Bioprospecting and Molecular Identification of Used Transformer Oil-Degrading Bacteria for Bioplastics Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Samples
2.2. Bacterial Isolation
2.3. Screening for PHA Production
2.4. Analysis of Used Transformer Oil Degradation
2.5. Shake Flask Fermentation and PHA Quantification
2.6. PHAs Monomers Analysis
2.7. Bacterial Identification
3. Results
3.1. Bacterial Isolation and Screening for PHA Production
3.2. Oil Degradation and PHAs Quantification
3.3. PHA Monomer Identification
3.4. Bacterial Identification and Diversity Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Możejko-Ciesielska, J.; Kiewisz, R. Bacterial polyhydroxyalkanoates: Still fabulous? Microbiol. Res. 2016, 192, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Chanprateep, S. Current trends in biodegradable polyhydroxyalkanoates. J. Biosci. Bioeng. 2010, 110, 621–632. [Google Scholar] [CrossRef]
- Lopez, G.; Artetxe, M.; Amutio, M.; Alvarez, J.; Bilbao, J.; Olazar, M. Recent advances in the gasification of waste plastics. A critical overview. Renew. Sustain. Energy Rev. 2018, 82, 576–596. [Google Scholar] [CrossRef]
- Prasanna Raj Yadav, S.; Saravanan, C.G.; Vallinayagam, R.; Vedharaj, S.; Roberts, W.L. Fuel and engine characterization study of catalytically cracked waste transformer oil. Energy Convers. Manag. 2015, 96, 490–498. [Google Scholar] [CrossRef]
- Koller, M. Biodegradable and biocompatible polyhydroxy-alkanoates (PHA): Auspicious microbial macromolecules for pharmaceutical and therapeutic applications. Molecules 2018, 23, 362. [Google Scholar] [CrossRef]
- Kunasundari, B.; Sudesh, K. Isolation and recovery of microbial polyhydroxyalkanoates. Express Polym. Lett. 2011, 5, 620–634. [Google Scholar] [CrossRef]
- Cavalheiro, J.M.B.T.; de Almeida, M.C.M.D.; Grandfils, C.; da Fonseca, M.M.R. Poly(3-hydroxybutyrate) production by Cupriavidus necator using waste glycerol. Process Biochem. 2009, 44, 509–515. [Google Scholar] [CrossRef]
- Teeka, J.; Imai, T.; Cheng, X.; Reungsang, A.; Higu, T.; Yamamoto, K.; Sekine, M. Screening of PHA-Producing Bacteria Using Biodiesel-Derived Waste Glycerol as a Sole Carbon Source. J. Water Environ. Technol. 2010, 8, 373–381. [Google Scholar] [CrossRef][Green Version]
- Loo, C.Y.; Lee, W.H.; Tsuge, T.; Doi, Y.; Sudesh, K. Biosynthesis and characterization of poly (3-hydroxybutyrate-co-3- hydroxyhexanoate) from palm oil products in a Wautersia eutropha mutant. Biotechnol. Lett. 2005, 27, 1405–1410. [Google Scholar] [CrossRef]
