The Diagnostic Challenges and Clinical and Serological Outcome in Patients Hospitalized for Suspected Lyme Neuroborreliosis
Abstract
1. Introduction
2. Materials and Methods
2.1. The Study Design
- Neurological symptoms suggestive of LNB without other obvious reasons.
- CSF pleocytosis.
- Intrathecal B. burgdorferi antibody production.
2.2. CSF Analysis
2.3. Serological Analysis
2.4. Data Analysis
3. Results
3.1. Patient Characteristics at Inclusion
3.2. Patient Diagnosis Classification
3.3. Clinical Data, Serology Results, and Imaging—Associations with Possible LNB Diagnosis
3.4. Differential Diagnosis
3.5. Therapy
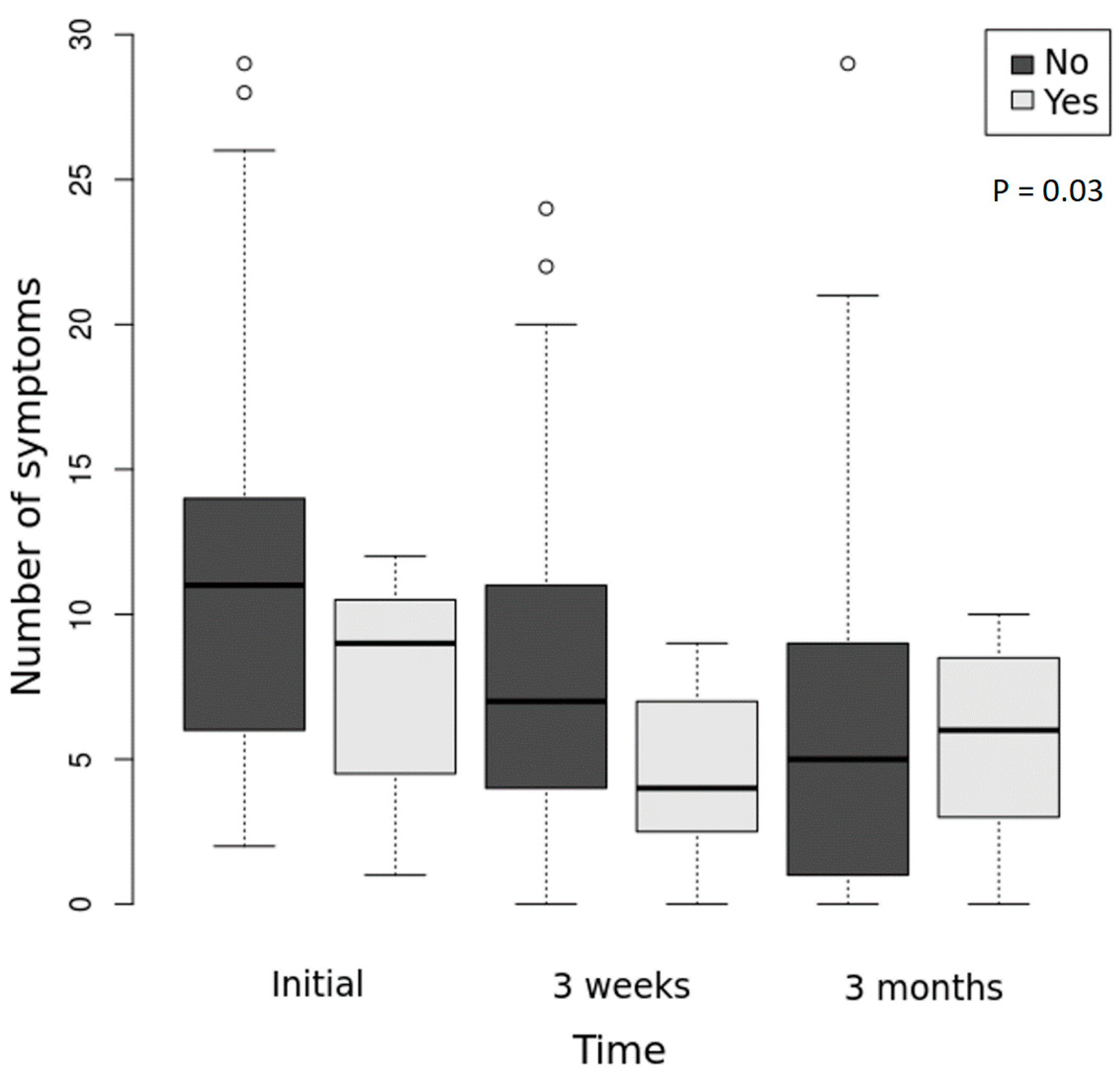
3.6. Clinical Follow-Up
3.7. Serological Follow-Up
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koedel, U.; Fingerle, V.; Pfister, H.-W. Lyme neuroborreliosis—Epidemiology, diagnosis and management. Nat. Rev. Neurol. 2015, 11, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Ljøstad, U.; Mygland, Å.; Chronic, L. Diagnostic and therapeutic challenges. Acta Neurol. Scand. Suppl. 2012, 127, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Mygland, A.; Ljøstad, U.; Fingerle, V.; Rupprecht, T.; Schmutzhard, E.; Steiner, I. EFNS guidelines on the diagnosis and management of European Lyme neuroborreliosis. Eur. J. Neurol. 2009, 17, 8-e4. [Google Scholar] [CrossRef] [PubMed]
