Methods to Evaluate Bacterial Motility and Its Role in Bacterial–Host Interactions
Abstract
1. Introduction
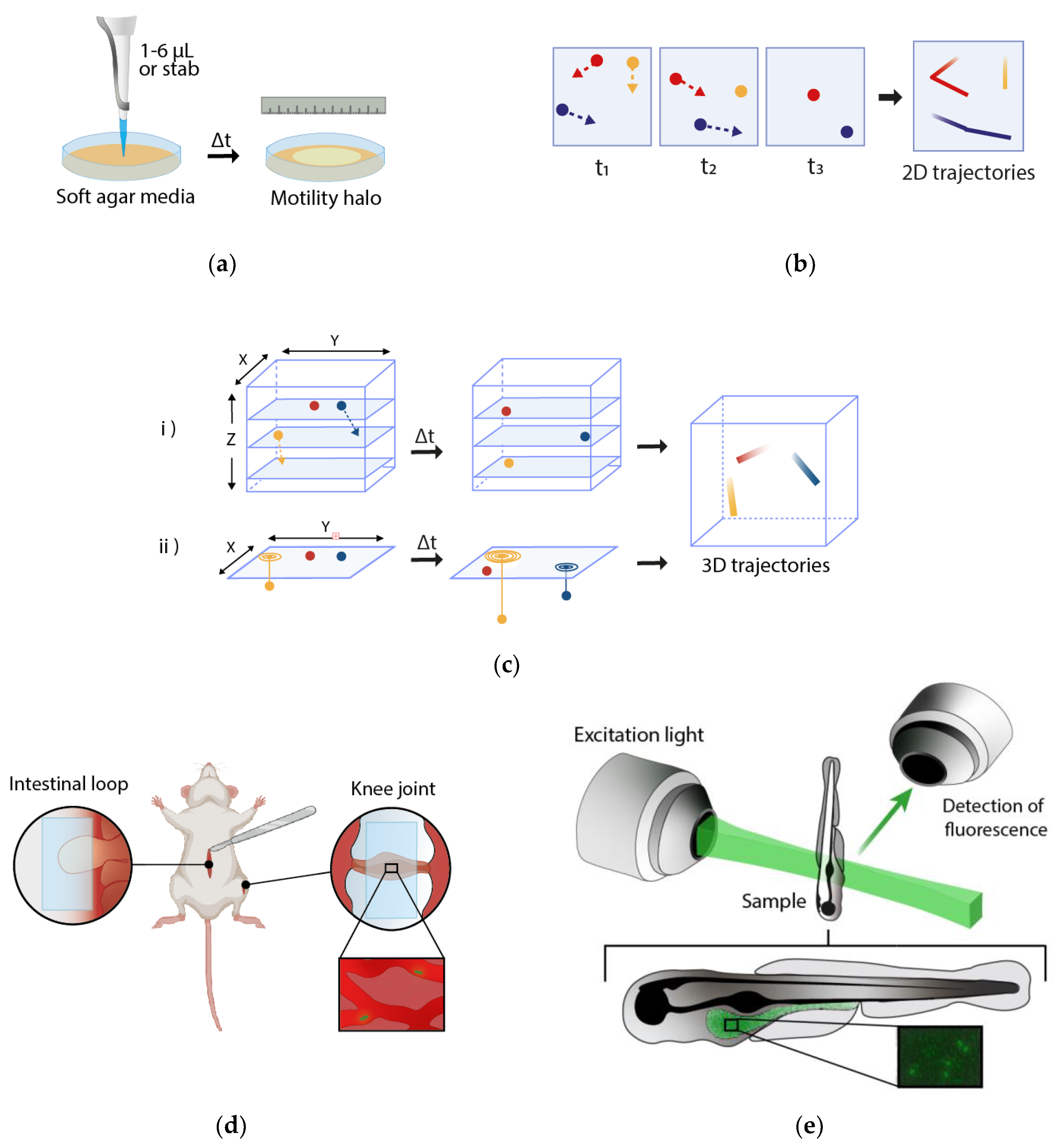
2. Macroscopic Techniques
3. Microscopic Techniques
4. Study of Bacterial Motility in Bacterial–Host Interactions
5. Discussion and Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Madigan, M.T.; Clark, D.P.; Stahl, D.; Martinko, J.M. Basic Principles of Microbiology. In Brock Biology of Microorganisms, 13th ed.; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2010; pp. 1–84. [Google Scholar]
- Josenhans, C.; Suerbaum, S. The Role of Motility as a Virulence Factor in Bacteria. Int. J. Med. Microbiol. 2002, 291, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Henrichsen, J. Bacterial Surface Translocation: A Survey and a Classification. Bacteriol. Rev. 1972, 36, 478–503. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, N.; Berg, H.C. Bacterial motility: Machinery and mechanisms. Nat. Rev. Microbiol. 2021, 1–13. [Google Scholar] [CrossRef]
- Kearns, D.B. A Field Guide to Bacterial Swarming Motility. Nat. Rev. Microbiol. 2010, 8, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Jarrell, K.F.; McBride, M.J. The Surprisingly Diverse Ways That Prokaryotes Move. Nat. Rev. Microbiol. 2008, 6, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Mattick, J.S. Type IV Pili and Twitching Motility. Annu. Rev. Microbiol. 2002, 56, 289–314. [Google Scholar] [CrossRef]
- Nan, B.; Zusman, D.R. Novel mechanisms power bacterial gliding motility. Mol. Microbiol. 2016, 101, 186–193. [Google Scholar] [CrossRef]
- Mattingly, A.E.; Weaver, A.A.; Dimkovikj, A.; Shrout, J.D. Assessing travel conditions: Environmental and host influences on bacterial surface motility. J. Bacteriol. 2018, 200, 1–17. [Google Scholar] [CrossRef]
- Shaevitz, J.W.; Lee, J.Y.; Fletcher, D.A. Spiroplasma swim by a processive change in body helicity. Cell 2005, 122, 941–945. [Google Scholar] [CrossRef]
- Wyman, M.; Gregory, R.P.; Carr, N.G. Novel Role for Phycoerythrin in a Marine Cyanobacterium, Synechococcus Strain DC2. Science 1985, 230, 818–820. [Google Scholar] [CrossRef]
- Ehlers, K.; Oster, G. On the mysterious propulsion of Synechococcus. PLoS ONE 2012, 7, e36081. [Google Scholar] [CrossRef] [PubMed]
- Wood, C.R.; Ohneck, E.J.; Edelmann, R.E.; Actis, L.A. A Light-Regulated Type I Pilus Contributes to Acinetobacter baumannii Biofilm, Motility, and Virulence Functions. Infect. Immun. 2018, 86, e00442-18. [Google Scholar] [CrossRef]
