Biological Control of Plant Pathogens: A Global Perspective
Abstract
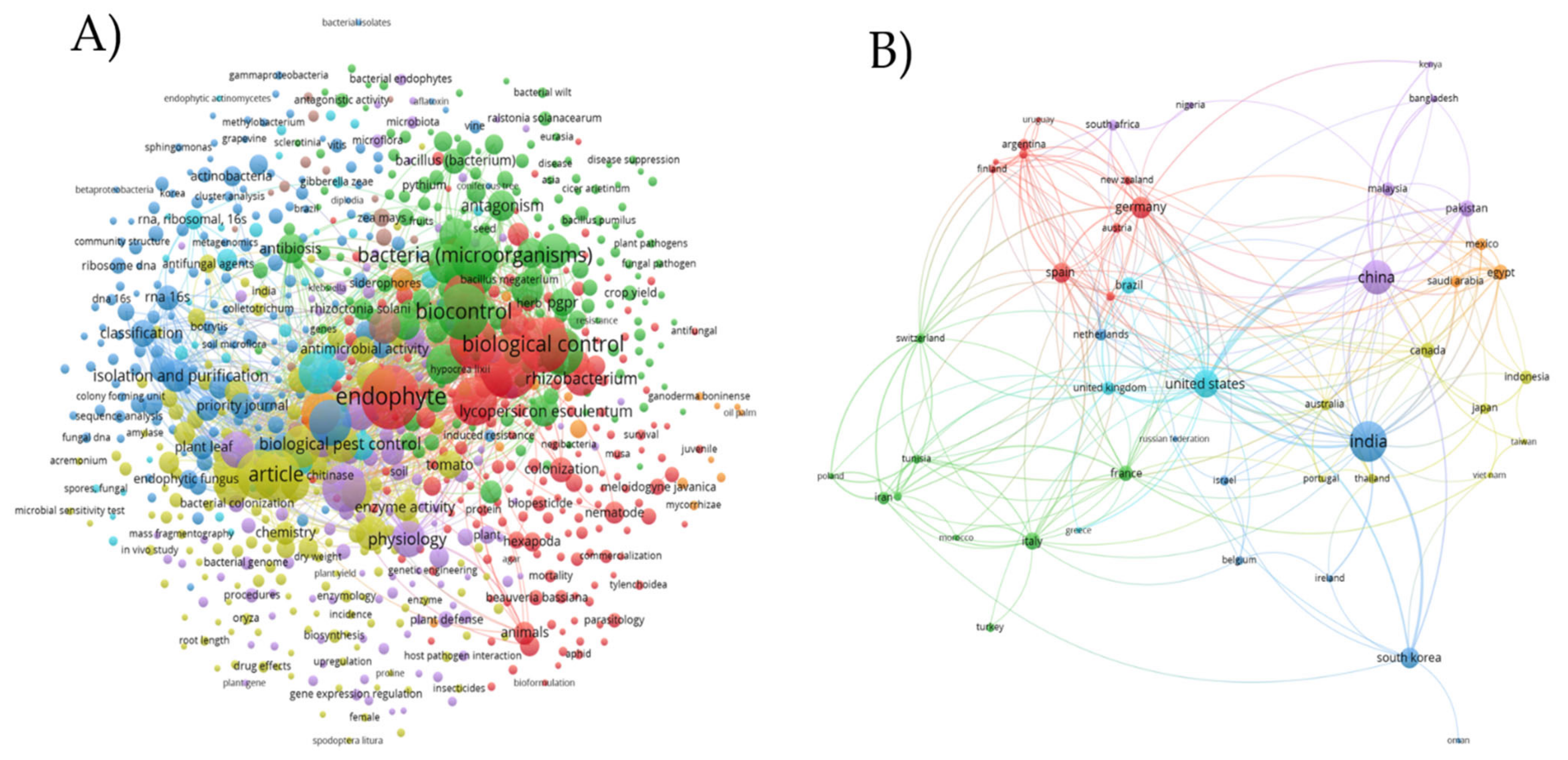
1. Introduction
2. Rhizosphere as a Potential Reservoir of Biopesticides
2.1. Beneficial Bacteria
2.2. Fungi and Yeasts
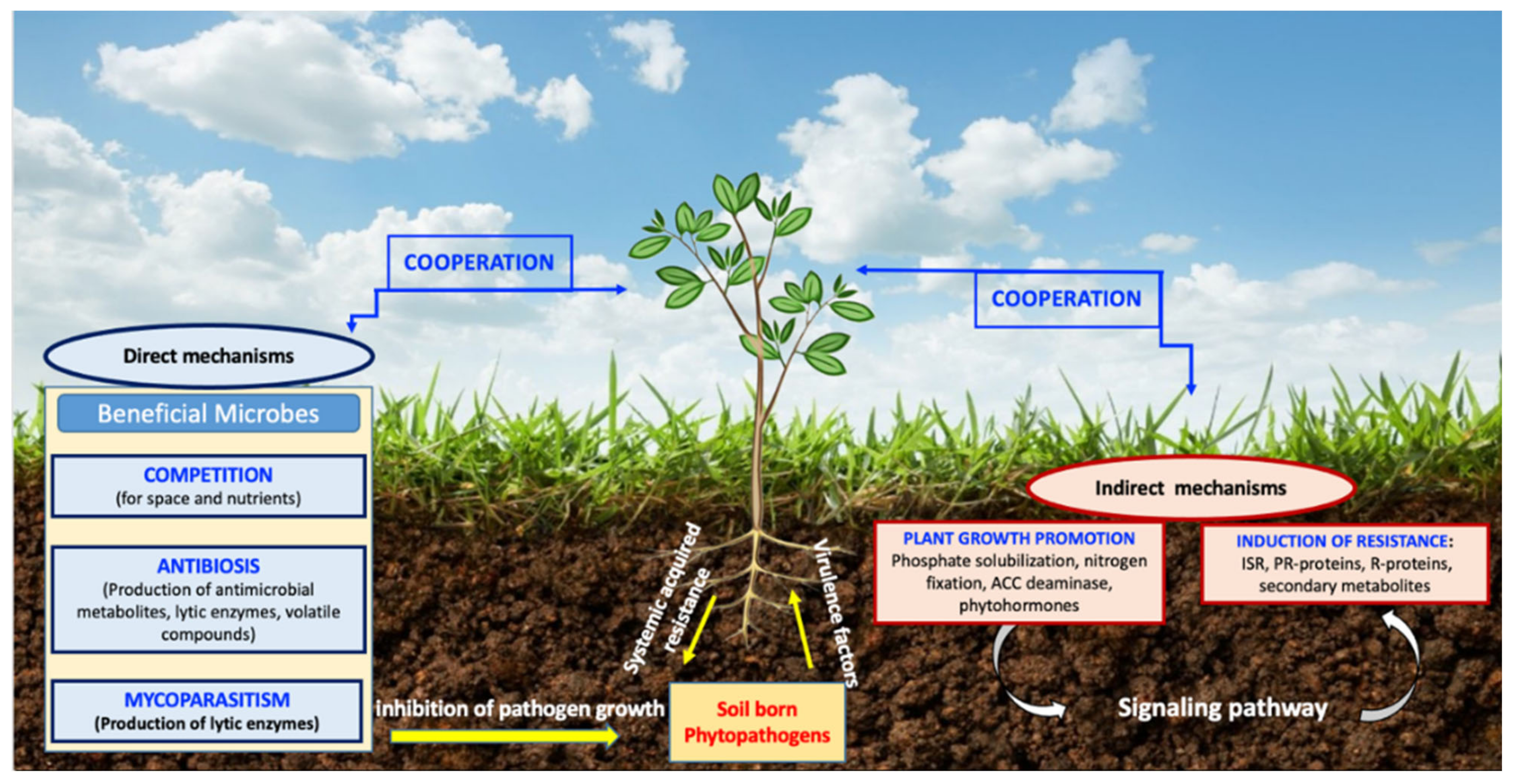
3. BCAs Modes of Action
3.1. Direct Mode of Action
3.1.1. Involvement of Lytic Enzymes
3.1.2. Antimicrobial Molecules
3.1.3. Biofilm Formation
3.1.4. Competition for Nutrients and Space
3.1.5. Parasitism
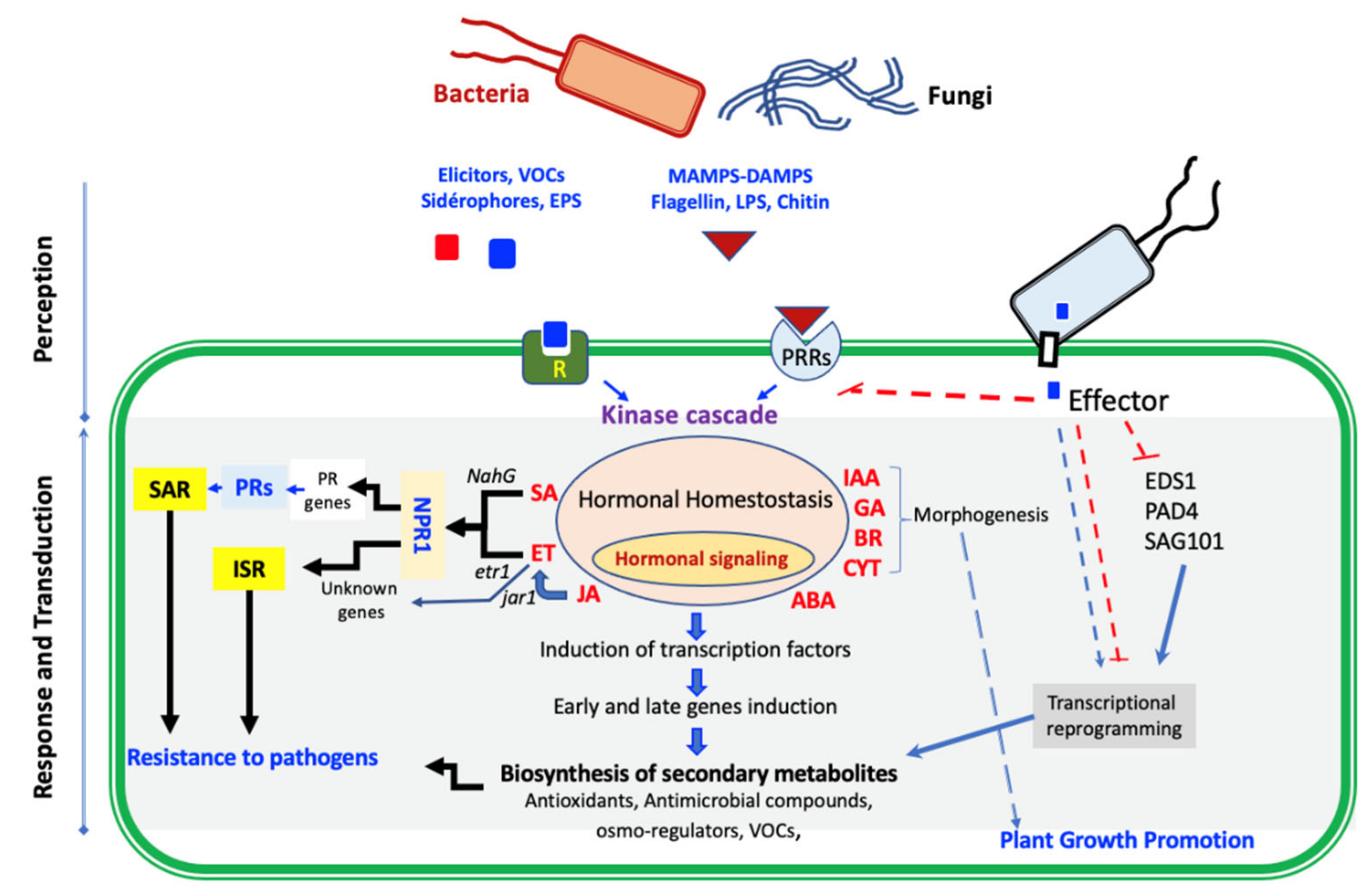
3.2. Indirect Mode of Action
3.2.1. Induced Resistance and Priming
3.2.2. Implication of Phytohormones
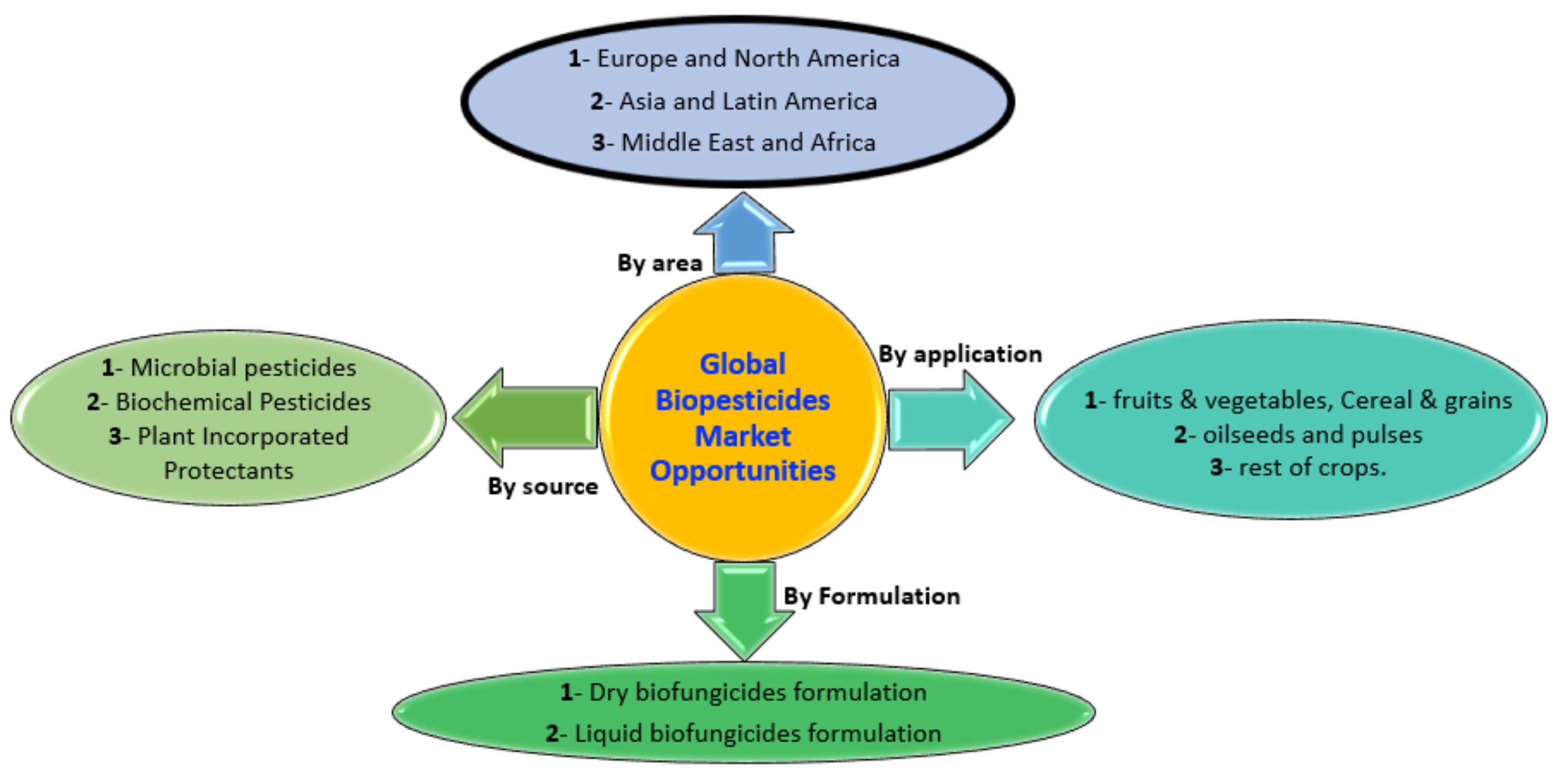
4. Biological Control: Facing Reality
4.1. Biopesticide from Lab to the Field
4.2. Limited Number of Registered Products
| Biopesticides Active Agents | Trade Name | Target Disease and/or Target Organism Pathogen | Crop | Manufacturer/Distributor |
|---|---|---|---|---|
| ||||
| Agrobacterium radiobacter strain k84 | Galltrol | Agrobacterium tumeifaciens | Ornamentals, Fruits, Nuts | AgBioChem, Los Molinos, CA, USA |
| Bacillus subtilis QST 713 | Serenade | Foliar pathogens, rots, Fire blight, and blights | Cherries, cucurbits, grapes, leafy vegetables, peppers, potatoes, tomatoes, and walnuts | AgraQuest, Davis, CA, USA |
| Bacillus firmus NCIM 2637 | Bionemagon | Root-knot nematode, Remiform nematode Cyst nematode, Burrowing nematode, Lesion nematode | Cereals, millets, pulses, oilseeds, fibre crops, sugar crops, forage crops, plantation crops, vegetables, fruits, etc. | ---- |
| Bacillus subtilis GB03 | Companion, Kodiak | Fusarium, Pythium, Rhizoctonia, Aspergillus, and others | Crop seeds, including seeds for cotton, peanuts, soybeans, wheat, barley, peas, and beans | Growth products, White Plains, NY, USA |
| Bacillus subtilis MBI 600 | Subtilex; Histick N/T | Damping-off Fusarium, Rhizoctonia, Alternaria, and Aspergillus | Cotton, beans, barley, wheat, corn, peas, peanuts, and soybeans | Becker Underwood, Ames, Iowa, USA; Premier Horticulture, Quakertown, PA, USA |
| Bacillus subtilis var. amyloliquefaciens strain FZB24 | Taegro | Rhizoctonia and Fusarium | Shade and forest tree seedlings, ornamentals, and shrubs | Earth BioSciences, Salem, OR, USA |
| Bacillus licheniformis strain SB3086 | Ecoguard; Novozymes Biofungicide GreenRelief | Foliar pathogens and blights | Ornamental plants and ornamental turf | Novozymes Biologicals, Davis, CA, USA |
| Bacillus pumilus strain GB34 | GB34 Concentrate Biological Fungicide | Rhizoctonia, Fusarium | Soybean | Gustafson, Inc, Plano, TX, USA |
| Bacillus thuringiensis | Bio-Dart, Biolep, Halt, Taciobio-Btk, Tacibio, Technar | Lepidopteran pests | Stored grains, fiber, and food crops | ---- |
| Bacillus thuringiensis tenebrionis | Novodor, Trident | Colorado potato beetle | Potato | ---- |
| Burkholderia spp. strain A396 | Venerate | Aphids, leafhopper, lygus, stink bug, thrips | Almonds, blueberry, citrus crops, cucurbits, fruiting, vegetables, grapes | ---- |
| Chromobacterium subtsugae | Grandevo WDG | Armyworms, Aphids, Asian Citrus Psyllid, Mites, Spotted Wing Drosophila, Thrips, Whiteflies. | Blueberry, citrus crops, cucurbits, fruiting vegetables, grapes, leafy greens | ---- |
| Pseudomonas chlororaphis strain 63–28 | AtEze | Pythium, Rhizoctonia solani, Fusarium oxysporum | Vegetables and ornamentals in greenhouses | EcoSoil Systems, San Diego, CA, USA |
| Psuedomonas fluoroscens strain A506 | Frostban | Fire blight, bunch rot | Fruit crop, tomato, potato | Plant Health Technologies, Burlington, CO, USA |
| Pseudomonas aureofaciens strain TX-1 | Bio–Ject, Spot Less | Rhizoctonia solani, Sclerotinia homeocarpa, Colletotrichum graminicola, Pythium aphanadermatum, Michrodochium nivale | Vegetables and ornamentals in greenhouses, golf course turf | EcoSoil system, Canyon Lake, TX, USA |
| Pseudomonas fluorescens A506 | BlighBan A506; Frostban | Frost damage, fire blight, bunch rot | Fruit crops, almond, potato, and tomato crops | Frost Technology Corporation, St Croix Falls, WI, USA; Plant Health Technologies, Burlington, CO, USA |
| ||||
| Ampelomyces quisqualis isolate M-10 | AQ10 BioFungicide | Powdery mildew | Fruit, vegetable, and ornamental crops | Ecogen, Grand Junction, CO, USA |
| Aspergillus flavus AF36 | Aspergillus flavus AF36 | Aspergillus flavus | Cotton | Arizona Cotton Research and Protection Council, Phoenix AZ, USA |
| Aspergillus flavus NRRL21, 882 | Afla-guard | Aspergillus flavus | Peanut | Circle One Global, Cuthbert, GA, USA |
| Beauveria spp. | Biosoft, ATEC Beauveria, Larvo-Guard, Biorin, Biolarvex, Biogrubex, Biowonder, Veera, Phalada 101B, Bioguard, Bio-power, Myco-Jaal | Coffeeberry borer, diamondback moth, thrips, grasshoppers, whiteflies, aphids, codling moth | Coffee berries, canola, mustard, cruciferous vegetables, and others | ---- |
| Chaetomium globosum | Ketomium | Rice blast, durian, and black Pepper rot, citrus rot, strawberry rot, anthracnose, and others | Rice, black pepper, citrus, strawberry, tomato, corn, and others | ---- |
| Coniothyrium minitans CON/M/91-08 | Contans WG; Intercept | Sclerotinia sclerotiorum and Sclerotinia minor | Agricultural soil | PROPHYTA Biologischer Pflanzenschutz GmbH, Wismar, Germany; Technology Sciences Group, Sacramento, CA, USA |
| Gliocladium catenulatum strain JI446 | Prima stop soil guard | Soil-borne pathogens | Vegetables, herbs, spices, turf, ornamentals, tree, and shrub seedlings | Kemira Agro Oy, Helsinki, Finland; RegWest Co., Holland, MI, USA |
| Gliocladium virens GL-21 | SoilGard | Soil-borne pathogens | Ornamentals, vegetables, cotton | Thermo Trilogy Corporation, WALTHAM, MA, USA |
| Gliocladium virens | SoilGard 12G | Clubroot Plasmodiophora brassicae | Canola and crucifer vegetable crops | Certis USA L.L.C., Columbia, MD, USA |
| Metarhizium anisopliae | Meta-Guard, Biomet, Biomagic, Meta, Biomet, Sun Agro Meta, BioMagic, ABTEC, Verticillium | Coleoptera and Lepidoptera, termites, mosquitoes, leafhoppers, beetles, grubs | Cotton, vegetable, field crops, and others | ---- |
| Pseudozyma flocculosa strain PF-A22UL | Sporodex L. | Powdery mildew | Roses and cucumbers in greenhouses | Plant Products Co., Leamington, ON, Canada; Technology Sciences Group, Washington DC, USA |
| Streptomyces lydicus | Actinovate AG | Clubroot Plasmodiophora brassicae | Canola and crucifer vegetable crops | Natural Industries Inc., Houston, TX, USA |
| Streptomyces griseoviridis strainK61 | Mycostop | Soil-borne pathogens | Ornamentals, tree seedlings | Kemira Oy, Helsinki, Finland |
| Trichoderma harzianum ATCC 20, 476 | Binab T | Tree wound pathogens | Wounds in ornamental, shade, and forest trees | BINAB Bio-Innovation AB, Helsingborg, Sweden |
| Trichoderma album 2.5% | Bio Zeid | Tomato wilt Fusarium oxysporum | Tomato | ---- |
| Trichoderma harzianum T39 | Trichodex | Botrytis cinerea | Most of the food crops | Bio works, Victor, NY, USA |
| Trichoderma harzianum T22 | Root shield, plant shield | Soil-borne pathogens | Greenhouse nurseries | Bio works, Victor, NY, USA |