- Posada, J.A.; Naranjo, J.M.; López, J.A.; Higuita, J.C.; Cardona, C.A. Design and analysis of poly-3-hydroxybutyrate production processes from crude glycerol. Process Biochem. 2011, 46, 310–317. [Google Scholar] [CrossRef]
- Teeka, J.; Imai, T.; Reungsang, A.; Cheng, X.; Yuliani, E.; Thiantanankul, J.; Poomipuk, N.; Yamaguchi, J.; Jeenanong, A.; Higuchi, T.; et al. Characterization of polyhydroxyalkanoates (PHAs) biosynthesis by isolated Novosphingobium sp. THA-AIK7 using crude glycerol. J. Ind. Microbiol. Biotechnol. 2012, 39, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Fukui, T.; Doi, Y. Efficient production of polyhydroxyalkanoates from plant oils by Alcaligenes eutrophus and its recombinant strain. Appl. Microbiol. Biotechnol. 1998, 49, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Cerrone, F.; Choudhari, S.K.; Davis, R.; Cysneiros, D.; O’Flaherty, V.; Duane, G.; Casey, E.; Guzik, M.W.; Kenny, S.T.; Babu, R.P.; et al. Medium chain length polyhydroxyalkanoate (mcl-PHA) production from volatile fatty acids derived from the anaerobic digestion of grass. Appl. Microbiol. Biotechnol. 2014, 98, 611–620. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, L.; Wen, Q.; Guo, Z. Efficient polyhydroxyalkanoate (PHA) accumulation by a new continuous feeding mode in three-stage mixed microbial culture (MMC) PHA production process. J. Biotechnol. 2015, 209, 68–75. [Google Scholar] [CrossRef]
- Pozo, C.; Martínez-Toledo, M.V.; Rodelas, B.; González-López, J. Effects of culture conditions on the production of polyhydroxyalkanoates by Azotobacter chroococcum H23 in media containing a high concentration of alpechín (wastewater from olive oil mills) as primary carbon source. J. Biotechnol. 2002, 97, 125–131. [Google Scholar] [CrossRef]
- Griffin, G.J.L. Chemistry and Technology of Biodegradable Polymers; Blackie Academic: London, UK, 1995; Volume 72, ISBN 0751400033. [Google Scholar]
- Philip, S.; Keshavarz, T.; Roy, I. Polyhydroxyalkanoates: Biodegradable polymers with a range of applications. J. Chem. Technol. Biotechnol. 2007, 82, 233–247. [Google Scholar] [CrossRef]
- Keshavarz, T.; Roy, I. Polyhydroxyalkanoates: Bioplastics with a green agenda. Curr. Opin. Microbiol. 2010, 13, 321–326. [Google Scholar] [CrossRef]
- Chee, J.; Yoga, S.; Lau, N.; Ling, S.; Abed, R.M.M. Bacterially Produced Polyhydroxyalkanoate (PHA): Converting Renewable Resources into Bioplastics. Curr. Res. Technol. Educ. Top. Appl. Microbiol. Microb. Biotechnol. 2010, 2, 1395–1404. [Google Scholar]