- Jaulhac, B.; Saunier, A.; Caumes, E.; Bouiller, K.; Gehanno, J.F.; Rabaud, C.; Perrot, S.; Eldin, C.; de Broucker, T.; Roblot, F.; et al. Lyme borreliosis and other tick-borne diseases. Guidelines from the French scientific societies (II). Biological diagnosis, treatment, persistent symptoms after documented or suspected Lyme borreliosis. Médecine Et Mal. Infect. 2019, 49, 335–346. [Google Scholar] [CrossRef]
- Rauer, S.; Kastenbauer, S.; Fingerle, V.; Hunfeld, K.P.; Huppertz, H.I.; Dersch, R. Clinical practice guideline: Lyme neuroborreliosis. Dtsch. Arztebl. Int. 2018, 115, 751–756. [Google Scholar]
- Rauer, S.; Kastenbauer, S.; Hofmann, H.; Fingerle, V.; Huppertz, H.I.; Hunfeld, K.P.; Krause, A.; Ruf, B.; Dersch, R.; Consensus Group. Guidelines for diagnosis and treatment in neurology—Lyme neuroborreliosis. GMS Ger. Med. Sci. 2020, 18, Doc03. [Google Scholar]
- Briciu, V.T.; Flonta, M.; Leucuţa, D.; Cârstina, D.; Ţăţulescu, D.F.; Lupşe, M. A Lyme borreliosis diagnosis probability score—No relation with antibiotic treatment response. Infect. Dis. 2017, 49, 373–379. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; ISBN 3-900051-07-0. Available online: http://www.R-project.org/ (accessed on 29 November 2021).