- Miyata, M.; Robinson, R.C.; Uyeda, T.Q.P.; Fukumori, Y.; Fukushima, S.; Haruta, S.; Homma, M.; Inaba, K.; Ito, M.; Kaito, C.; et al. Tree of Motility—A Proposed History of Motility Systems in the Tree of Life. Genes Cells 2020, 25, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Murray, T.S.; Kazmierczak, B.I. Pseudomonas aeruginosa Exhibits Sliding Motility in the Absence of Type IV Pili and Flagella. J. Bacteriol. 2008, 190, 2700–2708. [Google Scholar] [CrossRef] [PubMed]
- Pollitt, E.J.G.; Diggle, S.P. Defining Motility in the Staphylococci. Cell. Mol. Life Sci. 2017, 74, 2943–2958. [Google Scholar] [CrossRef]
- Zawiah, W.A.N.; Abdullah, W.A.N.; Mackey, B.M. High Phenotypic Variability among Representative Strains of Common Salmonella enterica Serovars with Possible Implications for Food Safety. J. Food Prot. 2018, 81, 93–104. [Google Scholar] [CrossRef]
- Macnab, R.M. Flagella and motility. In Escherichia coli and Salmonella: Cellular and Molecular Biology, 2nd ed.; Neidhardt, C., Curtiss, R., Ingraham, J.L., Lin, E.C.C., Low, K.B., Magasanik, B., Reznikoff, W.S., Riley, M., Schaechter, M., Umbarger, H.E., Eds.; ASM Press: Washington, DC, USA, 1996; pp. 123–145. [Google Scholar]
- Ottemann, K.M.; Miller, J.F. Roles for motility in bacterial–host interactions. Mol. Microbiol. 1997, 24, 1109–1117. [Google Scholar] [CrossRef]
- Lovely, P.S.; Dahlquist, F.W. Statistical Measures of Bacterial Motility. J. Theor. Biol. 1974, 50, 477–496. [Google Scholar] [CrossRef]
- Berg, H.C. Random Walks in Biology; Princeton University Press: Princeton, NJ, USA, 1983. [Google Scholar]
- Fisher, R.A. The wave of advance of advantageous genes. Ann. Eugen. 1937, 7, 355–369. [Google Scholar] [CrossRef]
- Kolmogorov, A.N.; Petrovsky, N.; Piscounov, N.S. A study of the equation of diffusion with increase in the quantity of matter, and its application to a biological problem. Mosc. Univ. Bull. Math. 1937, 1, 1–25. [Google Scholar]
- Gandhi, S.R.; Yurtsev, E.A.; Korolev, K.S.; Gore, J. Range Expansions Transition from Pulled to Pushed Waves as Growth Becomes More Cooperative in an Experimental Microbial Population. Proc. Natl. Acad. Sci. USA 2016, 113, 6922–6927. [Google Scholar] [CrossRef]
- Cremer, J.; Honda, T.; Tang, Y.; Wong-Ng, J.; Vergassola, M.; Hwa, T. Chemotaxis as a Navigation Strategy to Boost Range Expansion. Nature 2019, 575, 658–663. [Google Scholar] [CrossRef]
- Tittsler, R.P.; Sandholzer, L.A. The Use of Semi-Solid Agar for the Detection of Bacterial Motility. J. Bacteriol. 1936, 31, 575–580. [Google Scholar] [CrossRef]