| Verticillium lecanii | Verisoft, ABTEC, Verticillium, Vert-Guard, Bioline, Biosappex, Versitile, Ecocil, Phalada 107 V, Biovert Rich, ROM Verlac, ROM Gurbkill, Sun Agro Verti, Bio-Catch | Whitefly, coffee green bug, homopteran pests | Coffee crops and others | ---- |
| ||||
| Candida oleophila | Aspire | Botrytis, Penicillium, Monilinia | Pome fruit, citrus, strawberry, stone fruit | Ecogen, Grand Junction, CO, USA |
| Candida oleophila isolate I-182 | Aspire | Postharvest diseases | Various fruits, vegetables, flowers, ornamentals, other plants | Ecogen, Grand Junction, CO, USA |
| Cryptococcus albidus | YieldPlus | Botrytis, Penicillium, Mucor | Pome fruit, citrus | Lallemand, Bellville, South Africa |
| Candida sake | Candifruit | Penicillium, Botrytis, Rhizopus | Pome fruit | IRTA/Sipcam-Inagra, Valencia Spain |
| Pseudomonas syringae | Biosave | Penicillium, Botrytis, Mucor | Pome fruit, citrus, strawberry, cherry, potato | Jet Harvest Solutions, Longwood, FL, USA |
| Bacillus subtilis Candida oleophila | Avogreen Nexy | Cercospora, Colletotrichum Botrytis, Penicillium | Avocado Pome fruit | South Africa Lesaffre Belgium |
| Aureobasisium pullulans | BoniProtect | Botrytis, Penicillium, Monilinia | Pome fruit | Bio-ferm, Herzogenburg, Austria |
| Pantoea agglomerans | Pantovital | Botrytis, Penicillium, Monilinia | Citrus, pome fruit | IRTA/Sipcam-Inagra, Valencia Spain |
| Metschnikowia fructicola | Shemer | Botrytis, Penicillium, Rhizopus, Aspergillus | Table grape, pome fruit, strawberry, stone fruit, sweet potato | Bayer/Koppert, The Netherlands |
4.3. Legislative Procedures
5. Factors Affecting the Success/Failure of Biological Control of Plant Pathogens
5.1. Effect of the Plant on Biocontrol Activity
5.2. Effect of the Pathogen on Biocontrol Activity
5.3. Effect of the Biocontrol Agent on Biocontrol Activity
5.4. Effect of the Environment on Biocontrol Activity
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Montagu, M. The future of plant biotechnology in a globalized and environmentally endangered world. Genet. Mol. Biol. 2020, 43, e20190040. [Google Scholar] [CrossRef] [PubMed]
- Godfray, H.C.J. The challenge of feeding 9–10 billion people equitably and sustainably. J. Agric. Sci. 2014, 152, 2–8. [Google Scholar] [CrossRef]
- Guillou, M.; Matheron, G. The World’s Challenge; Springer: Dordrecht, The Netherlands, 2014; ISBN 978-94-017-8568-6. [Google Scholar]
- Dwivedi, S.L.; Lammerts van Bueren, E.T.; Ceccarelli, S.; Grando, S.; Upadhyaya, H.D.; Ortiz, R. Diversifying Food Systems in the Pursuit of Sustainable Food Production and Healthy Diets. Trends Plant Sci. 2017, 22, 842–856. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, P.R.; Sayre, K.; Gupta, R. The role of conservation agriculture in sustainable agriculture. Philos. Trans. R. Soc. B Biol. Sci. 2007, 363, 543–555. [Google Scholar] [CrossRef]
- Ngoune Liliane, T.; Shelton Charles, M. Factors Affecting Yield of Crops. In Agronomy—Climate Change and Food Security; InTech Open: London, UK, 2020; Volume 32, pp. 137–144. [Google Scholar]
- Hazell, P.; Wood, S. Drivers of change in global agriculture. Philos. Trans. R. Soc. B Biol. Sci. 2007, 363, 495–515. [Google Scholar] [CrossRef]
- Purvis, B.; Mao, Y.; Robinson, D. Three pillars of sustainability: In search of conceptual origins. Sustain. Sci. 2019, 14, 681–695. [Google Scholar] [CrossRef]
- Köhl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of Action of Microbial Biological Control Agents against Plant Diseases: Relevance Beyond Efficacy. Front. Plant Sci. 2019, 10, 845. [Google Scholar] [CrossRef]
- Rauscher, C.I.E.D.E.M.; Rauscher, M. International Trade, Factor Movements, and the Environment; Clarendon Press: Oxford, UK, 1997; ISBN 9780198290506. [Google Scholar]
- Pilet-Nayel, M.L.; Moury, B.; Caffier, V.; Montarry, J.; Kerlan, M.C.; Fournet, S.; Durel, C.E.; Delourme, R. Quantitative resistance to plant pathogens in pyramiding strategies for durable crop protection. Front. Plant Sci. 2017, 8, 1838. [Google Scholar] [CrossRef]
- Usta, C. Microorganisms in Biological Pest Control—A Review (Bacterial Toxin Application and Effect of Environmental Factors). In Current Progress in Biological Research; InTech Open: London, UK, 2013; Volume 32, pp. 137–144. [Google Scholar]
- Compant, S.; Duffy, B.; Nowak, J.; Clément, C.; Ait Barka, E. Biocontrol of plant diseases using plant growth-promoting bacteria (PGPB): Principles, mechanisms of action and future prospects. Appl. Environ. Microbiol. 2005, 71, 4951–4959. [Google Scholar] [CrossRef]
- O’Brien, P.A. Biological control of plant diseases. Australas. Plant Pathol. 2017, 46, 293–304. [Google Scholar] [CrossRef]
- Barratt, B.I.P.; Moran, V.C.; Bigler, F.; Van Lenteren, J.C. The status of biological control and recommendations for improving uptake for the future. BioControl 2018, 63, 155–167. [Google Scholar] [CrossRef]
- Macfadyen, S.; Hardie, D.C.; Fagan, L.; Stefanova, K.; Perry, K.D.; DeGraaf, H.E.; Holloway, J.; Spafford, H.; Umina, P.A. Reducing Insecticide Use in Broad-Acre Grains Production: An Australian Study. PLoS ONE 2014, 9, e89119. [Google Scholar] [CrossRef]
- Pérez-García, A.; Romero, D.; De Vicente, A. Plant protection and growth stimulation by microorganisms: Biotechnological applications of Bacilli in agriculture. Curr. Opin. Biotechnol. 2011, 22, 187–193. [Google Scholar] [CrossRef]
- Ruiu, L. Microbial Biopesticides in Agroecosystems. Agronomy 2018, 8, 235. [Google Scholar] [CrossRef]
- Angelopoulou, D.J.; Naska, E.J.; Paplomatas, E.J.; Tjamos, S.E. Biological control agents (BCAs) of verticillium wilt: Influence of application rates and delivery method on plant protection, triggering of host defence mechanisms and rhizosphere populations of BCAs. Plant Pathol. 2014, 63, 1062–1069. [Google Scholar] [CrossRef]
- Abd-Elgawad, M.M.M.; Askary, T.H. Factors affecting success of biological agents used in controlling the plant-parasitic nematodes. Egypt. J. Biol. Pest Control 2020, 30, 17. [Google Scholar] [CrossRef]
- Arthurs, S.; Dara, S.K. Microbial biopesticides for invertebrate pests and their markets in the United States. J. Invertebr. Pathol. 2019, 165, 13–21. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Blagodatskaya, E. Microbial hotspots and hot moments in soil: Concept & review. Soil Biol. Biochem. 2015, 83, 184–199. [Google Scholar]
- Lahlali, R.; Ibrahim, D.S.S.; Belabess, Z.; Kadir Roni, M.Z.; Radouane, N.; Vicente, C.S.L.; Menéndez, E.; Mokrini, F.; Barka, E.A.; Galvão de Melo e Mota, M.; et al. High-throughput molecular technologies for unraveling the mystery of soil microbial community: Challenges and future prospects. Heliyon 2021, 7, e08142. [Google Scholar] [CrossRef]
- Mohanram, S.; Kumar, P. Rhizosphere microbiome: Revisiting the synergy of plant-microbe interactions. Ann. Microbiol. 2019, 69, 307–320. [Google Scholar] [CrossRef]
- Shaikh, S.S.; Wani, S.J.; Sayyed, R.Z. Impact of Interactions between Rhizosphere and Rhizobacteria: A Review. J. Bacteriol. Mycol. 2018, 5, 1058. [Google Scholar]
- Tena, G. Recruiting microbial bodyguards. Nat. Plants 2018, 4, 857. [Google Scholar] [CrossRef]
- Ciancio, A.; Pieterse, C.M.J.; Mercado-blanco, J. Editorial: Harnessing Useful Rhizosphere Microorganisms for Pathogen and Pest Biocontrol—Second Edition. Front. Microbiol. 2019, 10, 1935. [Google Scholar] [CrossRef]