- Hristea, A.; Hristescu, S.; Ciufecu, C.; Vasile, A. Seroprevalence of Borrelia burgdorferi in Romania. Eur. J. Epidemiol. 2001, 17, 891–896. [Google Scholar] [CrossRef]
- Mygland, Å.; Skarpaas, T.; Ljøstad, U. Chronic polyneuropathy and Lyme disease. Eur. J. Neurol. 2006, 13, 1213–1215. [Google Scholar] [CrossRef]
- Briciu, V.T.; Meyer, F.; Sebah, D.; Tăţulescu, D.F.; Coroiu, G.; Lupşe, M.; Carstina, D.; Mihalca, A.D.; Hizo-Teufele, C.; Klier, C.; et al. Real-time PCR-based identification of Borrelia burgdorferi sensu lato species in ticks collected from humans in Romania. Ticks Tick Borne Dis. 2014, 5, 575–581. [Google Scholar] [CrossRef]
- Vatne, A.; Mygland, A.; Ljostad, U. Multiple sclerosis in Vest-Agder County, Norway. Acta Neurol. Scand. 2011, 123, 396–399. [Google Scholar] [CrossRef]
- Galbussera, A.; Tremolizzo, L.; Isella, V.; Gelosa, G.; Vezzo, R.; Vigore, L.; Brenna, M.; Ferrarese, C.; Appollonio, I. Lack of evidence for Borrelia burgdorferi seropositivity in Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2008, 22, 308. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, M.; Bedlack, R.; Cudkowicz, M. Lyme disease serology in amyotrophic lateral sclerosis. Muscle Nerve 2009, 40, 626–628. [Google Scholar] [CrossRef] [PubMed]
- Coyle, P.K.; Krupp, L.B.; Doscher, C. Signifcance of reactive Lyme serology in multiple sclerosis. Ann. Neurol. 1993, 34, 745–747. [Google Scholar] [CrossRef] [PubMed]
- Weiss, N.L.; Saddock, V.A.; Sigal, L.H.; Phillips, M.; Merryman, P.F.; Abramson, S.B. False positive seroreactivity to Borrelia burgdorferi in systemic lupus erythematous: The value of immunoblot analysis. Lupus 1995, 4, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Garment, A.R.; Demopoulos, B.P. False-positive seroreactivity to Borrelia burgdorferi in a patient with thyroiditis. Int. J. Infect. Dis. 2010, 14, e373. [Google Scholar] [CrossRef]
- Seriburi, V.; Ndukwe, N.; Chang, Z.; Cox, M.E.; Wormser, G.P. High frequency of false positive IgM immunoblots for Borrelia burgdorferi in clinical practice. Clin. Microbiol. Infect. 2012, 18, 1236–1240. [Google Scholar] [CrossRef]
- Hunfeld, K.P.; Kraiczy, P. When to order a western blot and how to interpret it. Curr. Probl. Dermatol. Basel Karger 2009, 37, 169–179. [Google Scholar]
- Polman, C.H.; Reingold, S.C.; Edan, G.; Filippi, M.; Hartung, H.P.; Kappos, L.; Lublin, F.D.; Metz, L.M.; McFarland, H.F.; O’Connor, P.W.; et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the “McDonald Criteria”. Ann. Neurol. 2011, 69, 292–302. [Google Scholar] [CrossRef]
- Halperin, J.J.; Luft, B.J.; Anand, A.K.; Roque, C.T.; Alvarez, O.; Volkman, D.J.; Dattwyler, R.J. Lyme neuroborreliosis: Central nervous system manifestations. Neurology 1989, 39, 753. [Google Scholar] [CrossRef]
- Morgen, K.; Martin, R.; Stone, R.D.; Grafman, J.; Kadom, N.; McFarland, H.F.; Marques, A. FLAIR and magnetization transfer imaging of patients with post-treatment Lyme disease syndrome. Neurology 2001, 57, 1980–1985. [Google Scholar] [CrossRef] [PubMed]
- Agosta, F.; Rocca, M.A.; Benedetti, B.; Capra, R.; Cordioli, C.; Filippi, M. MR imaging assessment of brain and cervical cord damage in patients with neuroborreliosis. Am. J. Neuroradiol. 2006, 27, 892–894. [Google Scholar] [PubMed]