- Pradeep, J.A.; Kishore Kumar, K.; Scholars, U.; Balasubramanian, D. Biofuel Production Using Butanol and Used Transformer Oil. Am. J. Eng. Res. 2016, 5, 285–293. [Google Scholar]
- Bahera, P. Experimental Studies on Utilization of Used Transformer Oil as an Alternative Fuel in a DI Diesel Engine. Ph.D. Thesis, National Institute of Technology, Rourkela, India, 2013; pp. 31–38. [Google Scholar] [CrossRef]
- Amirul, A.A.; Yahya, A.R.M.; Sudesh, K.; Azizan, M.N.M.; Majid, M.I.A. Biosynthesis of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) copolymer by Cupriavidus sp. USMAA1020 isolated from Lake Kulim, Malaysia. Bioresour. Technol. 2008, 99, 4903–4909. [Google Scholar] [CrossRef]
- Spiekermann, P.; Rehm, B.H.A.; Kalscheuer, R.; Baumeister, D.; Steinbüchel, A. A sensitive, viable colony staining method using Nile red for direct screening of bacteria that accumulate polyhydroxyalkanoic acids and other lipid storage compounds. Arch. Microbiol. 1999, 171, 73–80. [Google Scholar] [CrossRef] [PubMed]
- López-Cortés, A.; Lanz-Landázuri, A.; García-Maldonado, J.Q. Screening and isolation of PHB-producing bacteria in a polluted marine microbial mat. Microb. Ecol. 2008, 56, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Fernández, D.; Rodríguez, E.; Bassas, M.; Viñas, M.; Solanas, A.M.; Llorens, J.; Marqués, A.M.; Manresa, A. Agro-industrial oily wastes as substrates for PHA production by the new strain Pseudomonas aeruginosa NCIB 40045: Effect of culture conditions. Biochem. Eng. J. 2005, 26, 159–167. [Google Scholar] [CrossRef]
- Jayashree, R.; Nithya, S.E.; Prasanna, P.R.; Krishnaraju, M. Biodegradation capability of bacterial species isolated from oil contaminated soil. J. Acad. Ind. Res. 2012, 1, 140–143. [Google Scholar]
- Huong, K.H.; Teh, C.H.; Amirul, A.A. Microbial-based synthesis of highly elastomeric biodegradable poly(3-hydroxybutyrate-co-4-hydroxybutyrate) thermoplastic. Int. J. Biol. Macromol. 2017, 101, 983–995. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Duan, J.; Geng, W.; Feng, J.; Wang, S.; Song, C. Comparison of medium-chain-length polyhydroxyalkanoates synthases from Pseudomonas mendocina NK-01 with the same substrate specificity. Microbiol. Res. 2013, 168, 231–237. [Google Scholar] [CrossRef]