- Sindhu, S.S.; Sehrawat, A.; Sharma, R.; Dahiya, A. Biopesticides: Use of Rhizosphere Bacteria for Biological Control of Plant Pathogens. Def. Life Sci. J. 2016, 1, 135–148. [Google Scholar] [CrossRef]
- Seenivasagan, R.; Babalola, O.O. Utilization of Microbial Consortia as Biofertilizers and Biopesticides for the Production of Feasible Agricultural Product. Biology 2021, 10, 1111. [Google Scholar] [CrossRef]
- Sheppard, A.W.; Paynter, Q.; Mason, P.; Murphy, S.; Stoett, P.; Cowan, P.; Brodeur, J.; Warner, K.; Villegas, C.; Shaw, R.; et al. The Application of Classical Biological Control for the Management of Established Invasive Alien Species Causing Environmental Impacts; IUCN SSC Invasive Species Specialist Group; Secretariat of the Convention on Biological Diversity: Montreal, QC, Canada, 2019; pp. 1–94. [Google Scholar]
- He, D.C.; He, M.H.; Amalin, D.M.; Liu, W.; Alvindia, D.G.; Zhan, J. Biological Control of Plant Diseases: An Evolutionary and Eco-Economic Consideration. Pathogens 2021, 10, 1311. [Google Scholar] [CrossRef]
- Umer, M.; Mubeen, M.; Iftikhar, Y.; Shad, M.A.; Usman, H.M.; Sohail, M.A.; Atiq, M.N.; Abbas, A.; Ateeq, M. Role of Rhizobacteria on Plants Growth and Biological Control of Plant Diseases: A Review. Plant Prot. 2021, 5, 59–73. [Google Scholar] [CrossRef]
- Labuschagne, N.; Pretorius, T.; Idris, A.H. Plant Growth Promoting Rhizobacteria as Biocontrol Agents Against Soil-Borne Plant Diseases. Microbiol. Monogr. 2010, 18, 211–230. [Google Scholar] [CrossRef]
- Van Lenteren, J.C.; Bolckmans, K.; Köhl, J.; Ravensberg, W.J.; Urbaneja, A. Biological control using invertebrates and microorganisms: Plenty of new opportunities. BioControl 2018, 63, 39–59. [Google Scholar] [CrossRef]
- Esmaeel, Q.; Jacquard, C.; Sanchez, L.; Clément, C.; Ait Barka, E. The mode of action of plant associated Burkholderia against grey mould disease in grapevine revealed through traits and genomic analyses. Sci. Rep. 2020, 10, 19393. [Google Scholar] [CrossRef]
- Anderson, A.J.; Kim, Y.C. Biopesticides produced by plant-probiotic Pseudomonas chlororaphis isolates. Crop Prot. 2018, 105, 62–69. [Google Scholar] [CrossRef]
- Mendes, R.; Kruijt, M.; de Bruijn, I.; Dekkers, E.; van der Voort, M.; Schneider, J.H.M.; Piceno, Y.M.; DeSantis, T.Z.; Andersen, G.L.; Bakker, P.A.H.M.; et al. Deciphering the Rhizosphere Microbiome for Disease-Suppressive Bacteria. Science 2011, 332, 1097–1100. [Google Scholar] [CrossRef] [PubMed]
- Sehrawat, A.; Sindhu, S.S. Potential of Biocontrol Agents in Plant Disease Control for Improving Food Safety. Def. Life Sci. J. 2019, 4, 220–225. [Google Scholar] [CrossRef]
- Sindhu, S.S.; Parmar, P.; Phour, M.; Kumari, K. Rhizosphere microorganisms for improvement in soil fertility and plant growth. In Microbes in the Service of Mankind; Nagpal, R., Kumar, A., Eds.; JBC Press: New Delhi, India, 2014; pp. 32–94. [Google Scholar]
- Sharma, P.; Kumawat, K.C.; Kaur, S. Plant Growth Promoting Rhizobacteria in Nutrient Enrichment: Current Perspectives. In Biofortification of Food Crops; Singh, U., Praharaj, C.S., Singh, S.S., Singh, N.P., Eds.; Springer: New Delhi, India, 2016; pp. 263–289. [Google Scholar]
- Patel, S.; Sayyed, R.Z.; Saraf, M. Bacterial Determinants and Plant Defense Induction: Their Role as Biocontrol Agents in Sustainable Agriculture. In Plant, Soil and Microbes Volume 2: Mechanisms and Molecular Interactions; Hakeem, K.R., Akhtar, M.S., Eds.; Springer: Cham, Switzerland, 2016; pp. 187–204. [Google Scholar]
- Shaikh, S.S.; Sayyed, R.Z.; Reddy, M.S. Plant Growth Promoting Rhizobacteria: A Sustainable Approach to Agro- Plant Growth-Promoting Rhizobacteria: An Eco-friendly Approach for Sustainable Agroecosystem. In Plant, Soil and Microbes Volume 2: Mechanisms and Molecular Interactions; Hakeem, K.R., Akhtar, M.S., Eds.; Springer: Cham, Switzerland, 2016; pp. 181–201. [Google Scholar]
- Shaikh, S.S.; Patel, P.R.; Patel, S.S.; Nikam, S.D.; Rane, T.U.; Sayyed, R.Z. Production of biocontrol traits by banana field fluorescent Pseudomonads and comparison with chemical fungicide. Indian J. Exp. Biol. 2014, 52, 917–920. [Google Scholar]
- Chauhana, H.; Bagyaraja, D.J.; Selvakumarb, G.; Sundaramc, S.P. Novel plant growth promoting rhizobacteria—Prospects and potential. Appl. Soil Ecol. 2015, 95, 38–53. [Google Scholar] [CrossRef]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant Growth-Promoting Rhizobacteria: Context, Mechanisms of Action, and Roadmap to Commercialization of Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef]
- Lyousfi, N.; Lahlali, R.; Letrib, C.; Belabess, Z.; Ouaabou, R.; Ennahli, S.; Blenzar, A.; Ait Barka, E. Improving the Biocontrol Potential of Bacterial Antagonists with Salicylic Acid against Brown Rot Disease and Impact on Nectarine Fruits Quality. Agronomy 2021, 11, 209. [Google Scholar] [CrossRef]
- Lahlali, R.; Mchachti, O.; Radouane, N.; Ezrari, S.; Belabess, Z.; Khayi, S.; Mentag, R.; Tahiri, A.; Ait Barka, E. The Potential of Novel Bacterial Isolates from Natural Soil for the Control of Brown Rot Disease (Monilinia fructigena) on Apple Fruits. Agronomy 2020, 10, 1814. [Google Scholar] [CrossRef]
- Ait Bahadou, S.; Ouijja, A.; Karfach, A.; Tahiri, A.; Lahlali, R. New potential bacterial antagonists for the biocontrol of fire blight disease (Erwinia amylovora) in Morocco. Microb. Pathog. 2018, 117, 7–15. [Google Scholar] [CrossRef]
- Lanteigne, C.; Gadkar, V.J.; Wallon, T.; Novinscak, A.; Filion, M. Production of DAPG and HCN by Pseudomonas sp. LBUM300 Contributes to the Biological Control of Bacterial Canker of Tomato. Biol. Control 2012, 102, 967–973. [Google Scholar] [CrossRef]
- St-Onge, R.; Gadkar, V.J.; Arseneault, T.; Goyer, C.; Filion, M. The ability of Pseudomonassp. LBUM223 to produce phenazine-1- carboxylic acid affects the growth of Streptomyces scabies, the expression of thaxtomin biosynthesis genes and the biological control potential against common scabof potato. FEMS Microbiol. Ecol. 2011, 75, 173–183. [Google Scholar] [CrossRef]
- Jisha, V.N.; Smitha, R.B.; Benjamin, S. An Overview on the Crystal Toxins from Bacillus thuringiensis. Adv. Microbiol. 2013, 3, 462–472. [Google Scholar] [CrossRef]
- Schünemann, R.; Knaak, N.; Fiuza, L.M. Mode of Action and Specificity of Bacillus thuringiensis Toxins in the Control of Caterpillars and Stink Bugs in Soybean Culture. ISRN Microbiol. 2014, 2014, e135675. [Google Scholar] [CrossRef]
- Chowdhury, S.P.; Uhl, J.; Grosch, R.; Alquéres, S.; Pittroff, S.; Dietel, K.; Schmitt-kopplin, P.; Borriss, R.; Hartmann, A. Cyclic Lipopeptides of Bacillus amyloliquefaciens subsp. plantarum Colonizing the Lettuce Rhizosphere Enhance Plant Defense Responses Toward the Bottom Rot Pathogen Rhizoctonia solani. Mol. Plant-Microbe Interact. 2015, 28, 984–995. [Google Scholar] [CrossRef]
