrelioses: Current Knowledge and Perspectives. Front. Cell. Infect. Microbiol. 2020, 10, 241. [Google Scholar] [CrossRef] [PubMed]
- Jaulhac, B.; Saunier, A.; Caumes, E.; Bouiller, K.; Gehanno, J.; Rabaud, C.; Perrot, S.; Eldin, C.; de Broucker, T.; Roblot, F.; et al. Lyme borreliosis and other tick-borne diseases. Guidelines from the French scientific societies (II). Biological diagnosis, treatment, persistent symptoms after documented or suspected Lyme borreliosis. Med. Mal. Infect. 2019, 49, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Eldin, C.; Raffetin, A.; Bouiller, K.; Hansmann, Y.; Roblot, F.; Raoult, D.; Parola, P. Review of European and American guidelines for the diagnosis of Lyme borreliosis. Med. Mal. Infect. 2019, 49, 121–132. [Google Scholar] [CrossRef]
- Nguala, S.; Baux, E.; Patrat-Delon, S.; Saunier, F.; Schemoul, J.; Tattevin, P.; Cazorla, C.; Eldin, C.; Bouiller, K.; Raffetin, A. Methodological Quality Assessment with the AGREE II Scale and a Comparison of European and American Guidelines for the Treatment of Lyme Borreliosis: A Systematic Review. Pathogens 2021, 10, 972. [Google Scholar] [CrossRef]
- Berende, A.; Ter Hofstede, H.J.; Vos, F.J.; van Middendorp, H.; Vogelaar, M.L.; Tromp, M.; Hoogen, F.H.V.D.; Donders, A.R.T.; Evers, A.; Kullberg, B.J. Randomized Trial of Longer-Term Therapy for Symptoms Attributed to Lyme Disease. N. Engl. J. Med. 2016, 374, 1209–1220. [Google Scholar] [CrossRef] [PubMed]
- Fallon, B.A.; Keilp, J.G.; Corbera, K.M.; Petkova, E.; Britton, C.B.; Dwyer, E.; Slavov, I.; Cheng, J.; Dobkin, J.; Nelson, D.R.; et al. A randomized, placebo-controlled trial of repeated IV antibiotic therapy for Lyme encephalopathy. Neurology 2008, 70, 992–1003. [Google Scholar] [CrossRef] [Green Version]
- Klempner, M.S.; Hu, L.T.; Evans, J.; Schmid, C.; Johnson, G.M.; Trevino, R.P.; Norton, D.; Levy, L.; Wall, D.; McCall, J.; et al. Two Controlled Trials of Antibiotic Treatment in Patients with Persistent Symptoms and a History of Lyme Disease. N. Engl. J. Med. 2001, 345, 85–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krupp, L.B.; Hyman, L.G.; Grimson, R.; Coyle, P.K.; Melville, P.; Ahnn, S.; Dattwyler, R.; Chandler, B. Study and treatment of post Lyme disease (STOP-LD): A randomized double masked clinical trial. Neurology 2003, 60, 1923–1930. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, R.F.; Trevino, R.P.; Johnson, G.M.; Levy, L.; Dornbush, R.; Hu, L.T.; Evans, J.; Weinstein, A.; Schmid, C.; Klempner, M.S. Cognitive function in post-treatment Lyme disease Do additional antibiotics help? Neurology 2003, 60, 1916–1922. [Google Scholar] [CrossRef]
- Raffetin, A.; Barquin, A.; Nguala, S.; Paoletti, G.; Rabaud, C.; Chassany, O.; Caraux-Paz, P.; Covasso, S.; Partouche, H. Perceptions, Representations, and Experiences of Patients Presenting Nonspecific Symptoms in the Context of Suspected Lyme Borreliosis. Microorganisms 2021, 9, 1515. [Google Scholar] [CrossRef]