- Adler, J. A method for measuring chemotaxis and use of the method to determine optimum conditions for chemotaxis by Escherichia coli. J. Gen. Microbiol. 1973, 74, 77–91. [Google Scholar] [CrossRef]
- Ditty, J.L.; Parales, R.E. Protocols for the Measurement of Bacterial Chemotaxis to Hydrocarbons. In Hydrocarbon and Lipid Microbiology Protocols; McGenity, T.J., Timmis, K.N., Nogales, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 7–42. [Google Scholar] [CrossRef]
- Robinson, C.D.; Klein, H.S.; Murphy, K.D.; Parthasarathy, R.; Guillemin, K.; Bohannan, B.J.M. Experimental Bacterial Adaptation to the Zebrafish Gut Reveals a Primary Role for Immigration. PLoS Biol. 2018, 16, e2006893. [Google Scholar] [CrossRef]
- Ball, R.J.; Sellers, W. Improved motility medium. Appl. Microbiol. 1966, 14, 670–673. [Google Scholar] [CrossRef]
- Morris, J.D.; Hewitt, J.L.; Wolfe, L.G.; Kamatkar, N.G.; Chapman, S.M.; Diener, J.M.; Courtney, A.J.; Leevy, W.M.; Shrout, J.D. Imaging and Analysis of Pseudomonas aeruginosa Swarming and Rhamnolipid Production. Appl. Environ. Microbiol. 2011, 77, 8310–8317. [Google Scholar] [CrossRef]
- Lee, W.Y.; Sanz, M.J.; Wong, C.H.; Hardy, P.O.; Salman-Dilgimen, A.; Moriarty, T.J.; Chaconas, G.; Marques, A.; Krawetz, R.; Mody, C.H.; et al. Invariant natural killer T cells act as an extravascular cytotoxic barrier for joint-invading Lyme Borrelia. Proc. Natl. Acad. Sci. USA 2014, 111, 13936–13941. [Google Scholar] [CrossRef]
- Millet, Y.A.; Alvarez, D.; Ringgaard, S.; von Andrian, U.H.; Davis, B.M.; Waldor, M.K. Insights into Vibrio cholerae Intestinal Colonization from Monitoring Fluorescently Labeled Bacteria. PLoS Pathog. 2014, 10, e1004405. [Google Scholar] [CrossRef]
- Partridge, J.D.; Harshey, R.M. Investigating Flagella-Driven Motility in Escherichia coli by Applying Three Established Techniques in a Series. J. Vis. Exp. 2020, 2020, 1–8. [Google Scholar] [CrossRef]
- Partridge, J.D.; Harshey, R.M. Swarming: Flexible Roaming Plans. J. Bacteriol. 2013, 195, 909–918. [Google Scholar] [CrossRef]
- Turnbull, L.; Whitchurch, C.B. Motility Assay: Twitching Motility. In Pseudomonas Methods and Protocols; Filloux, A., Ramos, J.-L., Eds.; Humana Press Inc.: New York, NY, USA, 2014; pp. 73–86. [Google Scholar] [CrossRef]
- Martínez, A.; Torello, S.; Kolter, R. Sliding Motility in Mycobacteria. J. Bacteriol. 1999, 181, 7331–7338. [Google Scholar] [CrossRef]
- Liu, Y.; Kyle, S.; Straight, P.D. Antibiotic Stimulation of a Bacillus subtilis Migratory Response. mSphere 2018, 3, e00586-17. [Google Scholar] [CrossRef]