- Hasan, N.; Farzand, A.; Heng, Z.; Khan, I.U.; Moosa, A.; Zubair, M.; Na, Y.; Ying, S.; Canming, T. Antagonistic Potential of Novel Endophytic Bacillus Strains and Mediation of Plant Defense against Verticillium Wilt in Upland Cotton. Plants 2020, 9, 1438. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, Y.; Lu, J.; Liu, S.; Min, Y.; Liu, X.; Qiu, Y.; Hu, H.; Zhou, R. Complete Genome Sequence of Bacillus badius NBPM-293, a Plant Growth-Promoting Strain Isolated from Rhizosphere Soil. Am. Soc. Microbiol. 2021, 10, e00977-21. [Google Scholar] [CrossRef]
- Anckaert, A.; Arias, A.A.; Hoff, G. The use of Bacillus spp. as bacterial biocontrol agents to control plant diseases. In Microbial Bioprotectants for Plant Disease Management; Köhl, J., Ravensberg, W., Eds.; Burleigh Dodds Science Publishing: Cambridge, UK, 2022; pp. 1–54. [Google Scholar]
- Sujayanand, G.K.; Akram, M.; Konda, A.; Nigam, A.; Bhat, S.; Dubey, J.; Kumar, K.; Muthusamy, S.K. Distribution and toxicity of Bacillus thuringiensis (Berliner) strains from different crop rhizosphere in Indo-Gangetic plains against polyphagous lepidopteran pests. Int. J. Trop. Insect Sci. 2021, 41, 2713–2731. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Rao, G.V.R.; Humayun, P.; Rao, V.R.; Alekhya, G.; Jacob, S.; Deepthi, K.; Vidya, M.S.; Srinivas, V.; Mamatha, L.; et al. Efficacy of botanical extracts and entomopathogens on control of Helicoverpa armigera and Spodoptera litura. Afr. J. Biotechnol. 2011, 10, 16667–16673. [Google Scholar] [CrossRef]
- De Curtis, F.; Ianiri, G.; Raiola, A.; Ritieni, A.; Succi, M.; Tremonte, P.; Castoria, R. Integration of biological and chemical control of brown rot of stone fruits to reduce disease incidence on fruits and minimize fungicide residues in juice. Crop Prot. 2019, 119, 158–165. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, L.; Dong, Y.; Chen, W.; Li, C.; Gao, X.; Chen, R.; Li, L.; Xu, Z. The antagonistic mechanism of Bacillus velezensis ZW10 against rice blast disease: Evaluation of ZW10 as a potential biopesticide. PLoS ONE 2021, 16, e0256807. [Google Scholar] [CrossRef]
- Anderson, A.J.; Kang, B.R.; Kim, Y.C. The Gac/Rsm Signaling Pathway of a Biocontrol Bacterium, Pseudomonas chlororaphis O6. Res. Plant Dis. 2017, 23, 212–227. [Google Scholar] [CrossRef]
- Poveda, J. Trichoderma as biocontrol agent against pests: New uses for a mycoparasite. Biol. Control 2021, 159, e104634. [Google Scholar] [CrossRef]
- Alfiky, A.; Weisskopf, L. Deciphering Trichoderma–Plant–Pathogen Interactions for Better Development of Biocontrol Applications. J. Fungi 2021, 7, 61. [Google Scholar] [CrossRef]
- Pfordt, A.; Schiwek, S.; Karlovsky, P.; von Tiedemann, A. Trichoderma Afroharzianum Ear Rot—A New Disease on Maize in Europe. Front. Agron. 2020, 2, e547758. [Google Scholar] [CrossRef]
- Degani, O.; Dor, S. Trichoderma Biological Control to Protect Sensitive Maize Hybrids Against Late Wilt Disease in the Field. J. Fungi 2021, 7, 315. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Montesinos, B.; Santos, M.; Moreno-Gavíra, A.; Marín-Rodulfo, T.; Gea, F.J.; Diánez, F. Biological Control of Fungal Diseases by Trichoderma aggressivum f. europaeum and Its Compatibility with Fungicides. J. Fungi 2021, 7, 598. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Wang, Z.; Zhang, Y.; Wang, Y.; Liu, Z. Biocontrol and growth-promoting effect of Trichoderma asperellum TaspHu1 isolate from Juglans mandshurica rhizosphere soil. Microbiol. Res. 2021, 242, e126596. [Google Scholar] [CrossRef] [PubMed]
- Reghmit, A.; Benzina-tihar, F.; Escudero, F.J.L.; Halouane-Sahir, F.; Oukali, Z.; Bensmail, S.; Ghozali, N. Trichoderma spp. isolates from the rhizosphere of healthy olive trees in northern Algeria and their biocontrol potentials against the olive wilt pathogen, Verticillium dahliae. Org. Agric. 2021, 11, 639–657. [Google Scholar] [CrossRef]
- Nandini, B.; Puttaswamy, H.; Saini, R.K.; Prakash, H.S.; Geetha, N. Trichovariability in rhizosphere soil samples and their biocontrol potential against downy mildew pathogen in pearl millet. Sci. Rep. 2021, 11, 9517. [Google Scholar] [CrossRef]
- Lima, G.; Arru, S.; De Curtis, F.; Arras, G. Influence of antagonist, host fruit and pathogen on the biological control of postharvest fungal diseases by yeasts. J. Ind. Microbiol. Biotechnol. 1999, 23, 223–229. [Google Scholar] [CrossRef]
- Fernandez-San Millan, A.; Larraya, L.; Farran, I.; Ancin, M.; Veramendi, J. Successful biocontrol of major postharvest and soil-borne plant pathogenic fungi by antagonistic yeasts. Biol. Control 2021, 160, 104683. [Google Scholar] [CrossRef]
- Mishra, R.K.; Bohra, A.; Kamaal, N.; Kumar, K.; Gandhi, K.; Sujayanand, G.K.; Saabale, P.R.; Satheesh Naik, S.J.; Sarma, B.K.; Kumar, D.; et al. Utilization of biopesticides as sustainable solutions for management of pests in legume crops: Achievements and prospects. Egypt. J. Biol. Pest Control 2018, 28, 3. [Google Scholar] [CrossRef]
- Ghorbanpour, M.; Omidvari, M.; Abbaszadeh-Dahaji, P.; Omidvar, R.; Kariman, K. Mechanisms underlying the protective effects of beneficial fungi against plant diseases. Biol. Control 2018, 117, 147–157. [Google Scholar] [CrossRef]
- Dukare, A.S.; Paul, S.; Nambi, V.E.; Gupta, R.K.; Singh, R.; Sharma, K.; Vishwakarma, R.K. Exploitation of microbial antagonists for the control of postharvest diseases of fruits: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1498–1513. [Google Scholar] [CrossRef]
- Farhana, S.; Ab, S.; Singh, E.; Pieterse, C.M.J.; Schenk, P.M. Emerging Microbial Biocontrol Strategies for Plant Pathogens. Plant Sci. 2018, 267, 102–111. [Google Scholar] [CrossRef]
- Raymaekers, K.; Ponet, L.; Holtappels, D.; Berckmans, B.; Cammue, B.P.A. Screening for novel biocontrol agents applicable in plant disease management—A review. Biol. Control 2020, 144, 104240. [Google Scholar] [CrossRef]
- Karlsson, M.; Atanasova, L.; Jensen, D.F.; Zeilinger, S. Necrotrophic mycoparasites and their genomes. Microbiol. Spectr. 2017, 5, 2–5. [Google Scholar] [CrossRef]
- Jadhav, H.P.; Shaikh, S.S.; Sayyed, R.Z. Role of hydrolytic enzymes of rhizoflora in biocontrol of fungal phytopathogens: An overview. In Rhizotrophs Plant Growth Promotion to Bioremediation; Springer: Singapore, 2017; pp. 183–203. [Google Scholar]
- Bubici, G.; Kaushal, M.; Prigigallo, M.I.; Cabanás, C.G.L.; Mercado-Blanco, J. Biological control agents against Fusarium wilt of banana. Front. Microbiol. 2019, 10, 616. [Google Scholar] [CrossRef]
- Molinari, S.; Leonetti, P. Bio-control agents activate plant immune response and prime susceptible tomato against root-knot nematodes. PLoS ONE 2019, 14, e0213230. [Google Scholar] [CrossRef]
- Yedidia, I.; Shoresh, M.; Kerem, Z.; Benhamou, N.; Kapulnik, Y.; Chet, I. Concomitant induction of systemic resistance to Pseudomonas syringae pv. lachrymans in cucumber by Trichoderma asperellum (T-203) and accumulation of phytoalexins. Appl. Environ. Microbiol. 2003, 69, 7343–7353. [Google Scholar] [CrossRef]
- Win, T.T.; Bo, B.; Malec, P.; Khan, S.; Fu, P. Newly isolated strain of Trichoderma asperellum from disease suppressive soil is a potential bio-control agent to suppress Fusarium soil borne fungal phytopathogens. J. Plant Pathol. 2021, 103, 549–561. [Google Scholar] [CrossRef]
- Nakatsuji, T.; Gallo, R.L. Antimicrobial peptides: Old molecules with new ideas. J. Investig. Dermatol. 2012, 132, 887–895. [Google Scholar] [CrossRef]