- Turner, S.; Pryer, K.M.; Miao, V.P.W.; Palmer, J.D. Investigating deep phylogenetic relationships among cyanobacteria and plastids by small subunit rRNA sequence analysis. J. Eukaryot. Microbiol. 1999, 46, 327–338. [Google Scholar] [CrossRef]
- Rédei, G.P. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Encycl. Genet. Genom. Proteom. Inform. 2008, 11, 376–377. [Google Scholar] [CrossRef]
- Follonier, S.; Riesen, R.; Zinn, M. Pilot-scale production of functionalized mcl-PHA from grape pomace supplemented with fatty acids. Chem. Biochem. Eng. Q. 2015, 29, 113–121. [Google Scholar] [CrossRef]
- Wang, H.H.; Li, X.T.; Chen, G.Q. Production and characterization of homopolymer polyhydroxyheptanoate (P3HHp) by a fadBA knockout mutant Pseudomonas putida KTOY06 derived from P. putida KT2442. Process Biochem. 2009, 44, 106–111. [Google Scholar] [CrossRef]
- Gmbh, C.L.; Industries, A.; Gmbh, C.L.; Industries, A. Industrial production of poly-/3-hydroxybutyrate. FEMS Microbiol. Rev. 1992, 103, 251–255. [Google Scholar]
- Safi, A.; Subhanullah, K.; Ayaz, M.; Attaullah; Khatak, B.; Akbar, N.; Khan, I.; Asif, M.; Khan, N.; Ullah, S. Isolation and Identification of Bacteria from Transformer Oil Contaminated Soil. Br. Microbiol. Res. J. 2015, 6, 207–214. [Google Scholar] [CrossRef]
- Ayandele, A.; Fagade, O. Effect of ammonium salts on the biodegradation of used transformer oil using locally isolated microorganisms. Agric. Biol. J. North Am. 2012, 3, 131–139. [Google Scholar] [CrossRef]
- Sobiecka, E.; Cedzynska, K.; Bielski, C.; Antizar-Ladislao, B. Biological treatment of transformer oil using commercial mixtures of microorganisms. Int. Biodeterior. Biodegrad. 2009, 63, 328–333. [Google Scholar] [CrossRef]
- Amirul, A.A.; Syairah, S.N.; Yahya, A.R.M.; Azizan, M.N.M.; Majid, M.I.A. Synthesis of biodegradable polyesters by Gram negative bacterium isolated from Malaysian environment. World J. Microbiol. Biotechnol. 2008, 24, 1327–1332. [Google Scholar] [CrossRef]
- Kung, S.S.; Chuang, Y.C.; Chen, C.H.; Chien, C.C. Isolation of polyhydroxyalkanoates-producing bacteria using a combination of phenotypic and genotypic approach. Lett. Appl. Microbiol. 2007, 44, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Mravec, F.; Obruca, S.; Krzyzanek, V.; Sedlacek, P.; Hrubanova, K.; Samek, O.; Kucera, D.; Benesova, P.; Nebesarova, J. Accumulation of PHA granules in Cupriavidus necator as seen by confocal fluorescence microscopy. FEMS Microbiol. Lett. 2016, 363, fnw094. [Google Scholar] [CrossRef] [PubMed]