- Tchoufag, J.; Ghosh, P.; Pogue, C.B.; Nan, B.; Mandadapu, K.K. Mechanisms for Bacterial Gliding Motility on Soft Substrates. Proc. Natl. Acad. Sci. USA 2019, 116, 25087–25096. [Google Scholar] [CrossRef]
- Tremblay, J.; Déziel, E. Improving the Reproducibility of Pseudomonas aeruginosa Swarming Motility Assays. J. Basic Microbiol. 2008, 48, 509–515. [Google Scholar] [CrossRef]
- Pottash, A.E.; McKay, R.; Virgile, C.R.; Ueda, H.; Bentley, W.E. TumbleScore: Run and Tumble Analysis for Low Frame-Rate Motility Videos. BioTechniques 2017, 62, 31–36. [Google Scholar] [CrossRef]
- Macnab, R.M. Examination of Bacterial Flagellation by Dark Field Microscopy. J. Clin. Microbiol. 1976, 4, 258–265. [Google Scholar] [CrossRef]
- Nakamura, S.; Islam, M.S. Motility of Spirochetes. Methods Mol. Biol. 2017, 1593, 243–251. [Google Scholar] [CrossRef]
- Cheong, F.C.; Wong, C.C.; Gao, Y.; Nai, M.H.; Cui, Y.; Park, S.; Kenney, L.J.; Lim, C.T. Rapid, High-Throughput Tracking of Bacterial Motility in 3D via Phase-Contrast Holographic Video Microscopy. Biophys. J. 2015, 108, 1248–1256. [Google Scholar] [CrossRef]
- Hook, A.L.; Flewellen, J.L.; Dubern, J.-F.; Carabelli, A.M.; Zaid, I.M.; Berry, R.M.; Wildman, R.D.; Russell, N.; Williams, P.; Alexander, M.R. Simultaneous Tracking of Pseudomonas aeruginosa Motility in Liquid and at the Solid-Liquid Interface Reveals Differential Roles for the Flagellar Stators. mSystems 2019, 4, e00390-19. [Google Scholar] [CrossRef]
- Smith, B.; Li, J.; Metruccio, M.; Wan, S.; Evans, D.; Fleiszig, S. Quantification of Bacterial Twitching Motility in Dense Colonies Using Transmitted Light Microscopy and Computational Image Analysis. Bio Protoc. 2018, 8, e2804. [Google Scholar] [CrossRef] [PubMed]
- Turner, L.; Ryu, W.S.; Berg, H.C. Real-Time Imaging of Fluorescent Flagellar Filaments. J. Bacteriol. 2000, 182, 2793–2801. [Google Scholar] [CrossRef] [PubMed]
- Wiles, T.J.; Wall, E.S.; Schlomann, B.H.; Hay, E.A.; Parthasarathy, R.; Guillemin, K. Modernized Tools for Streamlined Genetic Manipulation and Comparative Study of Wild and Diverse Proteobacterial Lineages. mBio 2018, 9, 1–19. [Google Scholar] [CrossRef]
- Toepfer, J.A.; Ford, R.M.; Metge, D.; Harvey, R.W. Impact of Fluorochrome Stains Used to Study Bacterial Transport in Shallow Aquifers on Motility and Chemotaxis of Pseudomonas Species. FEMS Microbiol. Ecol. 2012, 81, 163–171. [Google Scholar] [CrossRef][Green Version]