- Berdy, J. Bioactive microbial metabolites. J. Antibiot. 2005, 58, 1–26. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wuertz, S. Bacteria and archaea on Earth and their abundance in biofilms. Nat. Rev. Microbiol. 2019, 17, 247–260. [Google Scholar] [CrossRef]
- Li, J.; Zhao, X. Effects of quorum sensing on the biofilm formation and viable but non-culturable state. Food Res. Int. 2020, 137, 109742. [Google Scholar] [CrossRef]
- Bais, H.P.; Fall, R.; Vivanco, J.M. Biocontrol of Bacillus subtilis against infection of Arabidopsis roots by Pseudomonas syringae is facilitated by biofilm formation and surfactin production. Plant Physiol. 2004, 134, 307–319. [Google Scholar] [CrossRef]
- Triveni, S.; Prasanna, R.; Shukla, L.; Saxena, A.K. Evaluating the biochemical traits of novel Trichoderma-based biofilms for use as plant growth-promoting inoculants. Ann. Microbiol. 2013, 63, 1147–1156. [Google Scholar] [CrossRef]
- Velmourougane, K.; Prasanna, R.; Supriya, P.; Ramakrishnan, B.; Thapa, S.; Saxena, A.K. Transcriptome profiling provides insights into regulatory factors involved in Trichoderma viride–Azotobacter chroococcum biofilm formation. Microbiol. Res. 2019, 227, 126292. [Google Scholar] [CrossRef]
- Pandin, C.; Darsonval, M.; Mayeur, C.; Le Coq, D.; Aymerich, S.; Briandet, R. Biofilm formation and synthesis of antimicrobial compounds by the biocontrol agent Bacillus velezensis QST713 in an Agaricus bisporus compost micromodel. Appl. Environ. Microbiol. 2019, 85, e00327-19. [Google Scholar] [CrossRef]
- Janisiewicz, W.J.; Tworkoski, T.J.; Sharer, C. Characterizing the mechanism of biological control of postharvest diseases on fruits with a simple method to study competition for nutrients. Phytopathology 2000, 90, 1196–1200. [Google Scholar] [CrossRef]
- Spadaro, D.; Ciavorella, A.; Dianpeng, Z.; Garibaldi, A.; Gullino, M.L. Effect of culture media and pH on the biomass production and biocontrol efficacy of a Metschnikowia pulcherrima strain to be used as a biofungicide for postharvest disease control. Can. J. Microbiol. 2010, 56, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Garrett, S.D. Cellulolysis rate and competitive saprophytic colonization of wheat straw by foot-rot fungi. Soil Biol. Biochem. 1975, 7, 323–327. [Google Scholar] [CrossRef]
- Shearer, C.A. Fungal competition. Can. J. Bot. 1995, 73, 1259–1264. [Google Scholar] [CrossRef]
- Fokkema, N.J. Opportunities and problems of control of foliar pathogens with micro-organisms. Pestic. Sci. 1993, 37, 411–416. [Google Scholar] [CrossRef]
- Bolton, M.D.; Thomma, B.P.H.J.; Nelson, B.D. Sclerotinia sclerotiorum (Lib.) de Bary: Biology and molecular traits of a cosmopolitan pathogen. Mol. Plant Pathol. 2006, 7, 1–16. [Google Scholar] [CrossRef]
- Williamson, B.; Tudzynski, B.; Tudzynski, P.; Van Kan, J.A.L. Botrytis cinerea: The cause of grey mould disease. Mol. Plant Pathol. 2007, 8, 561–580. [Google Scholar] [CrossRef]
- Zhou, T. Application of Epicoccum purpurascens Spores to Control White Mold of Snap Bean. Plant Dis. 1989, 73, 639. [Google Scholar] [CrossRef]
- Lima, G.; Ippolito, A.; Nigro, F.; Salerno, M. Effectiveness of Aureobasidium pullulans and Candida oleophila against postharvest strawberry rots. Postharvest Biol. Technol. 1997, 10, 169–178. [Google Scholar] [CrossRef]
- Köhl, J.; Gerlagh, M.; De Haas, B.H.; Krijger, M.C. Biological control of Botrytis cinerea in cyclamen with Ulocladium atrum and Gliocladium roseum under commercial growing conditions. Phytopathology 1998, 88, 568–575. [Google Scholar] [CrossRef]
- Li, G.Q.; Huang, H.C.; Acharya, S.N. Antagonism and biocontrol potential of Ulocladium atrum on Sclerotinia sclerotiorum. Biol. Control 2003, 28, 11–18. [Google Scholar] [CrossRef]
- Wszelaki, A.L.; Mitcham, E.J. Effect of combinations of hot water dips, biological control and controlled atmospheres for control of gray mold on harvested strawberries. Postharvest Biol. Technol. 2003, 27, 255–264. [Google Scholar] [CrossRef]
- Karabulut, O.A.; Tezcan, H.; Daus, A.; Cohen, L.; Wiess, B.; Droby, S. Control of preharvest and postharvest fruit rot in Strawberry by Metschnikowia fructicola. Biocontrol Sci. Technol. 2004, 14, 513–521. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, X.; Yu, T. Biological control of postharvest diseases of peach with Cryptococcus laurentii. Food Control 2007, 18, 287–291. [Google Scholar] [CrossRef]
- Handelsman, J.; Parke, J.L. Mechanisms of biocontrol of soilborne plant pathogens. Plant Soil 1989, 129, 85–92. [Google Scholar]
- Spadaro, D.; Gullino, M.L. State of the art and future prospects of the biological control of postharvest fruit diseases. Int. J. Food Microbiol. 2004, 91, 185–194. [Google Scholar] [CrossRef]
- Heydari, A.; Pessarakli, M. A review on biological control of fungal plant pathogens using microbial antagonists. J. Biol. Sci. 2010, 10, 273–290. [Google Scholar] [CrossRef]
- Roca-Couso, R.; Flores-Félix, J.D.; Rivas, R. Mechanisms of Action of Microbial Biocontrol Agents against Botrytis cinerea. J. Fungi 2021, 7, 1045. [Google Scholar] [CrossRef]
- Zhao, Q.; Shen, Q.; Ran, W.; Xiao, T.; Xu, D.; Xu, Y. Inoculation of soil by Bacillus subtilis Y-IVI improves plant growth and colonization of the rhizosphere and interior tissues of muskmelon (Cucumis melo L.). Biol. Fertil. Soils 2011, 47, 507–514. [Google Scholar] [CrossRef]
- Li, S.; Zhang, N.; Zhang, Z.; Luo, J.; Shen, B.; Zhang, R.; Shen, Q. Antagonist Bacillus subtilis HJ5 controls Verticillium wilt of cotton by root colonization and biofilm formation. Biol. Fertil. Soils 2013, 49, 295–303. [Google Scholar] [CrossRef]
- Vellasamy, S.; Hariharan, H. Controlling Strategies (Diagnosis/Quarantine/Eradication) of Plant Pathogenic Bacteria. Sustain. Approaches Control Plant Pathog. Bact. 2015, 2015, 98–127. [Google Scholar]
- Calvente, V.; De Orellano, M.E.; Sansone, G.; Benuzzi, D.; Sanz de Tosetti, M.I. Effect of nitrogen source and pH on siderophore production by Rhodotorula strains and their application to biocontrol of phytopathogenic moulds. J. Ind. Microbiol. Biotechnol. 2001, 26, 226–229. [Google Scholar] [CrossRef]
- Sulochana, M.B.; Jayachandra, S.Y.; Anil Kumar, S.; Dayanand, A. Antifungal attributes of siderophore produced by the Pseudomonas aeruginosa JAS-25. J. Basic Microbiol. 2014, 54, 418–424. [Google Scholar] [CrossRef]
- Weller, D.M.; Raaijmakers, J.M.; Gardener, B.B.M.; Thomashow, L.S. Microbial populations responsible for specific soil suppressiveness to plant pathogens. Annu. Rev. Phytopathol. 2002, 40, 309–348. [Google Scholar] [CrossRef]
- Yu, X.; Ai, C.; Xin, L.; Zhou, G. The siderophore-producing bacterium, Bacillus subtilis CAS15, has a biocontrol effect on Fusarium wilt and promotes the growth of pepper. Eur. J. Soil Biol. 2011, 47, 138–145. [Google Scholar] [CrossRef]
- Demoz, B.T.; Korsten, L. Bacillus subtilis attachment, colonization, and survival on avocado flowers and its mode of action on stem-end rot pathogens. Biol. Control 2006, 37, 68–74. [Google Scholar] [CrossRef]
- Wang, X.Q.; Zhao, D.L.; Shen, L.L.; Jing, C.L.; Zhang, C.S. Application and Mechanisms of Bacillus subtilis in Biological Control of Plant Disease. In Role of Rhizospheric Microbes in Soil; Meena, V.S., Ed.; Springer: Singapore, 2018; pp. 225–250. [Google Scholar]