- Redzwan, G.; Gan, S.; Tan, I.K.P. Short Communication: Isolation of polyhydroxyalkanoate-producing bacteria from an integrated-farming pond and palm-oil mill ef ¯ uent ponds. World J. Microbiol. Biotechnol. 1997, 13, 707–709. [Google Scholar] [CrossRef]
- Tyo, K.E.; Zhou, H.; Stephanopoulos, G.N. High-throughput screen for poly-3-hydroxybutyrate in Escherichia coli and Synechocystis sp. strain PCC6803. Appl. Environ. Microbiol. 2006, 72, 3412–3417. [Google Scholar] [CrossRef]
- Chaudhry, W.N.; Jamil, N.; Ali, I.; Ayaz, M.H.; Hasnain, S. Screening for polyhydroxyalkanoate (PHA)-producing bacterial strains and comparison of PHA production from various inexpensive carbon sources. Ann. Microbiol. 2011, 61, 623–629. [Google Scholar] [CrossRef]
- Motamedi, H.; Ardakani, M.R.; Mayeli, N. Isolation and screening of native polyhydroxyalkanoate producing bacteria from oil contaminated soils of Abadan refinery. Biol. J. Microorg. 2015, 3, 93–104. [Google Scholar]
- Tan, W.A.; Wijaya, I.; Purwadaria, T. Bioprospecting of polyhydroxyalkanoates-producing bacteria from Indonesian marine environment. Biodiversitas 2019, 20, 1309–1315. [Google Scholar] [CrossRef]
- Cammarota, M.C.; Freire, D.M.G. A review on hydrolytic enzymes in the treatment of wastewater with high oil and grease content. Bioresour. Technol. 2006, 97, 2195–2210. [Google Scholar] [CrossRef] [PubMed]
- Ghazali, F.M.; Rahman, R.N.Z.A.; Salleh, A.B.; Basri, M. Biodegradation of hydrocarbons in soil by microbial consortium. Int. Biodeterior. Biodegrad. 2004, 54, 61–67. [Google Scholar] [CrossRef]
- Chandra, S.; Sharma, R.; Singh, K.; Sharma, A. Application of bioremediation technology in the environment contaminated with petroleum hydrocarbon. Ann. Microbiol. 2013, 63, 417–431. [Google Scholar] [CrossRef]
- Ashby, R.D.; Solaiman, D.K.Y.; Foglia, T.A. Bacterial poly(hydroxyalkanoate) polymer production from the biodiesel co-product stream. J. Polym. Environ. 2004, 12, 105–112. [Google Scholar] [CrossRef]
- Koller, M. A review on established and emerging fermentation schemes for microbial production of polyhydroxyalkanoate (PHA) biopolyesters. Fermentation 2018, 4, 30. [Google Scholar] [CrossRef]
- Koller, M.; Bona, R.; Braunegg, G.; Hermann, C.; Horvat, P.; Kroutil, M.; Martinz, J.; Neto, J.; Pereira, L.; Varila, P. Production of polyhydroxyalkanoates from agricultural waste and surplus materials. Biomacromolecules 2005, 6, 561–565. [Google Scholar] [CrossRef]
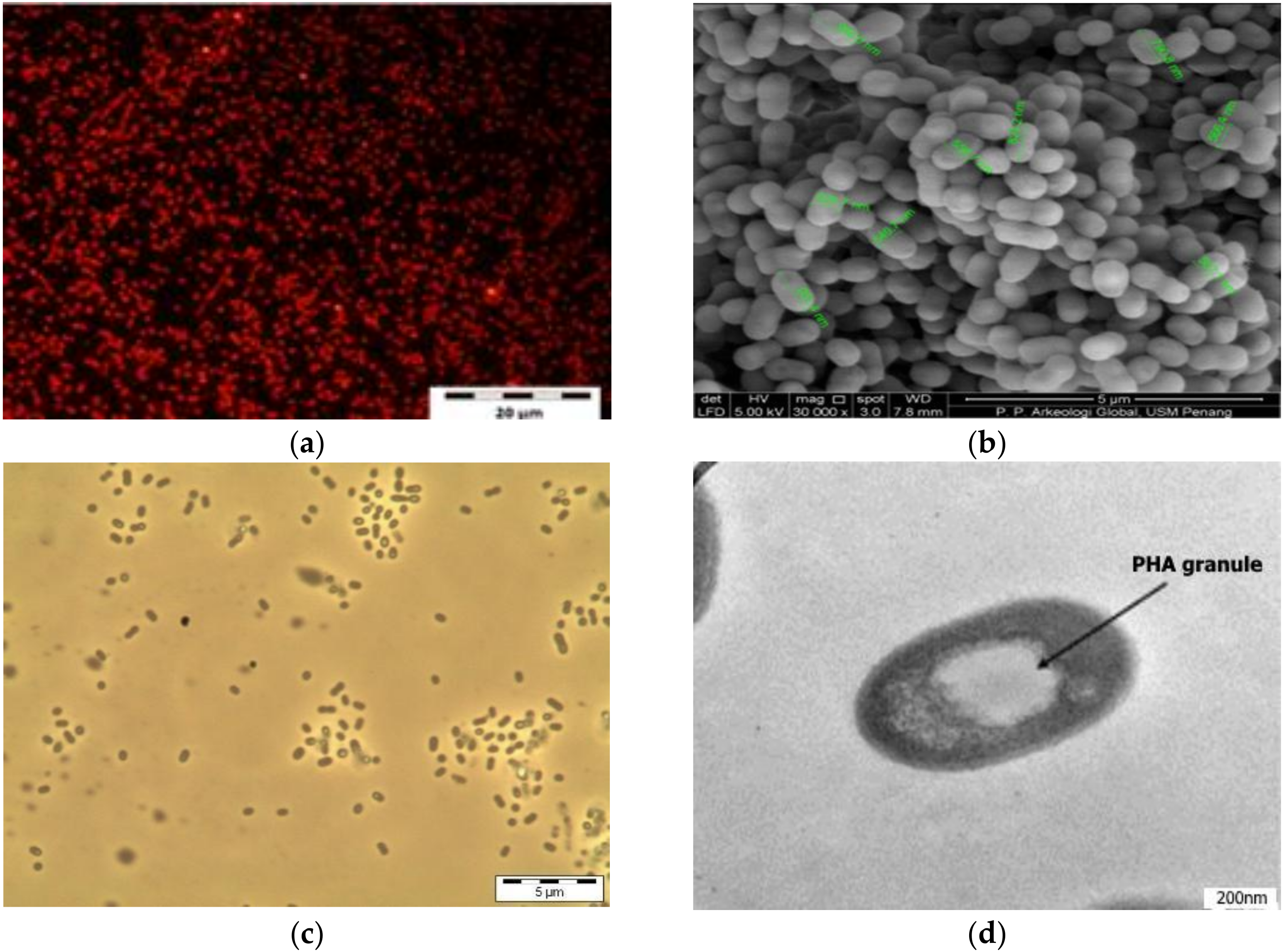