- Agarwal, R.; Sze, G. Neuro-lyme disease: MR imaging findings. Radiology 2009, 253, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Djukic, M.; Schmidt-Samoa, C.; Nau, R.; von Steinbuchel, N.; Eiffert, H.; Schmidt, H. The diagnostic spectrum in patients with suspected chronic Lyme neuroborreliosis—The experience from one year of a university hospital’s Lyme neuroborreliosis outpatients clinic. Eur. J. Neurol. 2011, 18, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Ettestad, P.J.; Campbell, G.L.; Welbel, S.F.; Genese, C.A.; Spitalny, K.C.; Marchetti, C.M.; Dennis, D.T.; Storgaard, M.; Larsen, K.; Blegvad, S.; et al. Biliary complications in the treatment of unsubstantiated Lyme disease. J. Infect. Dis. 1995, 171, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.C.; Schoen, R.T.; Evans, J.; Rosenberg, J.C.; Horwitz, R.I. The consequences of overdiagnosis and overtreatment of Lyme disease: An observational study. Ann. Intern. Med. 1998, 128, 354–362. [Google Scholar] [CrossRef]
- Holzbauer, S.M.; Kemperman, M.M.; Lynfield, R. Death due to community-associated Clostridium difficile in a woman receiving prolonged antibiotic therapy for suspected Lyme disease. Clin. Infect. Dis. 2010, 51, 369–370. [Google Scholar] [CrossRef]
- Picha, D.; Moravcova, L.; Lasikova, S.; Holeckova, D.; Maresova, V. Symptoms of post-Lyme syndrome in longterm outcome of patients with neuroborreliosis. Scand. J. Infect. Dis. 2006, 38, 747–748. [Google Scholar] [CrossRef]
- Klempner, M.; Hu, L.; Evans, J.; Schmid, C.; Johnson, G.; Trevino, R.; Norton, D.; Levy, L.; Wall, D.; McCall, J.; et al. Two controlled trials of antibiotic treatment in patients with persistent symptoms and a history of Lyme disease. N. Engl. J. Med. 2001, 345, 85–92. [Google Scholar] [CrossRef]
- Kaplan, R.F.; Trevino, R.P.; Johnson, G.M.; Levy, L.; Dornbush, R.; Hu, L.T.; Evans, J.; Weinstein, A.; Schmid, C.H.; Klempner, M.S. Cognitive function in post-treatment Lyme disease: Do additional antibiotics help? Neurology 2003, 60, 1916–1922. [Google Scholar] [CrossRef]
- Krupp, L.B.; Hyman, L.G.; Grimson, R.; Coyle, P.K.; Melville, P.; Ahnn, S.; Dattwyler, R.; Chandler, B. Study and treatment of post Lyme disease (STOP-LD): A randomized double masked clinical trial. Neurology 2003, 60, 1923–1930. [Google Scholar] [CrossRef] [PubMed]
- Fallon, B.A.; Keilp, J.G.; Corbera, K.M.; Petkova, E.; Britton, C.B.; Dwyer, E.; Slavov, I.; Cheng, J.; Dobkin, J.; Nelson, D.R.; et al. A randomized, placebo-controlled trial of repeated IV antibiotic therapy for Lyme encephalopathy. Neurology 2008, 70, 992–1003. [Google Scholar] [CrossRef] [PubMed]
- Brorson, O.; Brorson, S.H. In vitro conversion of Borrelia burgdorferi to cystic forms in spinal fluid, and transformation to mobile spirochetes by incubation in BSK-H medium. Infection 1998, 26, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Hassett, A.L.; Radvanski, D.C.; Buyske, S.; Savage, S.V.; Gara, M.; Escobar, J.I.; Sigal, L.H. Role of psychiatric comorbidity in chronic Lyme disease. Arthritis. Care Res. 2008, 59, 1742–1749. [Google Scholar] [CrossRef] [PubMed]
- Straubinger, R.K.; Summers, B.A.; Chang, Y.F.; Appel, M.J. Persistence of Borrelia burgdorferi in experimentally infected dogs after antibiotic treatment. J. Clin. Microbiol. 1997, 35, 111–116. [Google Scholar] [CrossRef]