- Drew, D.; Hewitt, H. A Qualitative Approach to Understanding Patients’ Diagnosis of Lyme Disease. Public Health Nurs. 2006, 23, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, C.R.; Lloyd, V.K.; Gould, O.N. Motivations and Experiences of Canadians Seeking Treatment for Lyme Disease Outside of the Conventional Canadian Health-Care System. J. Patient Exp. 2017, 5, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Vitulano, L.; Lee, R.; Weiss, T.R.; Colson, E.R. Experiences of patients identifying with chronic Lyme disease in the healthcare system: A qualitative study. BMC Fam. Pr. 2014, 15, 79. [Google Scholar] [CrossRef] [Green Version]
- Coumou, J.; Herkes, E.; Brouwer, M.; van de Beek, D.; Tas, S.; Casteelen, G.; van Vugt, M.; Starink, M.; de Vries, H.; de Wever, B.; et al. Ticking the right boxes: Classification of patients suspected of Lyme borreliosis at an academic referral center in the Netherlands. Clin. Microbiol. Infect. 2015, 21, 368.e11–368.e20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacquet, C.; Goehringer, F.; Baux, E.; Conrad, J.; Devonec, M.G.; Schmutz, J.; Mathey, G.; Tronel, H.; Moulinet, T.; Chary-Valckenaere, I.; et al. Multidisciplinary management of patients presenting with Lyme disease suspicion. Med. Mal. Infect. 2019, 49, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Gynthersen, R.M.; Tetens, M.M.; Ørbæk, M.; Haahr, R.; Fana, V.; Hansen, K.; Mens, H.; Andersen, A.B.; Lebech, A.-M. Classification of patients referred under suspicion of tick-borne diseases, Copenhagen, Denmark. Ticks Tick-Borne Dis. 2021, 12, 101591. [Google Scholar] [CrossRef] [PubMed]
- Kortela, E.; Kanerva, M.; Kurkela, S.; Oksi, J.; Järvinen, A. Suspicion of Lyme borreliosis in patients referred to an infectious diseases clinic: What did the patients really have? Clin. Microbiol. Infect. 2020, 27, 1022–1028. [Google Scholar] [CrossRef] [PubMed]
- Dessau, R.; Van Dam, A.; Fingerle, V.; Gray, J.; Hovius, J.; Hunfeld, K.-P.; Jaulhac, B.; Kahl, O.; Kristoferitsch, W.; Lindgren, P.-E.; et al. To test or not to test? Laboratory support for the diagnosis of Lyme borreliosis—Author’s reply. Clin. Microbiol. Infect. 2018, 24, 211–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steere, A.C.; Strle, F.; Wormser, G.P.; Hu, L.T.; Branda, J.A.; Hovius, J.W.R.; Li, X.; Mead, P.S. Lyme borreliosis. Nat. Rev. Dis. Primer. 2016, 2, 16090. [Google Scholar] [CrossRef] [PubMed]
- Haute Autorité de Santé (HAS). Borréliose de Lyme et Autres Maladies Vectorielles à Tiques; Haute Autorité de Santé: Saint-Denis, France, 2018; pp. 1–52. Available online: https://www.has-sante.fr/upload/docs/application/pdf/2018-06/reco266_rbp_borreliose_de_lyme_cd_2018_06_13__recommandations.pdf (accessed on 11 March 2022).