- Heo, M.; Nord, A.L.; Chamousset, D.; van Rijn, E.; Beaumont, H.J.E.; Pedaci, F. Impact of Fluorescent Protein Fusions on the Bacterial Flagellar Motor. Sci. Rep. 2017, 7, 12583. [Google Scholar] [CrossRef]
- Taute, K.M.; Gude, S.; Tans, S.J.; Shimizu, T.S. High-Throughput 3D Tracking of Bacteria on a Standard Phase Contrast Microscope. Nat. Commun. 2015, 6, 8776. [Google Scholar] [CrossRef]
- Berg, H.; Brown, D. Chemotaxis in Escherichia coli analysed by Three-dimensional Tracking. Nature 1972, 239, 500–504. [Google Scholar] [CrossRef]
- Speidel, M.; Jonáš, A.; Florin, E.-L. Three-Dimensional Tracking of Fluorescent Nanoparticles with Subnanometer Precision by Use of off-Focus Imaging. Opt. Lett. 2003, 28, 69. [Google Scholar] [CrossRef]
- Wu, M.; Roberts, J.W.; Kim, S.; Koch, D.L.; Delisa, M.P. Collective Bacterial Dynamics Revealed Using a Three-Dimensional Population-Scale Defocused Particle Tracking Technique. Appl. Environ. Microbiol. 2006, 72, 4987–4994. [Google Scholar] [CrossRef]
- Deforet, M.; Van Ditmarsch, D.; Carmona-Fontaine, C.; Xavier, J.B. Hyperswarming Adaptations in a Bacterium Improve Collective Motility without Enhancing Single Cell Motility. Soft Matter 2014, 10, 2405–2413. [Google Scholar] [CrossRef]
- Kim, M.K. Principles and Techniques of Digital Holographic Microscopy. SPIE Rev. 2010, 1, 018005. [Google Scholar] [CrossRef]
- Molaei, M.; Sheng, J. Imaging Bacterial 3D Motion Using Digital In-Line Holographic Microscopy and Correlation-Based de-Noising Algorithm. Opt. Express 2014, 22, 32119. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Garmann, R.F.; Manoharan, V.N. Tracking E. coli Runs and Tumbles with Scattering Solutions and Digital Holographic Microscopy. Opt. Express 2016, 24, 23719. [Google Scholar] [CrossRef] [PubMed]
- Acres, J.; Nadeau, J. 2D vs. 3D tracking in bacterial motility analysis. AIMS Biophys. 2021, 8, 385–399. [Google Scholar] [CrossRef]
- Levoy, M.; Zhang, Z.; McDowall, I. Recording and Controlling the 4D Light Field in a Microscope Using Microlens Arrays. J. Microsc. 2009, 235, 144–162. [Google Scholar] [CrossRef]
- Truong, T.; Holland, D.B.; Madaan, S.; Andreev, A.; Keomanee-Dizon, K.; Troll, J.; Koo, D.E.S.; McFall-Ngai, M.J.; Fraser, S.E. High-Contrast, Synchronous Volumetric Imaging with Selective Volume Illumination Microscopy. Commun. Biol. 2020, 3, 74. [Google Scholar] [CrossRef]
- Wilson, L.G.; Martinez, V.A.; Tailleur, J.; Bryant, G.; Pusey, P.N.; Poon, W.C.K. Differential Dynamic Microscopy of Bacterial Motility. Phys. Rev. Lett. 2011, 106, 018101. [Google Scholar] [CrossRef]