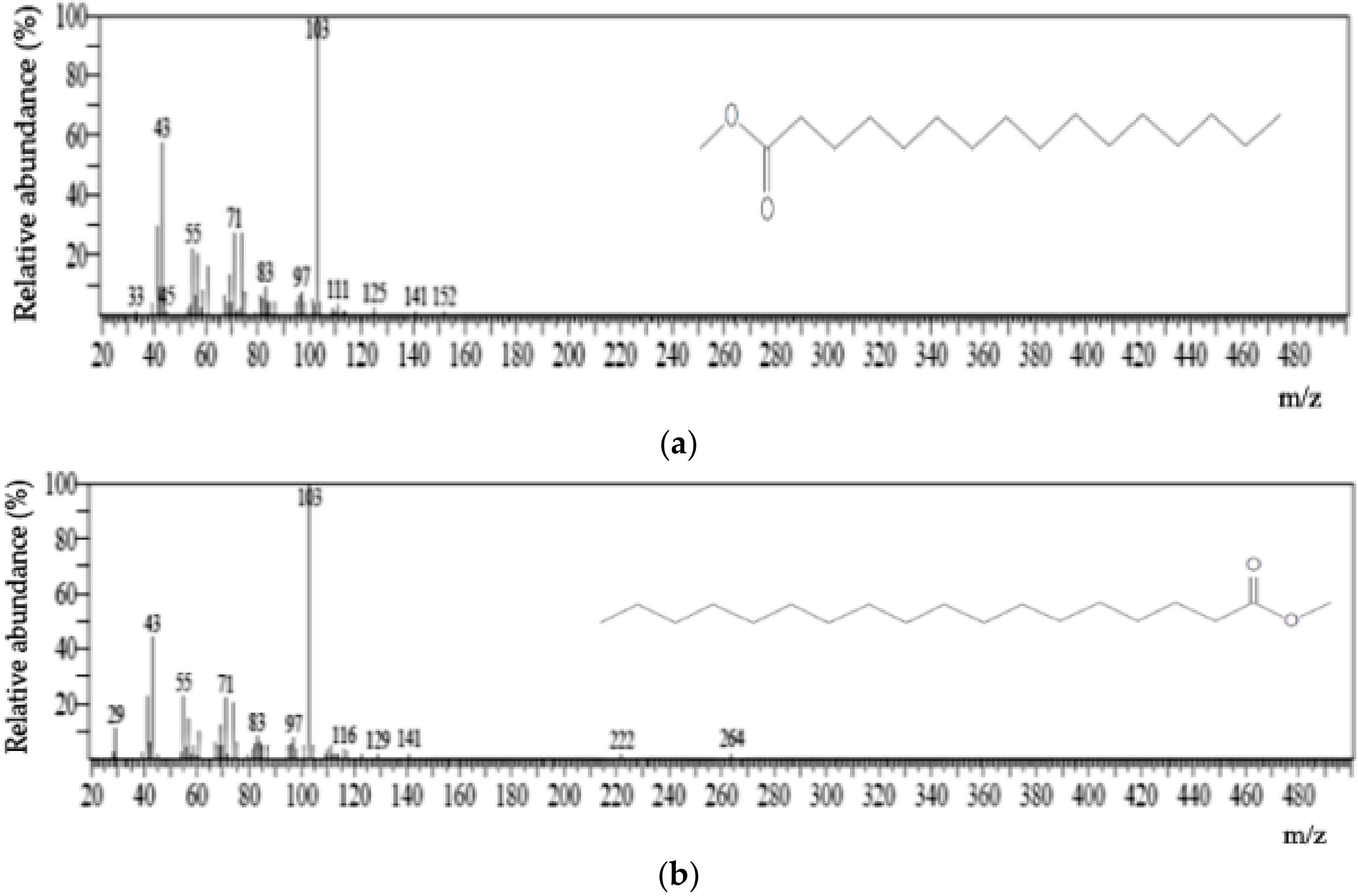

| Sample Code | Sample Type | Sampling Site | GPS | Number of MDC | |
|---|---|---|---|---|---|
| Lat. | Longt. | ||||
| PBA | Wastewater | Pulau Burung | 5°11′53″ | 100°25′40″ | 5 |
| PBB | Wastewater | Pulau Burung | 5°11′31″ | 100°25′88″ | 3 |
| SCA | Wastewater | Sungai Chenaam | 5°08′31″ | 100°24′12″ | 6 |
| SCC | Sediment | Sungai Chenaam | 5°08′31″ | 100°24′12″ | 3 |
| SPA | Wastewater | Sungai Pinang | 5°08′58″ | 100°24′38″ | 8 |
| SPD | Soil | Sungai Pinang | 5°08′57″ | 100°24′38″ | 5 |
| SJA | Wastewater | Sungai Jelutong | 5°24′24″ | 100°18′50″ | 8 |
| SJB | Wastewater | Sungai Jelutong | 5°24′24″ | 100°18′50″ | 5 |
| BFA | Water | Batu Ferringi Beach | 5°24′45″ | 100°20′23″ | 2 |
| BFC | Sediment | Batu Ferringi Beach | 5°24′45″ | 100°20′23″ | 6 |
| PPB | Water | Penang Bridge | 5°21′28″ | 100°18′58″ | 3 |
| PPD | Soil | Penang Bridge | 5°21′28″ | 100°18′58″ | 5 |
| UTO | Used Oil | TNBR Malaysia | NA | NA | 3 |
| 62 | |||||
| Sample Code | Sample Type | Number of Isolates Screened | Number of Positive Isolates |
|---|---|---|---|
| PBA | Wastewater | 5 | 1 |
| PBB | Wastewater | 3 | 1 |
| SCA | Wastewater | 6 | 2 |
| SCC | Sediment | 3 | 1 |
| SPA | Wastewater | 8 | 0 |
| SPD | Soil | 5 | 3 |
| SJA | Wastewater | 8 | 0 |
| SJB | Wastewater | 5 | 4 |
| BFA | Water | 2 | 0 |
| BFC | Sediment | 6 | 2 |
| PPB | Water | 3 | 1 |
| PPD | Soil | 5 | 1 |
| UTO | Used Oil | 3 | 0 |
| 62 | 16 (26%) |
| Isolate Code | Oil Degradation (%) | PHA Content (%) | CDW(g/L) |
|---|---|---|---|
| BFC1 | 23.78 ± 0.46 | 33.06 ± 0.19 | 2.22 ± 0.29 |
| BFC4 | 33.21 ± 1.96 | 17.62 ± 0.24 | 3.49 ± 0.98 |
| PBA4 | 19.58 ± 1.73 | 13.75 ± 0.15 | 2.27 ± 0.24 |
| PBB2 | 53.65 ± 0.29 | 8.15 ± 0.07 | 1.41 ± 1.10 |
| PPD5 | 52.92 ± 2.35 | 16.39 ± 0.42 | 2.64 ± 0.13 |
| SCA1 | 32.15 ± 0.29 | 21.33 ± 0.19 | 0.26 ± 2.25 |
| SCA6 | 35.12 ± 0.29 | 6.95 ± 0.07 | 3.22 ± 0.71 |
| SCC1 | 53.46 ± 0.29 | 19.77 ± 0.17 | 2.29 ± 0.22 |
| SJA7 | 54.29 ± 0.29 | 14.42 ± 0.32 | 4.82 ± 2.31 |
| SJB1 | 48.08 ± 0.29 | 18.41 ± 0.62 | 0.31 ± 2.20 |
| SJB2 | 57.51 ± 2.06 | 12.31 ± 0.14 | 3.91 ± 1.40 |
| SJB3 | 30.05 ± 1.75 | 11.13 ± 0.13 | 1.70 ± 0.81 |
| SB5 | 46.96 ± 1.62 | 20.88 ± 0.06 | 3.91 ± 1.40 |
| SPD2 | 45.42 ± 1.09 | 31.12 ± 0.12 | 4.19 ± 1.68 |
| SPD3 | 27.12 ± 1.14 | 21.51 ± 0.17 | 1.12 ± 1.39 |
| SPD4 | 28.58 ± 1.20 | 12.32 ± 0.07 | 2.37 ± 0.14 |