- Montgomery, S.A.; Åsberg, M. A New Depression Scale Designed to be Sensitive to Change. Br. J. Psychiatry 1979, 134, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Rush, A.; Trivedi, M.H.; Ibrahim, H.M.; Carmody, T.J.; Arnow, B.; Klein, D.N.; Markowitz, J.C.; Ninan, P.; Kornstein, S.; Manber, R.; et al. The 16-Item quick inventory of depressive symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): A psychometric evaluation in patients with chronic major depression. Biol. Psychiatry 2003, 54, 573–583. [Google Scholar] [CrossRef]
- Spielberger, C.D.; Gorsuch, R.L.; Lushene, R.E.; Vagg, P.R.; Jacobs, G.A. State-Trait Anxiety Inventory; Consulting Psychologists Press: Palo Alto, CA, USA, 1983. [Google Scholar]
- Singh, S.K.; Girschick, H.J. Lyme borreliosis: From infection to autoimmunity. Clin. Microbiol. Infect. 2004, 10, 598–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arvikar, S.L.; Steere, A.C. Diagnosis and Treatment of Lyme Arthritis. Infect. Dis. Clin. North Am. 2015, 29, 269–280. [Google Scholar] [CrossRef] [Green Version]
| Final Diagnoses | Diagnoses Implicating LB (N = 249/569) | Diagnoses with No Links with LB (N = 320/569) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Confirmed LB (n,%) | Possible LB (n,%) | PTLDS/Sequelae * (n,%) | Other Diagnoses (n,%) | Other Differential Diagnoses (n,%) | |||||
| PTLDS | Sequelae of LB | Failure of the Antibiotics Test ** | Complete Recovery of a Treated LB | Monitoring after a Tick-Bite | |||||
| Total (N = 569, 100%) | 72 (12.7) | 43 (7.6) | 51 (9.0) | 7 (1.2) | 24 (4.2) | 39 (6.9) | 13 (2.3) | 320 (56.2) | |
| EM | 26 (4.6) *** | - | 14 (2.5) **** | - | - | - | - | - | |
| Lymphocytoma | 2 (0.4) | - | - | - | - | - | - | - | |
| Early LNB | 17 (3.0) | 8 (1.4) | 9 (1.6) | 3 (0.5) | 2 (0.4) | - | - | - | |
| Early Lyme arthritis | 2 (0.4) | 3 (0.5) | - | 1 (0.2) | - | - | - | - | |
| Early disseminated non-specific LB | 2 (0.4) | 3 (0.5) | 8 (1.4) | - | 2 (0.4) | - | - | - | |
| Late LNB | 16 (2.8) | 19 (3.3) | 6 (1.1) | 3 (0.5) | 7 (1.2) | - | - | - | |
| Late Lyme arthritis | 0 (0) | 2 (0.4) | - | - | 1 (0.2) | - | - | - | |
| Early cardiac LB | 4 (0.7) | - | - | - | - | - | - | - | |
| ACA | 3 (0.5) ***** | - | - | - | - | - | - | - | |
| Late disseminated non-specific LB | - | 6 (1.1) | 4 (0.7) | - | 3 (0.5) | - | - | - | |
| Unknown | - | 2 (0.4) | 11 (1.9) | - | 9 (1.6) | - | - | - | |
| Other Infectious Diseases | 68/569 (12.0) |
|---|---|
| Other tick-borne diseases (rickettsiosis, tularemia etc.) | 9 (1.6) |
| Other bacterial infections (cutaneous infectious, tuberculosis, pneumonia etc.) | 14 (2.5) |
| Viral infections (Epstein Barr Virus, Herpes Virus, Cytomegalovirus etc.) | 22 (3.9) |
| Parasitic infections (larva migrans, schistosoma, toxocara etc.) | 10 (1.8) |
| Post-infectious syndrome | 13 (2.3) |