- Martinez, V.A.; Besseling, R.; Croze, O.A.; Tailleur, J.; Reufer, M.; Schwarz-Linek, J.; Wilson, L.G.; Bees, M.A.; Poon, W.C.K. Differential Dynamic Microscopy: A High-Throughput Method for Characterizing the Motility of Microorganisms. Biophys. J. 2012, 103, 1637–1647. [Google Scholar] [CrossRef]
- Rosko, J.; Martinez, V.A.; Poon, W.C.K.; Pilizota, T. Osmotaxis in Escherichia coli through Changes in Motor Speed. Proc. Natl. Acad. Sci. USA 2017, 114, E7969–E7976. [Google Scholar] [CrossRef]
- Frangipane, G.; Dell’arciprete, D.; Petracchini, S.; Maggi, C.; Saglimbeni, F.; Bianchi, S.; Vizsnyiczai, G.; Bernardini, M.L.; Di Leonardo, R. Dynamic density shaping of photokinetic E. coli. eLife 2018, 7, e36608. [Google Scholar] [CrossRef]
- Liang, X.; Lu, N.; Chang, L.; Nguyen, T.H.; Massoudieh, A. Evaluation of Bacterial Run and Tumble Motility Parameters through Trajectory Analysis. J. Contam. Hydrol. 2018, 211, 26–38. [Google Scholar] [CrossRef]
- Vissers, T.; Koumakis, N.; Hermes, M.; Brown, A.T.; Schwarz-Linek, J.; Dawson, A.; Poon, W. Dynamical analysis of bacteria in microscopy movies. PLoS ONE 2019, 14, e0217823. [Google Scholar] [CrossRef]
- Ellison, C.K.; Dalia, T.N.; Dalia, A.B.; Brun, Y.V. Real-time microscopy and physical perturbation of bacterial pili using maleimide-conjugated molecules. Nat. Protoc. 2019, 14, 1803–1819. [Google Scholar] [CrossRef]
- Qin, Z.; Zhang, P. Studying bacterial chemosensory array with CryoEM. Biochem. Soc. Trans. 2021, 49, 2081–2089. [Google Scholar] [CrossRef]
- Kühn, M.J.; Schmidt, F.K.; Eckhardt, B.; Thormann, K.M. Bacteria exploit a polymorphic instability of the flagellar filament to escape from traps. Proc. Natl. Acad. Sci. USA 2017, 114, 6340–6345. [Google Scholar] [CrossRef]
- Talà, L.; Fineberg, A.; Kukura, P.; Persat, A. Pseudomonas aeruginosa orchestrates twitching motility by sequential control of type IV pili movements. Nat. Microbiol. 2019, 4, 774–780. [Google Scholar] [CrossRef]
- Sun, E.; Liu, S.; Hancock, R.E.W. Surfing Motility: A Conserved yet Diverse Adaptation among Motile Bacteria. J. Bacteriol. 2018, 200, e00394-18. [Google Scholar] [CrossRef]
- Yeung, A.T.Y.; Parayno, A.; Hancock, R.E.W. Mucin Promotes Rapid Surface Motility in Pseudomonas aeruginosa. mBio 2012, 3, e00073-12. [Google Scholar] [CrossRef]
- Meng, Y.; Li, Y.; Galvani, C.D.; Hao, G.; Turner, J.N.; Burr, T.J.; Hoch, H.C. Upstream Migration of Xylella fastidiosa via Pilus-Driven Twitching Motility. J. Bacteriol. 2005, 187, 5560–5567. [Google Scholar] [CrossRef]