- Hodzic, E.; Feng, S.; Holden, K.; Freet, K.J.; Barthold, S.W. Persistence of Borrelia burgdorferi following antibiotic treatment in mice. Antimicrob. Agents Chemother. 2008, 52, 1728–1736. [Google Scholar] [CrossRef]
- Embers, M.E.; Barthold, S.W.; Borda, J.T.; Bowers, L.; Doyle, L.; Hodzic, E.; Jacobs, M.B.; Hasenkampf, N.R.; Martin, D.S.; Narasimhan, S.; et al. Persistence of Borrelia burgdorferi in Rhesus Macaques following Antibiotic Treatment of Disseminated Infection. PLoS ONE 2012, 7, e29914. [Google Scholar] [CrossRef]
- Ljostad, U.; Mygland, A. Remaining complaints 1 year after treatment for acute Lyme neuroborreliosis; frequency, pattern and risk factors. Eur. J. Neurol. 2009, 17, 118–123. [Google Scholar] [CrossRef]
- Jaulhac, B.; Saunier, A.; Caumes, E.; Bouiller, K.; Gehanno, J.F.; Rabaud, C.; Perrot, S.; Eldin, C.; de Broucker, T.; Roblot, F.; et al. Lyme borreliosis and other tick-borne diseases. Guidelines from the French scientific societies. Médecine Et Mal. Infect. 2019, 49, 296–317. [Google Scholar]
- Puéchal, X.; Sibilia, J. What should be done in case of persistent symptoms after adequate antibiotic treatment for Lyme disease? Curr. Probl. Dermatol. 2009, 37, 191–199. [Google Scholar]
- Kalmár, Z.; Dumitrache, M.; D’Amico, G.; Matei, I.A.; Ionică, A.M.; Gherman, C.M.; Lupse, M.; Mihalca, A.D. Multiple Tick-borne pathogens in Ixodes ricinus ticks collected from humans in Romania. Pathogens 2020, 19, 390. [Google Scholar] [CrossRef] [PubMed]
- Kalish, R.A.; McHugh, G.; Granquist, J.; Shea, B.; Ruthazer, R.; Steere, A.C. Persistence of immunoglobulin M or immunoglobulin G antibody responses to Borrelia burgdorferi 10–20 years after active Lyme disease. Clin. Infect. Dis. 2001, 33, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Coumou, J.; Herkes, E.A.; Brouwer, M.C.; van de Beek, D.; Tas, S.W.; Casteelen, G.; van Vugt, M.; Starink, M.V.; de Vries, H.J.; de Wever, B.; et al. Ticking the right boxes: Classification of patients suspected of Lyme borre-liosis at an academic referral center in the Netherlands. Clin. Microbiol. Infect. 2015, 21, e311–e320. [Google Scholar] [CrossRef] [PubMed]
- Haddad, E.; Chabane, K.; Jaureguiberry, S.; Monsel, G.; Pourcher, V.; Caumes, E. Holistic approach in patients with presumed Lyme borreliosis leads to less than 10% of confirmation and more than 80% of antibiotics failure. Clin. Infect. Dis. 2018, 68, 2060–2066. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, C.; Goehringer, F.; Baux, E.; Conrad, J.A.; Ganne Devonec, M.O.; Schmutz, J.L.; Mathey, G.; Tronel, H.; Moulinet, T.; Chary-Valckenaere, I.; et al. Multidisciplinary management of patients presenting with Lyme disease suspicion. Med. Mal. Infect. 2019, 49, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Bouiller, K.; Klopfenstein, T.; Chirouze, C. Consultation for presumed Lyme borre-liosis: The need for a multidisciplinary management. Clin. Infect. Dis. 2019, 68, 1974. [Google Scholar] [CrossRef]
- Cooper, J.D.; Feder, H.M., Jr. Inaccurate information about Lyme disease on the internet. Pediatr. Infect. Dis. J. 2004, 23, 1105–1108. [Google Scholar]
- CNCSBT. Analysis of the Evolution of Communicable Diseases under Surveillance. Available online: https://www.cnscbt.ro/index.php/rapoarte-anuale (accessed on 29 November 2021).