| Rheumatological and auto-immune diseases | 228/569 (40.1) |
| Chronic inflammatory rheumatism (spondylarthritis, rheumatoid arthritis etc.) | 55 (9.7) |
| Arthrosis and complications | 59 (10.4) |
| Tunnel syndrome | 47 (8.3) |
| Tendinopathy | 24 (4.2) |
| Other rheumatological diseases | 12 (2.1) |
| Auto-immune diseases (Gougerot-Sjogren disease, multiple sclerosis, lupus etc.) | 31 (5.5) |
| Neurological disorders | 109/569 (19.2) |
| Peripheral neuropathy | 26 (4.6) |
| Dementia | 10 (1.8) |
| Optical neuritis | 5 (0.9) |
| Sequelae of stroke | 5 (0.8) |
| Others (parkinsonism, Charcot’s disease etc.) | 10 (1.8) |
| Vitamin deficiencies (B9, D, PP, C etc.) | 98/569 (17.2) |
| Psychiatric disorders | 68/569 (12.0) |
| Anxiety and/or depression | 43 (7.6) |
| Psychotic disorders | 11 (1.9) |
| Panic disorder | 6 (1.1) |
| Others (addiction, post-traumatic syndrome, bipolar disorders etc.) | 14 (2.5) |
| Iatrogenism linked to a prolonged antibiotic therapy | 65/569 (11.4) |
| Bodily Distress Syndrome | 52/569 (9.1) |
| Endocrinopathy (thyroid disorders, adrenal disorders etc.) | 21/569 (3.7) |
| Others (cancers, sleep apnea syndrome, genetic diseases, cardiovascular diseases etc.) | 67/569 (11.8) |
| No specific diagnosis | 51/569 (9.0) |
| Epidemiological Characteristics of the Patients | Total N = 569 (%) | Confirmed LB N = 72 (%) | Possible LB N = 43 (%) | PTLDS or Sequelae N = 58 (%) | Other Diagnoses N = 396 (%) | p-Value |
|---|---|---|---|---|---|---|
| Age, years (median [IQ 25,75]) | 48 (35.61) | 52.5 (36.65) | 52 (46.59) | 47.5 (36.64) | 47 (34.60) | 0.14 |
| Male | 220 (38.7) | 42 (58.3) | 19 (44.2) | 15 (25.9) | 144 (36.4) | 0.001 |
| Life style | 0.74 | |||||
| Home in a rural area | 121 (21.2) | 12 (16.7) | 13 (30.2) | 14 (24.1) | 82 (20.7) | |
| Employment in rural areas/forest | 30 (5.3) | 4 (5.6) | 1 (2.3) | 3 (5.2) | 22 (5.6) | |
| Forest-based leisure activities | 399 (70) | 55 (76.4) | 28 (65.1) | 40 (69.0) | 276 (69.7) | |
| No exposure | 20 (3.5) | 1 (1.4) | 1 (2.3) | 1 (1.7) | 16 (4.0) | |
| Past history of tick-bite | 372 (65.3) | 59 (81.9) | 33 (76.7) | 46 (79.3) | 234 (59.1) | <0.001 |
| Past history of erythema migrans | 145 (25.4) | 39 (54.2) | 18 (41.9) | 25 (43.9) | 64 (16.2) | <0.001 |
| Patients referred by a physician with a letter | 516 (90.7) | 69 (95.8) | 42 (97.7) | 51 (87.9) | 354 (89.4) | 0.016 |
| General Practitioner | 401 (70.4) | 46 (63.9) | 36 (83.7) | 46 (79.3) | 273 (68.9) | |
| Specialist physician | 94 (16.5) | 17 (23.6) | 4 (9.3) | 5 (8.6) | 68 (17.2) | |
| Emergency unit physician | 21 (3.7) | 6 (8.3) | 2 (4.7) | 0 (0.0) | 13 (3.3) | |
| No letter, patient self-referral | 53 (9.5) | 3 (4.2) | 1 (2.33) | 7 (12.1) | 42 (10.6) | |
| Duration (days) of chief complaints prior to examination at TBD-RC (median [IQ 25,75]) | 512 [156,1392.5] | 123.5 (37,233) | 296 (132,1138) | 374.5 (167,1078) | 735 (219,1778) | <0.001 |