- Poppe, L.; Vanhoutte, S.; Höfte, M. Modes of action of Pantoea agglomerans CPA-2, an antagonist of postharvest pathogens on fruits. Eur. J. Plant Pathol. 2003, 109, 963–973. [Google Scholar] [CrossRef]
- Bencheqroun, S.K.; Bajji, M.; Massart, S.; Labhilili, M.; El Jaafari, S.; Jijakli, M.H. In vitro and in situ study of postharvest apple blue mold biocontrol by Aureobasidium pullulans: Evidence for the involvement of competition for nutrients. Postharvest Biol. Technol. 2007, 46, 128–135. [Google Scholar] [CrossRef]
- Spadaro, D.; Droby, S. Development of biocontrol products for postharvest diseases of fruit: The importance of elucidating the mechanisms of action of yeast antagonists. Trends Food Sci. Technol. 2016, 47, 39–49. [Google Scholar] [CrossRef]
- Bonaterra, A.; Mari, M.; Casalini, L.; Montesinos, E. Biological control of Monilinia laxa and Rhizopus stolonifer in postharvest of stone fruit by Pantoea agglomerans EPS125 and putative mechanisms of antagonism. Int. J. Food Microbiol. 2003, 84, 93–104. [Google Scholar] [CrossRef]
- Kerry, B.R. Rhizosphere interactions and the exploitation of microbial agents for the biological control of plant-parasitic nematodes. Annu. Rev. Phytopathol. 2000, 38, 423–441. [Google Scholar] [CrossRef]
- Xu, X.; Hu, X. The effect of aggregation of pathogen and biocontrol microbe propagules on biocontrol potential: A simple modelling study. Phytopathol. Res. 2020, 2, 5. [Google Scholar] [CrossRef]
- Johnson, K.B. Pathogen refuge: A key to understanding biological control. Annu. Rev. Phytopathol. 2010, 48, 141–160. [Google Scholar] [CrossRef] [PubMed]
- Freimoser, F.M.; Rueda-Mejia, M.P.; Tilocca, B.; Migheli, Q. Biocontrol yeasts: Mechanisms and applications. World J. Microbiol. Biotechnol. 2019, 35, 154. [Google Scholar] [CrossRef] [PubMed]
- Elad, Y.; Chet, I.; Boyle, P.; Henis, Y. Parasitism of Trichoderma spp. on Rhizoctonia solani and Sclerotium rolfsii-scanning electron microscopy and fluorescence microscopy. Phytopathology 1983, 73, 85–88. [Google Scholar] [CrossRef]
- Harman, G.; Khadka, R.; Doni, F.; Uphoff, N. Benefits to plant health and productivity from enhancing plant microbial symbionts. Front. Plant Sci. 2021, 11, 610065. [Google Scholar] [CrossRef]
- Howell, C.R. Mechanisms employed by Trichoderma species in the biological control of plant diseases: The history and evolution of current concepts. Plant Dis. 2003, 87, 4–10. [Google Scholar] [CrossRef]
- Abdullah, N.S.; Doni, F.; Mispan, M.S.; Saiman, M.Z.; Yusuf, Y.M.; Oke, M.A.; Suhaimi, N.S.M. Harnessing Trichoderma in agriculture for productivity and sustainability. Agronomy 2021, 11, 2559. [Google Scholar] [CrossRef]
- Wisniewski, M.; Biles, C.; Droby, S.; McLaughlin, R.; Wilson, C.; Chalutz, E. Mode of action of the postharvest biocontrol yeast, Pichia guilliermondii. I. Characterization of attachment to Botrytis cinerea. Physiol. Mol. Plant Pathol. 1991, 39, 245–258. [Google Scholar] [CrossRef]
- Ezrari, S.; Mhidra, O.; Radouane, N.; Tahiri, A.; Polizzi, G.; Lazraq, A.; Lahlali, R. Potential role of rhizobacteria isolated from citrus rhizosphere for biological control of citrus dry root rot. Plants 2021, 10, 872. [Google Scholar] [CrossRef]
- Olanrewaju, O.S.; Glick, B.R.; Babalola, O.O. Mechanisms of action of plant growth promoting bacteria. World J. Microbiol. Biotechnol. 2017, 33, 197. [Google Scholar] [CrossRef]
- Shafi, J.; Tian, H.; Ji, M. Bacillus species as versatile weapons for plant pathogens: A review. Biotechnol. Biotechnol. Equip. 2017, 31, 446–459. [Google Scholar] [CrossRef]
- Li, N.; Han, X.; Feng, D.; Yuan, D.; Huang, L.-J. Signaling crosstalk between salicylic acid and ethylene/jasmonate in plant defense: Do we understand what they are whispering? Int. J. Mol. Sci. 2019, 20, 671. [Google Scholar] [CrossRef]
- Ghozlan, M.H.; Eman, E.-A.; Tokgöz, S.; Lakshman, D.K.; Mitra, A. Plant Defense against Necrotrophic Pathogens. J. Plant Sci. 2020, 11, 2122–2138. [Google Scholar] [CrossRef]
- Van Loon, L.C.; Bakker, P.; Pieterse, C.M.J. Systemic resistance induced by rhizosphere bacteria. Annu. Rev. Phytopathol. 1998, 36, 453–483. [Google Scholar] [CrossRef]
- Numan, M.; Bashir, S.; Khan, Y.; Mumtaz, R.; Shinwari, Z.K.; Khan, A.L.; Khan, A.; AL-Harrasi, A. Plant growth promoting bacteria as an alternative strategy for salt tolerance in plants: A review. Microbiol. Res. 2018, 209, 21–32. [Google Scholar] [CrossRef]
- Ezrari, S.; Radouane, N.; Tahiri, A.; El Housni, Z.; Mokrini, F.; Özer, G.; Lazraq, A.; Belabess, Z.; Amiri, S.; Lahlali, R. Dry root rot disease, an emerging threat to citrus industry worldwide under climate change: A review. Physiol. Mol. Plant Pathol. 2022, 117, 101753. [Google Scholar] [CrossRef]
- Yu, Y.; Gui, Y.; Li, Z.; Jiang, C.; Guo, J.; Niu, D. Induced Systemic Resistance for Improving Plant Immunity by Beneficial Microbes. Plants 2022, 11, 386. [Google Scholar] [CrossRef]
- Saxena, A.; Mishra, S.; Ray, S.; Raghuwanshi, R.; Singh, H.B. Differential reprogramming of defense network in Capsicum annum L. plants against colletotrichum truncatum infection by phyllospheric and rhizospheric trichoderma strains. J. Plant Growth Regul. 2020, 39, 751–763. [Google Scholar] [CrossRef]
- Gkizi, D.; Poulaki, E.G.; Tjamos, S.E. Towards Biological Control of Aspergillus carbonarius and Botrytis cinerea in Grapevine Berries and Transcriptomic Changes of Genes Encoding Pathogenesis-Related (PR) Proteins. Plants 2021, 10, 970. [Google Scholar] [CrossRef]
- Guo, Q.; Li, Y.; Lou, Y.; Shi, M.; Jiang, Y.; Zhou, J.; Sun, Y.; Xue, Q.; Lai, H. Bacillus amyloliquefaciens Ba13 induces plant systemic resistance and improves rhizosphere microecology against tomato yellow leaf curl virus disease. Appl. Soil Ecol. 2019, 137, 154–166. [Google Scholar] [CrossRef]
- Wang, M.; Xue, J.; Ma, J.; Feng, X.; Ying, H.; Xu, H. Streptomyces lydicus M01 regulates soil microbial community and alleviates foliar disease caused by Alternaria alternata on cucumbers. Front. Microbiol. 2020, 11, 942. [Google Scholar] [CrossRef]
- Walters, D.R.; Ratsep, J.; Havis, N.D. Controlling crop diseases using induced resistance: Challenges for the future. J. Exp. Bot. 2013, 64, 1263–1280. [Google Scholar] [CrossRef]
- Ross, A.F. Systemic acquired resistance induced by localized virus infections in plants. Virology 1961, 14, 340–358. [Google Scholar] [CrossRef]
- Kloepper, J.W.; Tuzun, S.; Kuć, J.A. Proposed definitions related to induced disease resistance. Biocontrol Sci. Technol. 1992, 2, 349. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.M.; Bakker, P.A.H.M. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef]
- Jones, J.D.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- Van Peer, R.; Niemann, G.J.; Schippers, B. Induced resistance and phytoalexin accumulation in biological control of Fusarium wilt of carnation by Pseudomonas sp. strain WCS 417 r. Phytopathology 1991, 81, 728–734. [Google Scholar] [CrossRef]