- Lupșe, M.; Briciu, V.; Flonta, M.; Nastase, V.; Todor, N.; Kullberg, B.J. Serological and clinical one year follow-up of patients with erythema migrans treated in a Romanian infectious disease hospital. Rev. Rom. Med. Lab. 2014, 22, 221–231. [Google Scholar] [CrossRef][Green Version]
- CNCSBT. Analysis of the Evolution of Communicable Diseases under Surveillance; The Report for 2011. Available online: https://www.cnscbt.ro/index.php/rapoarte-anuale/545-analiza-evolutiei-bolilor-transmisibile-aflate-in-supraveghere-raport-pentru-anul-2011/file (accessed on 29 November 2021).
- Ţilea, B.; Cristina Gârbovan, C.; Ţilea, I. Characteristics of patients with Lyme neuroborreliosis in central Romania. Acta Med. Transilv. 2013, 18, 263–269. [Google Scholar]
- ECDC. ECDC Comment: European Commission Updates Communicable Disease Surveillance List—Lyme Neuroborreliosis Now under EU/EEA Surveillance. Available online: https://www.ecdc.europa.eu/en/news-events/ecdc-comment-european-commission-updates-communicable-disease-surveillance-list-lyme (accessed on 29 November 2021).
- van den Wijngaard, C.C.; Hofhuis, A.; Simões, M.; Rood, E.; van Pelt, W.; Zeller, H.; Van Bortel, W. Surveillance perspective on Lyme borreliosis across the European Union and European Economic Area. Eurosurveillance 2017, 22, 30569. [Google Scholar] [CrossRef]

| Characteristics | |
|---|---|
| Number of adults: children | 41:1 |
| Age (years): mean ± SD (min-max) | 35.83 ± 13.85 (4–63) |
| Female: male, number (%) | 33 (78.57): 9 (21.43) |
| Urban: rural residence, number (%) | 34 (80.9): 8 (9.1) |
| Possible LNB | Invalidated LNB | Not Classified LNB | Total | |||
|---|---|---|---|---|---|---|
| Pleocytosis | No Pleocytosis | Pleocytosis | No Pleocytosis | No Pleocytosis | ||
| CSF antibodies | 15 | |||||
| VIAI > 1.5 | 0 | 3 | 0 | 0 | 0 | 3 |
| IAI < 1.3 | 0 | 0 | 0 | 9 | 0 | 9 |
| IAI not determined | 1 | 0 | 0 | 0 | 2 | 3 |
| No CSF antibodies | 3 | 0 | 0 | 24 | 0 | 27 |
| Total | 4 | 3 | 0 | 33 | 2 | 42 |
| Characteristics | Total | Possible LNB n (%) | LNB Invalidated n (%) | p-Value |
|---|---|---|---|---|
| Tick bite recalled | 19 | 1 (14.3) | 18 (54.5) | 0.095 |
| Erythema migrans | 5 | 2 (28.6) | 3 (9.1) | 0.204 |
| Signs and symptoms | ||||
| Cervical pain | 11 | 0 (0) | 11 (33.33) | 0.159 |
| Decrease in occupational activity | 16 | 3 (42.86) | 13 (39.39) | 1 |
| Decrease in visual acuity | 9 | 1 (14.29) | 8 (24.24) | 1 |
| Diplopia | 5 | 0 (0) | 5 (15.15) | 0.565 |
| Facial paresis | 2 | 0 (0) | 2 (6.06) | 1 |
| Fatigue | 27 | 4 (57.14) | 23 (69.7) | 0.662 |
| Gait disorders | 14 | 0 (0) | 14 (42.42) | 0.075 |
| Headache | 23 | 5 (71.43) | 18 (54.55) | 0.677 |
| Joint pain | 21 | 2 (28.57) | 19 (57.58) | 0.226 |
| Joint tumefaction | 3 | 0 (0) | 3 (9.09) | 1 |
| Memory impairment | 13 | 1 (14.29) | 12 (36.36) | 0.393 |
| Myalgia | 18 | 5 (71.43) | 13 (39.39) | 0.211 |