| Patient’s chief complaint | <0.001 | |||||
| Erythema migrans | 17 (3) | 8 (11.1) | 0 (0.0) | 1 (1.7) | 8 (2.0) | |
| Clinical signs/symptoms implicating early disseminated LB (>six months) | 159 (27.9) | 40 (55.6) | 17 (39.5) | 19 (32.8) | 83 (21.0) | |
| Clinical signs/symptoms implicating late disseminated LB (>six months) | 382 (67.2) | 24 (33.3) | 26 (60.5) | 38 (65.5) | 294 (74.2) | |
| Questions after a tick-bite | 6 (1.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 6 (1.5) | |
| Positive serological test with no clinical signs | 5 (0.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 5 (1.26) | |
| Serological test | <0.001 | |||||
| IgM and/or IgG positive in ELISA and WB | 180 (31.6) | 54 (75.0) | 18 (41.9) | 34 (58.6) | 74 (18.7) | |
| IgG positive in ELISA only | 75 (13.2) | 5 (6.9) | 10 (23.3) | 9 (15.5) | 51 (12.9) | |
| IgM and IgG negative in ELISA | 276 (48.5) | 7 (9.7) | 15 (34.9) | 15 (25.9) | 239 (60.4) | |
| No serology (suspicion of erythema migrans) | 38 (6.7) | 6 (8.3) | 0 (0.00) | 0 (0.00) | 32 (8.1) | |
| Antibiotic therapy prescribed before TBD-RC | 369 (64.9) | 51 (70.8) | 27 (62.8) | 58 (100.0) | 233 (58.8) | <0.001 |
| Antibiotic therapy > four weeks | 117 (22.6) | 15 (20.8) | 4 (9.3) | 29 (50.0) | 69 (17.4) | <0.001 |
| Non-recommended treatments (>eight weeks of antibiotics and/or associated antimicrobials) | 101 (17.8) | 7 (9.7) | 1 (2.3) | 23 (39.7) | 70 (17.7) | <0.001 |
| Clinical Signs | Total N = 569 (%) | Confirmed LB N = 72 (%) | Possible LB N = 43 (%) | PTLDS or Sequelae N = 58 (%) | Other Diagnoses N = 396 (%) | p-Value |
|---|---|---|---|---|---|---|
| Polymyalgia | 213 (37.4) | 22 (30.6) | 17 (39.5) | 21 (36.2) | 153 (38.6) | 0.611 |
| Polyarthralgia | 300 (52.7) | 34 (47.2) | 22 (51.2) | 30 (51.7) | 214 (54.0) | 0.749 |
| Asthenia | 380 (66.8) | 48 (66.7) | 35 (81.4) | 48 (82.8) | 249 (62.9) | 0.004 |
| Fever, chills | 49 (8.6) | 2 (2.8) | 7 (16.3) | 1 (1.7) | 39 (9.9) | 0.014 |
| Night Sweat | 50 (8.8) | 1 (1.4) | 3 (7.0) | 3 (5.2) | 43 (10.9) | 0.043 |
| Paresthesia | 225 (39.5) | 27 (37.5) | 26 (60.5) | 21 (36.2) | 151 (38.1) | 0.035 |
| Headache | 141 (35.6) | 26 (36.1) | 18 (41.9) | 24 (41.4) | 209 (36.7) | 0.740 |
| Insomnia | 96 (16.9) | 11 (15.3) | 7 (16.3) | 9 (15.5) | 69 (17.4) | 0.959 |
| Loss of weight | 72 (12.7) | 3 (4.2) | 7 (16.3) | 4 (6.9) | 58 (14.7) | 0.039 |
| Arthritis-small joints | 39 (6.9) | 2 (2.8) | 0 (0.0) | 7 (12.1) | 30 (7.6) | 0.050 |
| Arthritis-large joints | 71 (12.5) | 8 (11.1) | 9 (20.9) | 9 (15.5) | 45 (11.4) | 0.275 |
| Facial palsy | 19 (3.4) | 10 (13.9) | 1 (2.3) | 4 (6.9) | 4 (1.0) | 0.001 |
| Neuropathic pain | 130 (22.9) | 22 (30.6) | 18 (41.9) | 14 (24.1) | 76 (19.2) | 0.003 |
| Memory impairment | 99 (17.4) | 8 (11.1) | 11 (25.6) | 13 (22.4) | 67 (16.9) | 0.167 |