- Ongena, M.; Duby, F.; Jourdan, E.; Beaudry, T.; Jadin, V.; Dommes, J.; Thonart, P. Bacillus subtilis M4 decreases plant susceptibility towards fungal pathogens by increasing host resistance associated with differential gene expression. Appl. Microbiol. Biotechnol. 2005, 67, 692–698. [Google Scholar] [CrossRef]
- Risoli, S.; Cotrozzi, L.; Sarrocco, S.; Nuzzaci, M.; Pellegrini, E.; Vitti, A. Trichoderma-Induced Resistance to Botrytis cinerea in Solanum Species: A Meta-Analysis. Plants 2022, 11, 180. [Google Scholar] [CrossRef]
- Frew, A.; Antunes, P.M.; Cameron, D.D.; Hartley, S.E.; Johnson, S.N.; Rillig, M.C.; Bennett, A.E. Plant herbivore protection by arbuscular mycorrhizas: A role for fungal diversity? New Phytol. 2022, 233, 1022–1031. [Google Scholar] [CrossRef]
- Conrath, U.; Beckers, G.J.M.; Langenbach, C.J.G.; Jaskiewicz, M.R. Priming for enhanced defense. Annu. Rev. Phytopathol. 2015, 53, 97–119. [Google Scholar] [CrossRef] [PubMed]
- Shoresh, M.; Harman, G.E.; Mastouri, F. Induced systemic resistance and plant responses to fungal biocontrol agents. Annu. Rev. Phytopathol. 2010, 48, 21–43. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Tang, S.; Meng, X.; Zhu, H.; Zhu, Y.; Liu, D.; Shen, Q. Proteomic Analysis Demonstrates a Molecular Dialog Between Trichoderma guizhouense NJAU 4742 and Cucumber (Cucumis sativus L.) Roots: Role in Promoting Plant Growth. Mol. Plant-Microbe Interact. 2021, 34, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Sarma, B.K.; Yadav, S.K.; Patel, J.S.; Singh, H.B. Molecular mechanisms of interactions of Trichoderma with other fungal species. Open Mycol. J. 2014, 8, 140–147. [Google Scholar] [CrossRef][Green Version]
- Pandey, V.; Shukla, A.; Kumar, J. Physiological and molecular signalling involved in disease management through Trichoderma: An effective biocontrol paradigm. In Current Trends in Plant Disease Diagnostics and Management Practices; Springer: Cham, Switzerland, 2016; pp. 317–346. [Google Scholar]
- Durrant, W.E.; Dong, X. Systemic acquired resistance. Annu. Rev. Phytopathol. 2004, 42, 185–209. [Google Scholar] [CrossRef]
- Pineda, A.; Zheng, S.-J.; van Loon, J.J.A.; Pieterse, C.M.J.; Dicke, M. Helping plants to deal with insects: The role of beneficial soil-borne microbes. Trends Plant Sci. 2010, 15, 507–514. [Google Scholar] [CrossRef]
- Alınç, T.; Cusumano, A.; Peri, E.; Torta, L.; Colazza, S. Trichoderma harzianum strain T22 modulates direct defense of tomato plants in response to Nezara viridula feeding activity. J. Chem. Ecol. 2021, 47, 455–462. [Google Scholar] [CrossRef]
- Leonetti, P.; Zonno, M.C.; Molinari, S.; Altomare, C. Induction of SA-signaling pathway and ethylene biosynthesis in Trichoderma harzianum-treated tomato plants after infection of the root-knot nematode Meloidogyne incognita. Plant Cell Rep. 2017, 36, 621–631. [Google Scholar] [CrossRef]
- Martínez-Medina, A.; Fernandez, I.; Lok, G.B.; Pozo, M.J.; Pieterse, C.M.J.; Van Wees, S.C.M. Shifting from priming of salicylic acid-to jasmonic acid-regulated defences by Trichoderma protects tomato against the root knot nematode Meloidogyne incognita. New Phytol. 2017, 213, 1363–1377. [Google Scholar] [CrossRef]
- Stenberg, J.A.; Sundh, I.; Becher, P.G.; Björkman, C.; Dubey, M.; Egan, P.A.; Friberg, H.; Gil, J.F.; Jensen, D.F.; Jonsson, M.; et al. When is it biological control? A framework of definitions, mechanisms, and classifications. J. Pest Sci. 2021, 94, 665–676. [Google Scholar] [CrossRef]
- Singh, A.; Singh, V.K.; Dwivedy, A.K.; Deepika; Tiwari, S.; Dwivedi, A.; Dubey, N.K. Biological Control of Plant Diseases: Opportunities and Limitations. In Plant Microbiome Paradigm; Springer International Publishing: Cham, Switzerland, 2020; pp. 121–146. [Google Scholar]
- Essiedu, J.A.; Adepoju, F.O.; Ivantsova, M.N. Benefits and limitations in using biopesticides: A review. AIP Conf. Proc. 2020, 2313, 080002. [Google Scholar]
- Navon, A. Bacillus thuringiensis Application in Agriculture; Springer: Dordrecht, The Netherlands, 2000. [Google Scholar]
- Akutse, K.S.; Subramanian, S.; Maniania, N.K.; Dubois, T.; Ekesi, S. Biopesticide Research and Product Development in Africa for Sustainable Agriculture and Food Security—Experiences from the International Centre of Insect Physiology and Ecology (icipe). Front. Sustain. Food Syst. 2020, 4, 152. [Google Scholar] [CrossRef]
- Chandler, D.; Bailey, A.S.; Tatchell, G.M.; Davidson, G.; Greaves, J.; Grant, W.P. The development, regulation and use of biopesticides for integrated pest management. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 1987–1998. [Google Scholar] [CrossRef]
- Hassen, A.I.; Bopape, F.L.; Sanger, L.K. Microbial Inoculants as Agents of Growth Promotion and Abiotic Stress Tolerance in Plants. In Microbial Inoculants in Sustainable Agricultural Productivity; Springer: New Deli, India, 2016; pp. 23–36. [Google Scholar] [CrossRef]
- Stamenković, S.; Beškoski, V.; Karabegović, I.; Lazić, M.; Nikolić, N. Microbial fertilizers: A comprehensive review of current findings and future perspectives. Span. J. Agric. Res. 2018, 16, 1. [Google Scholar] [CrossRef]
- Prashar, P.; Kapoor, N.; Sachdeva, S. Rhizosphere: Its structure, bacterial diversity and significance. Rev. Environ. Sci. Biotechnol. 2014, 13, 63–77. [Google Scholar] [CrossRef]
- Fravel, D.R. Commercialization and Implementation of Biocontrol. Annu. Rev. Phytopathol. 2005, 43, 337–359. [Google Scholar] [CrossRef]
- Junaid, J.M.; Dar, N.A.; Bhat, T.A.; Bhat, A.H.; Bhat, M.A. Commercial Biocontrol Agents and Their Mechanism of Action in the Management of Plant Pathogens. Int. J. Mod. Plant Anim. Sci. 2013, 1, 39–57. [Google Scholar]
- Gehlot, J.S.P. Fungi and Their Role in Sustainable Development: Current Perspectives; Springer: Singapore, 2018; 779p. [Google Scholar] [CrossRef]
- Bisht, N.; Mishra, S.K.; Chauhan, P.S. Bacillus amyloliquefaciens inoculation alters physiology of rice (Oryza sativa L. var. IR-36) through modulating carbohydrate metabolism to mitigate stress induced by nutrient starvation. Int. J. Biol. Macromol. 2019, 143, 937–951. [Google Scholar] [CrossRef]
- Sundh, I.; Goettel, M.S. Regulating biocontrol agents: A historical perspective and a critical examination comparing microbial and macrobial agents. BioControl 2013, 58, 575–593. [Google Scholar] [CrossRef]
- Taylor, P.; Deacon, J.W. Significance of ecology in the development of biocontrol agents against soil-borne plant pathogens. Biocontrol Sci. Technol. 2008, 1, 37–41. [Google Scholar] [CrossRef]
- Lacey, L.A.; Grzywacz, D.; Frutos, R.; Brownbridge, M.; Goettel, M.S. JIP-15-82 Formerly with Agriculture and Agri-Food Canada, Lethbridge Research Centre, Lethbridge. J. Invertebr. Pathol. 2015, 132, 1–41. [Google Scholar] [CrossRef]
- Holah, J.C.; Alexander, H.M. Soil pathogenic fungi have the potential to affect the co- existence of two tallgrass prairie species. J. Ecol. 1999, 598–608. [Google Scholar] [CrossRef]
- Shtienberg, D.; Elad, Y. Incorporation of Weather Forecasting in Integrated, Biological-Chemical Management of Botrytis cinerea. Phytopathology 1996, 87, 332–340. [Google Scholar] [CrossRef]
- Guetsky, R.; Shtienberg, D.; Elad, Y.; Dinoor, A. Combining Biocontrol Agents to Reduce the Variability of Biological Control. Phytopathology 2001, 91, 621–627. [Google Scholar] [CrossRef]