- Courson, D.S.; Pokhrel, A.; Scott, C.; Madrill, M.; Rinehold, A.J.; Tamayo, R.; Cheney, R.E.; Purcell, E.B. Single Cell Analysis of Nutrient Regulation of Clostridioides (Clostridium) difficile Motility. Anaerobe 2019, 59, 205–211. [Google Scholar] [CrossRef]
- Mills, D.C.; Gundogdu, O.; Elmi, A.; Bajaj-elliott, M.; Taylor, P.W.; Wren, B.W.; Dorrell, N. Increase in Campylobacter jejuni Invasion of Intestinal Epithelial Cells under Low-Oxygen Coculture Conditions That Reflect the In Vivo Environment. Infect. Immun. 2012, 80, 1690–1698. [Google Scholar] [CrossRef]
- Szymanski, C.M.; King, M.; Haardt, M.; Armstrong, G.D. Campylobacter jejuni Motility and Invasion of Caco-2 Cells. Infect. Immun. 1995, 63, 4295–4300. [Google Scholar] [CrossRef]
- Higashi, D.L.; Lee, S.W.; Snyder, A.; Weyand, N.J.; Bakke, A.; So, M. Dynamics of Neisseria gonorrhoeae Attachment: Microcolony Development, Cortical Plaque Formation, and Cytoprotection. Infect. Immun. 2007, 75, 4743–4753. [Google Scholar] [CrossRef]
- Toley, B.J.; Forbes, N.S. Motility Is Critical for Effective Distribution and Accumulation of Bacteria in Tumor Tissue. Integr. Biol. 2012, 4, 165–176. [Google Scholar] [CrossRef]
- Jubelin, G.; Desvaux, M.; Schüller, S.; Etienne-Mesmin, L.; Muniesa, M.; Blanquet-Diot, S. Modulation of Enterohaemorrhagic Escherichia coli Survival and Virulence in the Human Gastrointestinal Tract. Microorganisms 2018, 6, 115. [Google Scholar] [CrossRef]
- Kortman, G.A.M.; Dutilh, B.E.; Maathuis, A.J.H.; Engelke, U.F.; Boekhorst, J.; Keegan, K.P.; Nielsen, F.G.G.; Betley, J.; Weir, J.C.; Kingsbury, Z.; et al. Microbial Metabolism Shifts towards an Adverse Profile with Supplementary Iron in the TIM-2 in Vitro Model of the Human Colon. Front. Microbiol. 2016, 6, 1481. [Google Scholar] [CrossRef]
- Guentzel, M.N.; Field, L.H.; Eubanks, E.R.; Berry, L.J. Use of Fluorescent Antibody in Studies of Immunity to Cholera in Infant Mice. Infect. Immun. 1977, 15, 539–548. [Google Scholar] [CrossRef]
- Masedunskas, A.; Milberg, O.; Porat-Shliom, N.; Sramkova, M.; Wigand, T.; Amornphimoltham, P.; Weigert, R. Intravital microscopy: A practical guide on imaging intracellular structures in live animals. Bioarchitecture 2012, 2, 143–157. [Google Scholar] [CrossRef]
- Moriarty, T.J.; Norman, M.U.; Colarusso, P.; Bankhead, T.; Kubes, P.; Chaconas, G. Real-Time High Resolution 3D Imaging of the Lyme Disease Spirochete Adhering to and Escaping from the Vasculature of a Living Host. PLoS Pathog. 2008, 4, 17–19. [Google Scholar] [CrossRef]