| Concentration impairment | 94 (16.5) | 5 (6.9) | 11 (25.6) | 14 (24.1) | 64 (16.2) | 0.020 |
| Radicular pain | 62 (10.9) | 7 (9.7) | 10 (23.3) | 8 (13.8) | 37 (9.3) | 0.039 |
| Spinal pain | 116 (20.4) | 11 (15.3) | 10 (23.3) | 7 (12.1) | 88 (22.2) | 0.198 |
| Vertigo | 85 (14.9) | 5 (6.9) | 9 (20.9) | 9 (15.5) | 62 (15.7) | 0.171 |
| Anxiety | 103 (18.1) | 9 (12.5) | 11 (25.6) | 4 (6.9) | 79 (20.0) | 0.030 |
| Sadness | 66 (11.6) | 5 (6.9) | 10 (23.3) | 6 (10.3) | 45 (11.4) | 0.062 |
| Psychotic disorders | 24 (4.2) | 0 (0.0) | 2 (4.7) | 0 (0.0) | 22 (5.6) | 0.058 |
| Cardiac conduction disturbances | 5 (0.9) | 3 (4.2) | 0 (0.0) | 0 (0.0) | 2 (0.5) | 0.015 |
| Risk Factor | N (n = 484) | n(%) Rapid Recovery at 3 Months | Crude OR [95% CI] | p-Value | Adjusted OR [95% CI] | p-Value |
|---|---|---|---|---|---|---|
| Age (years) | 0.23 | 0.26 | ||||
| <35 | 127 | 39 (30.7) | 1 | 1 | ||
| 35–48 | 121 | 25 (20.7) | 0.59 (0.33–1.05) | 0.56 (0.29–1.06) | ||
| 48–61 | 114 | 29 (25.4) | 0.77 (0.44–1.36) | 0.72 (0.38–1.38] | ||
| >61 | 122 | 37 (30.3) | 0.98 (0.57–1.68) | 0.95 (0.51–1.76] | ||
| Sex | 0.17 | 0.98 | ||||
| Male | 188 | 57 (30.3) | 1.33 (0.88–2.00) | 1.01 (0.63–1.60] | ||
| Female | 296 | 73 (24.6) | 1 | 1 | ||
| History of tick-bite | 0.19 | - | - | |||
| Yes | 328 | 94 (28.7) | 1.34 (0.86–2.08) | |||
| No | 156 | 36 (23.1) | 1 | |||
| History of erythema migrans | 0.023 | - | - | |||
| Yes | 120 | 43 (35.8) | 1.67 (1.08–2.58) | |||
| No | 331 | 76 (23.0) | 1 | |||
| Serology | 0.004 | - | - | |||
| Positive serology in ELISA and WB | 154 | 45 (29.2) | 1.51 (0.95–2.40) | |||
| Positive serology in ELISA only | 65 | 19 (29.2) | 1.51 (0.81–2.79) | |||
| Negative serology in ELISA | 237 | 51 (21.5) | 1 | |||
| Patient with no serology (erythema migrans) | 28 | 15 (53.6) | 4.21 (1.88–9.41) | |||
| Delay 1st symptoms-1st consultation at the TBD-RC | <0.001 | 0.001 | ||||
| 0–155 days (0.0–0.4 year) | 131 | 53 (40.5) | 1 | 1 | ||
| 155–512 days (0.4–1.4 years) | 129 | 41 (31.8) | 0.69 (0.41–1.14) | 0.86 (0.48–1.52) | ||
| 512–1393 days (1.4–3.8 years) | 115 | 24 (20.9) | 0.39 (0.22–0.69) | 0.51 (0.26–0.97) | ||
| >1393 days (>3.8 years) | 108 | 12 (11.1) | 0.18 (0.09–0.37) | 0.22 (0.10–0.47) | ||
| Delay 1st consultation at the TBD-RC-final diagnosis | <0.001 | <0.001 | ||||
| 0 day | 189 | 80 (42.3) | 1 | 1 | ||
| 1–15 days | 30 | 10 (33.3) | 0.68 (0.30–1.53) | 0.62 (0.26–1.49) | ||
| 15–83 days | 142 | 23 (16.2) | 0.26 (0.15–0.45) | 0.27 (0.15–0.49) | ||
| >83 days | 123 | 17 (13.8) | 0.22 (0.12–0.39) | 0.28 (0.15–0.53) | ||
| Final diagnosis | 0.008 | 0.22 | ||||
| Confirmed LB | 69 | 29 (42.0) | 2.08 (1.21–3.56) | 0.93 (0.49–1.77) | ||
| Possible LB | 37 | 9 (24.3) | 0.92 (0.42–2.03) | 1.13 (0.46–2.75) | ||
| PTLDS or sequelae | 53 | 8 (15.1) | 0.51 (0.23–1.13) | 0.40 (0.17–0.96) | ||
| Other diagnoses | 325 | 84 (25.9) | 1 | 1 | ||