| IsolateCode | Top Hit of the NCBI BLAST Search | Similarity (%) | Organism Identified | GenBank Accession Number |
|---|---|---|---|---|
| BFC1 | Acinetobacter sp. Strain Gamma-15 | 99.45 | Acinetobacter sp. | MZ411707 |
| BFC4 | Acinetobacter sp. Strain WWW203 | 99.01 | Acinetobacter sp. | MZ411708 |
| PBA4 | Bacterium Stain Glm4 | 99.79 | Acinetobacter sp. | MZ411699 |
| PBB2 | Serratia marcescens Strain KS10 | 99.79 | Serratia marcescens | MZ411696 |
| PPD5 | Acinetobacter sp. HS-B1 | 99.51 | Acinetobacter sp. | MZ411697 |
| SCA1 | Bacterium Stain CH-12 | 99.38 | Acinetobacter sp. | MZ411704 |
| SCA6 | Proteus Vulgaris | 81.74 | Proteus sp. | MZ411698 |
| SCC1 | Acinetobacter sp. Strain PrPc065 | 99.86 | Acinetobacter sp. | MZ411695 |
| SJA7 | Uncultured Bacterium Clone SH201207-62 | 99.65 | Acinetobacter sp. | MZ411705 |
| SJB1 | Serratia marcescens Strain RS | 99.93 | Serratia marcescens | MZ411693 |
| SJB2 | Bacterium Stain N13.7 | 98.03 | Acinetobacter sp. | MZ411694 |
| SJB3 | Serratia marcescens Strain KS10 | 99.51 | Serratia marcescens | MZ411702 |
| SJB5 | Acinetobacter bereziniae Strain XH901 | 99.72 | Acinetobacter sp. | MZ411703 |
| SPD2 | Acinetobacter sp. Strain PrPc065 | 99.44 | Acinetobacter sp. | MZ411700 |
| SPD3 | Uncultured Gamma Proteobacterium Clone FTL260 | 99.72 | Serratia sp. | MZ411706 |
| SPD4 | Bacillus aryabhattai Strain WH6 | 95.78 | Bacillus sp. | MZ411701 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Idris, S.; Abdul Rahim, R.; Abdullah Amirul, A.-A. Bioprospecting and Molecular Identification of Used Transformer Oil-Degrading Bacteria for Bioplastics Production. Microorganisms 2022, 10, 583. https://doi.org/10.3390/microorganisms10030583
Idris S, Abdul Rahim R, Abdullah Amirul A-A. Bioprospecting and Molecular Identification of Used Transformer Oil-Degrading Bacteria for Bioplastics Production. Microorganisms. 2022; 10(3):583. https://doi.org/10.3390/microorganisms10030583
Chicago/Turabian StyleIdris, Shehu, Rashidah Abdul Rahim, and Al-Ashraf Abdullah Amirul. 2022. "Bioprospecting and Molecular Identification of Used Transformer Oil-Degrading Bacteria for Bioplastics Production" Microorganisms 10, no. 3: 583. https://doi.org/10.3390/microorganisms10030583
APA StyleIdris, S., Abdul Rahim, R., & Abdullah Amirul, A.-A. (2022). Bioprospecting and Molecular Identification of Used Transformer Oil-Degrading Bacteria for Bioplastics Production. Microorganisms, 10(3), 583. https://doi.org/10.3390/microorganisms10030583