- Mark, G.L.; Morrissey, J.P.; Higgins, P.; Gara, F.O. Molecular-based strategies to exploit Pseudomonas biocontrol strains for environmental biotechnology applications. FEMS Microbiol. Ecol. 2006, 56, 167–177. [Google Scholar] [CrossRef]
- Guerrero, V.; Guigon, C.; Berlanga, D.; Ojeda, D. Complete control of Penicillium expansum on apple fruit using a combination of antagonistic yeast Candida oleophila. Chil. J. Agric. Res. 2014, 74, 427–431. [Google Scholar] [CrossRef]
- Pal, K.K.; Scholar, V.; Gardener, B.M. Biological Control of Plant Pathogens; American Psychopathological Society: St. Paul, MN, USA, 2006; pp. 1–25. [Google Scholar] [CrossRef]
- Prajapati, S.; Kumar, N.; Kumar, S.; Iakharan, L.; Maurya, S. Biological control a sustainable approach for plant disease management: A review. J. Pharmacogn. Phytochem. 2020, 9, 1514–1523. [Google Scholar]
- Tunde, D.; Molnár, I.; Rakosy-Tican, E. New insights into the interaction between cultivated potato and Phytophthora infestans. Stud. Biol. UBB 2015, 1, 2015–2165. [Google Scholar]
- Stojanović, S.S.; Karabegović, I.; Beškoski, V.; Nikolić, N.; Lazić, M. Bacillus based microbial formulations: Optimization of the production process. Hem. Ind. 2019, 73, 169–182. [Google Scholar] [CrossRef]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; Mcroberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019. 3, 430–439. [CrossRef]
- Berling, M.; Blachere-lopez, C.; Soubabere, O.; Lery, X.; Bonhomme, A.; Sauphanor, B.; Lopez-ferber, M. Cydia pomonella granulovirus Genotypes Overcome Virus Resistance in the Codling Moth and Improve Virus Efficiency by Selection against Resistant Hosts. Appl. Environ. Microbiol. 2009, 75, 925–930. [Google Scholar] [CrossRef]
- Zhan, J.; Mcdonald, B.A. Field-based experimental evolution of three cereal pathogens using a mark—Release–recapture strategy. Plant Pathol. 2013, 62, 106–114. [Google Scholar] [CrossRef]
- Sundh, I.; Eilenberg, J. Why Has the Authorization of Microbial Biological Control Agents Been Slower in the EU than in Comparable Jurisdictions? Pest Manag. Sci. 2021, 77, 2170–2178. [Google Scholar] [CrossRef] [PubMed]
- Abou-Zeid, N.M.; Mahmoud, N.A.; Eid, H.T.; Abbas, M.S.; Soliman, A.S. Induction of systemic resistance against Tomato wilt disease under greenhouse conditions in Egypt. Biosci. Res. 2018, 15, 4559–4570. [Google Scholar]
- Dhakal, R. Biopesticides: A Key to Sustainable Agriculture. Int. J. Pure Appl. Biosci. 2019, 7, 391–396. [Google Scholar] [CrossRef]
- Peng, G.; Mcgregor, L.; Lahlali, R.; Gossen, B.D.; Hwang, S.F.; Adhikari, K.K.; Strelkov, S.E.; Mcdonald, M.R. Potential biological control of clubroot on canola and crucifer vegetable crops. Plant Pathol. 2011, 60, 566–574. [Google Scholar] [CrossRef]
- Kean, S.; Soytong, K.; To-anun, C. Application of biological fungicides to control citrus root rot under field condition in Cambodia. J. Agric. Technol. 2010, 6, 219–230. [Google Scholar]
- Soytong, K.; Song, J.J.; Tongon, R. Agricultural Inputs for Organic Agriculture. Proceeding of International Seminar on Promoting Local Resources for Sustainable Agriculture and Development (ISPLRSAD 2020). Adv. Biol. Sci. Res. 2021, 13, 523–532. [Google Scholar] [CrossRef]
- Wisniewski, M.; Droby, S.; Norelli, J.; Liu, J.; Schena, L. Alternative management technologies for postharvest disease control: The journey from simplicity to complexity. Postharvest Biol. Technol. 2016, 122, 3–10. [Google Scholar] [CrossRef]
- Mudgal, S.; De Toni, A.; Tostivint, C.; Hokkanen, H.; Chandler, D. Scientific support, literature review and data collection and analysis for risk assessment on microbial organisms used as active substance in plant protection products—Lot 1 Environmental Risk characterisation. EFSA Support. Publ. 2017, 10, 518E. [Google Scholar] [CrossRef]
- Villaverde, J.J.; Sevilla-Morán, B.; Sandín-España, P.; López-Goti, C.; Alonso-Prados, J.L. Biopesticides in the framework of the European Pesticide Regulation (EC) No. 1107/2009. Pest Manag. Sci. 2014, 70, 2–5. [Google Scholar] [CrossRef]
- Raveau, R.; Fontaine, J.; Lounès-Hadj Sahraoui, A. Essential oils as potential alternative biocontrol products against plant pathogens and weeds: A review. Foods 2020, 9, 365. [Google Scholar] [CrossRef]
- Nicot, P.C.; Alabouvette, C.; Bardin, M.; Blum, B.; Köhl, J.; Ruocco, M. Review of factors influencing the success or failure of biocontrol: Technical, industrial and socio-economic perspectives. Biol. Control Fungal Bact. Plant Pathog. 2012, 78, 95–98. [Google Scholar]
- Timper, P. Conserving and Enhancing Biological Control of Nematodes. J. Nematol. 2014, 46, 75–89. [Google Scholar]
- Bardin, M.; Ajouz, S.; Comby, M.; Lopez-Ferber, M.; Graillot, B.; Siegwart, M.; Nicot, P.C. Is the efficacy of biological control against plant diseases likely to be more durable than that of chemical pesticides? Front. Plant Sci. 2015, 6, 566. [Google Scholar] [CrossRef]
- Hassan, M.N.; Afghan, S.; Hafeez, F.Y. Biological control of red rot in sugarcane by native pyoluteorin-producing Pseudomonas putida strain NH-50 under field conditions and its potential modes of action. Pest Manag. Sci. 2011, 67, 1147–1154. [Google Scholar] [CrossRef]
- Singh, J.S.; Vimal, S.R. Microbial Services in Restoration Ecology, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Sare, A.R.; Jijakli, M.H.; Jijakli, M.H.; Massart, S. Microbial ecology to support integrative efficacy improvement of biocontrol agents for postharvest diseases management. Postharvest Biol. Technol. 2021, 179, 111572. [Google Scholar] [CrossRef]
- Askary, T.H.; Martinelli, P.R.P. Biocontrol Agents of Phytonematodes; Askary, T.H., Martinelli, P.R.P., Eds.; CABI: Wallingford, UK, 2015. [Google Scholar]
- Crow, W.T.; Luc, J.E.; Giblin-Davis, R.M. Evaluation of EconemTM, a Formulated Pasteuria sp. Bionematicide, for Management of Belonolaimus longicaudatus on Golf Course Turf. J. Nematol. 2011, 43, 101–109. [Google Scholar]
- Wilson, M.J.; Jackson, T.A. Progress in the commercialisation of bionematicides. BioControl 2013, 58, 715–722. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lahlali, R.; Ezrari, S.; Radouane, N.; Kenfaoui, J.; Esmaeel, Q.; El Hamss, H.; Belabess, Z.; Barka, E.A. Biological Control of Plant Pathogens: A Global Perspective. Microorganisms 2022, 10, 596. https://doi.org/10.3390/microorganisms10030596
Lahlali R, Ezrari S, Radouane N, Kenfaoui J, Esmaeel Q, El Hamss H, Belabess Z, Barka EA. Biological Control of Plant Pathogens: A Global Perspective. Microorganisms. 2022; 10(3):596. https://doi.org/10.3390/microorganisms10030596
Chicago/Turabian StyleLahlali, Rachid, Said Ezrari, Nabil Radouane, Jihane Kenfaoui, Qassim Esmaeel, Hajar El Hamss, Zineb Belabess, and Essaid Ait Barka. 2022. "Biological Control of Plant Pathogens: A Global Perspective" Microorganisms 10, no. 3: 596. https://doi.org/10.3390/microorganisms10030596
APA StyleLahlali, R., Ezrari, S., Radouane, N., Kenfaoui, J., Esmaeel, Q., El Hamss, H., Belabess, Z., & Barka, E. A. (2022). Biological Control of Plant Pathogens: A Global Perspective. Microorganisms, 10(3), 596. https://doi.org/10.3390/microorganisms10030596