| Number of diagnosis per patient | <0.001 | 0.004 | ||||
| 1 diagnosis | 254 | 93 (36.6) | 1 | 1 | ||
| 2 diagnoses | 139 | 24 (17.3) | 0.36 (0.22–0.60) | 0.43 (0.25–0.75) | ||
| ≥3 diagnoses | 91 | 13 (14.3) | 0.29 [0.15–0.55) | 0.46 (0.23–0.93) | ||
| Antibiotics prescribed before the TBD-RC | 0.50 | - | - | |||
| Yes | 317 | 82 (25.9) | 0.87 (0.57–1.32) | |||
| No | 167 | 48 (28.7) | 1 | |||
| History of non-recommended antibiotics | <0.001 | 0.023 | ||||
| Yes | 83 | 10 (12.1) | 0.32 (0.16–0.64) | 0.41 (0.19–0.88) | ||
| No | 401 | 120 (29.9) | 1 | 1 | ||
| First line of antibiotics prescribed at the TBD-RC | 0.036 | - | - | |||
| Yes | 140 | 47 (33.6) | 1.59 (1.03–2.44) | |||
| No | 344 | 83 (24.1) | 1 | |||
| Second line of antibiotics at the TBD-RC | 0.75 | - | - | |||
| Yes | 17 | 4 (23.5) | 0.83 (0.27–2.60) | |||
| No | 467 | 126 (27.0) | 1 |
| Risk Factor | N (n = 528) | n(%) Cured Patients at 12 Months | Crude OR [95% CI] | p-Value | Adjusted OR [95% CI] | p-Value |
|---|---|---|---|---|---|---|
| Age (years) | 0.50 | 0.41 | ||||
| <35 | 138 | 60 (43.5) | 1 | 1 | ||
| 35–47 | 126 | 50 (39.7) | 0.86 (0.52–1.40) | 0.78 (0.46–1.34) | ||
| 48–61 | 129 | 46 (35.7) | 0.72 (0.44–1.18) | 0.65 (0.37–1.12) | ||
| >61 | 135 | 59 (43.7) | 1.01 (0.63–1.63) | 0.93 (0.55–1.58) | ||
| Gender | 0.022 | 0.17 | ||||
| Male | 200 | 94 (47.0) | 1.52 (1.06–2.17) | 1.31 (0.89–1.95) | ||
| Female | 328 | 121 (36.9) | 1 | 1 | ||
| History of tick-bite | 0.017 | - | - | |||
| Yes | 352 | 156 (44.3) | 1.58 (1.08–2.30) | |||
| No | 176 | 59 (33.5) | 1 | |||
| History of EM | <0.001 | 0.020 | ||||
| Yes | 143 | 77 (53.9) | 2.08 (1.41–3.07) | 1.70 (1.09–2.65) | ||
| No | 384 | 138 (35.9) | 1 | 1 | ||
| Serology | 0.028 | - | - | |||
| Positive serology in ELISA and WB | 170 | 82 (48.2) | 1.79 (1.20–2.66) | |||
| Positive serology in ELISA only | 70 | 30 (42.9) | 1.44 (0.84–2.47) | |||
| Negative serology in ELISA | 254 | 87 (34.3) | 1 | |||
| Patient with no serology (erythema migrans) | 34 | 16 (47.1) | 1.71 (0.83–3.51) | |||
| Delay 1st symptoms-1st consultation at the TBD-RC | <0.001 | <0.001 | ||||
| 0–154 days (0.0–0.4 year) | 131 | 78 (59.5) | 1 | 1 | ||
| 155–511 days (0.4–1.4 years) | 138 | 67 (48.6) | 0.64 (0.40–1.04) | 0.82 (0.49–1.37) | ||
| 512–1393 days (1.4–3.8 years) | 130 | 44 (33.9) | 0.35 (0.21–0.58) | 0.47 (0.28–0.81) | ||
| >1393 days (>3.8 years) | 128 | 26 (20.3) | 0.17 (0.10–0.30) | 0.26 (0.14–0.46) | ||
| Delay 1st consultation at the TBD-RC-final diagnosis | 0.064 | - | - | |||
| 0 day | 228 | 107 (46.9) | 1 | |||
| 1–14 days | 33 | 14 (42.4) | 0.83 (0.40–1.74) | |||
| 15–83 days | 133 | 45 (33.8) | 0.58 (0.37–0.90) | |||
| >83 days | 134 | 49 (36.6) | 0.65 (0.42–1.01) | |||
| Final diagnosis | <0.001 | 0.004 | ||||
| Confirmed LB | 68 | 51 (75.0) | 5.69 (3.15–10.26) | 3.13 (1.64–5.96) | ||