| Optic neuropathy | 2 | 0 (0) | 2 (6.06) | 1 |
| Paresthesia | 32 | 3 (42.86) | 29 (87.88) | 0.02 |
| Photophobia | 6 | 1 (14.29) | 5 (15.15) | 1 |
| Speech disorders | 15 | 2 (28.57) | 13 (39.39) | 0.691 |
| Tremor | 12 | 1 (14.29) | 11 (33.33) | 0.652 |
| Vertigo | 19 | 3 (42.86) | 16 (48.48) | 1 |
| Serology | ||||
| Negative ELISA + positive WB | 13 | 1 (14.3) | 12 (36.4) | 0.393 |
| Positive ELISA + positive WB | 27 | 6 (85.7) | 21 (63.6) | |
| Demyelinating lesions on cerebral MRI | 20 | 4 (57.1) | 16 (57.1) | 1 |
| Neurological Diagnosis | |
| Possible LNB | Right peripheral vestibular disorder. Vascular encephalopathy. |
| Demyelinating cerebral lesions of unknown etiology. | |
| Acute encephalitis, right hemiparesis, expressive aphasia. | |
| LNB invalidated | Incomplete thoracic myelitis with left hemicorporeal paresthesia syndrome and sensory level at the sixth dorsal segment. |
| Tension-type headache, lumbar discopathy with radiculalgia. | |
| Vertebrobasilar stroke, right hemiparesis, transient ischemic attack. | |
| Demyelinating disease, persistent headache, severe hypotonia in the lower limbs. | |
| Demyelinating disease. | |
| Demyelinating disease. | |
| Suspicion of MS, progressive bulbar palsy- confirmed during follow-up as ALS. | |
| Cephalalgia. | |
| Central vestibular disorder. | |
| Left sacral 1 radiculopathy. | |
| Fibromyalgia. | |
| Axonal peripheral polyneuropathy. | |
| Right brachial plexus paresis, right Cervical 7 radiculopathy. | |
| Migraine without aura. | |
| Suspected ALS-confirmed during follow-up as ALS. | |
| Left peripheral facial palsy. | |
| Suspected MS-confirmed during follow-up as MS. | |
| Suspected MS, left peripheral facial palsy. | |
| Benign intracranial hypertension, right hemiparesis, anxiety disorder with somatization. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Briciu, V.; Flonta, M.; Leucuța, D.; Lupșe, M. The Diagnostic Challenges and Clinical and Serological Outcome in Patients Hospitalized for Suspected Lyme Neuroborreliosis. Microorganisms 2022, 10, 1392. https://doi.org/10.3390/microorganisms10071392
Briciu V, Flonta M, Leucuța D, Lupșe M. The Diagnostic Challenges and Clinical and Serological Outcome in Patients Hospitalized for Suspected Lyme Neuroborreliosis. Microorganisms. 2022; 10(7):1392. https://doi.org/10.3390/microorganisms10071392
Chicago/Turabian StyleBriciu, Violeta, Mirela Flonta, Daniel Leucuța, and Mihaela Lupșe. 2022. "The Diagnostic Challenges and Clinical and Serological Outcome in Patients Hospitalized for Suspected Lyme Neuroborreliosis" Microorganisms 10, no. 7: 1392. https://doi.org/10.3390/microorganisms10071392
APA StyleBriciu, V., Flonta, M., Leucuța, D., & Lupșe, M. (2022). The Diagnostic Challenges and Clinical and Serological Outcome in Patients Hospitalized for Suspected Lyme Neuroborreliosis. Microorganisms, 10(7), 1392. https://doi.org/10.3390/microorganisms10071392