| Possible LB | 42 | 20 (47.6) | 1.72 (0.91–3.28) | 1.34 (0.67–2.68) | ||
| PTLDS or sequelae | 56 | 19 (33.9) | 0.97 (0.54–1.76) | 0.85 (0.44–1.62) | ||
| Other diagnoses | 362 | 125 (34.5) | 1 | 1 | ||
| Number of diagnosis per patient | 0.005 | - | - | |||
| one diagnosis | 286 | 134 (46.9) | 1 | |||
| two diagnoses | 149 | 53 (35.6) | 0.63 (0.42–0.94) | |||
| ≥three diagnoses | 93 | 28 (30.1) | 0.49 (0.30–0.81) | |||
| Antibiotics prescribed before the TBD-RC | 0.52 | - | - | |||
| Yes | 345 | 137 (39.7) | 0.89 (0.62–1.28) | |||
| No | 183 | 78 (42.6) | 1 | |||
| History of non-recommended antibiotics | <0.001 | 0.05 | ||||
| Yes | 96 | 25 (26.0) | 0.45 (0.27–0.73) | 0.58 (0.34–1.01) | ||
| No | 432 | 190 (44.0) | 1 | 1 | ||
| First line of antibiotics prescribed at the TBD-RC | <0.001 | - | - | |||
| Yes | 143 | 84 (58.7) | 2.76 [1.86–4.09) | |||
| No | 385 | 131 (34.0) | 1 | |||
| Second line of antibiotics at the TBD-RC | 0.64 | - | - | |||
| Yes | 15 | 7 (46.7) | 1.28 (0.46–3.59) | |||
| No | 513 | 208 (40.6) | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raffetin, A.; Schemoul, J.; Chahour, A.; Nguala, S.; Caraux-Paz, P.; Paoletti, G.; Belkacem, A.; Medina, F.; Fabre, C.; Gallien, S.; et al. Multidisciplinary Management of Suspected Lyme Borreliosis: Clinical Features of 569 Patients, and Factors Associated with Recovery at 3 and 12 Months, a Prospective Cohort Study. Microorganisms 2022, 10, 607. https://doi.org/10.3390/microorganisms10030607
Raffetin A, Schemoul J, Chahour A, Nguala S, Caraux-Paz P, Paoletti G, Belkacem A, Medina F, Fabre C, Gallien S, et al. Multidisciplinary Management of Suspected Lyme Borreliosis: Clinical Features of 569 Patients, and Factors Associated with Recovery at 3 and 12 Months, a Prospective Cohort Study. Microorganisms. 2022; 10(3):607. https://doi.org/10.3390/microorganisms10030607
Chicago/Turabian StyleRaffetin, Alice, Julien Schemoul, Amal Chahour, Steve Nguala, Pauline Caraux-Paz, Giulia Paoletti, Anna Belkacem, Fernanda Medina, Catherine Fabre, Sébastien Gallien, and et al. 2022. "Multidisciplinary Management of Suspected Lyme Borreliosis: Clinical Features of 569 Patients, and Factors Associated with Recovery at 3 and 12 Months, a Prospective Cohort Study" Microorganisms 10, no. 3: 607. https://doi.org/10.3390/microorganisms10030607
APA StyleRaffetin, A., Schemoul, J., Chahour, A., Nguala, S., Caraux-Paz, P., Paoletti, G., Belkacem, A., Medina, F., Fabre, C., Gallien, S., Vignier, N., Madec, Y., & on the behalf of the Tick-Borne Diseases Reference Center-Paris and Northern Region Working Group. (2022). Multidisciplinary Management of Suspected Lyme Borreliosis: Clinical Features of 569 Patients, and Factors Associated with Recovery at 3 and 12 Months, a Prospective Cohort Study. Microorganisms, 10(3), 607. https://doi.org/10.3390/microorganisms10030607