- Schuh, C.D.; Haenni, D.; Craigie, E.; Ziegler, U.; Weber, B.; Devuyst, O.; Hall, A.M. Long Wavelength Multiphoton Excitation Is Advantageous for Intravital Kidney Imaging. Kidney Int. 2016, 89, 712–719. [Google Scholar] [CrossRef]
- Belperron, A.A.; Mao, J.; Bockenstedt, L.K. Two Photon Intravital Microscopy of Lyme Borrelia in Mice. In Borrelia burgdorferi. Methods in Molecular Biology; Pal, U., Buyuktanir, O., Eds.; Humana Press: New York, NY, USA, 2018; Volume 1690, pp. 279–290. [Google Scholar] [CrossRef]
- López Nadal, A.; Ikeda-Ohtsubo, W.; Sipkema, D.; Peggs, D.; McGurk, C.; Forlenza, M.; Wiegertjes, G.F.; Brugman, S. Feed, Microbiota, and Gut Immunity: Using the Zebrafish Model to Understand Fish Health. Front. Immunol. 2020, 11, 114. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The Zebrafish Reference Genome Sequence and Its Relationship to the Human Genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef]
- Sunyer, J.O. Fishing for Mammalian Paradigms in the Teleost Immune System. Nat. Immunol. 2013, 14, 320–326. [Google Scholar] [CrossRef]
- Van der Sar, A.M.; Musters, R.J.P.; van Eeden, F.J.M.; Appelmelk, B.J.; Vandenbroucke-Grauls, C.M.; Bitter, W. Zebrafish Embryos as a Model Host for the Real Time Analysis of Salmonella typhimurium Infections. Cell. Microbiol. 2003, 5, 601–611. [Google Scholar] [CrossRef]
- O’Toole, R.; von Hofsten, J.; Rosqvist, R.; Olsson, P.E.; Wolf-Watz, H. Visualisation of Zebrafish Infection by GFP-Labelled Vibrio anguillarum. Microb. Pathog. 2004, 37, 41–46. [Google Scholar] [CrossRef]
- Rawls, J.F.; Mahowald, M.A.; Goodman, A.L.; Trent, C.M.; Gordon, J.I. In Vivo Imaging and Genetic Analysis Link Bacterial Motility and Symbiosis in the Zebrafish Gut. Proc. Natl. Acad. Sci. USA 2007, 104, 7622–7627. [Google Scholar] [CrossRef]
- Caruffo, M.; Navarrete, N.; Salgado, O.; Díaz, A.; López, P.; García, K.; Feijóo, C.G.; Navarrete, P. Potential Probiotic Yeasts Isolated from the Fish Gut Protect Zebrafish (Danio rerio) from a Vibrio anguillarum Challenge. Front. Microbiol. 2015, 6, 1093. [Google Scholar] [CrossRef]
- Jemielita, M.; Taormina, M.J.; Burns, A.R.; Hampton, J.S.; Rolig, A.S.; Guillemin, K.; Parthasarathy, R. Spatial and Temporal Features of the Growth of a Bacterial Species Colonizing the Zebrafish Gut. mBio 2014, 5, 1–8. [Google Scholar] [CrossRef]
- Wiles, T.J.; Jemielita, M.; Baker, R.P.; Schlomann, B.H.; Logan, S.L.; Ganz, J.; Melancon, E.; Eisen, J.S.; Guillemin, K.; Parthasarathy, R. Host Gut Motility Promotes Competitive Exclusion within a Model Intestinal Microbiota. PLoS Biol. 2016, 14, e1002517. [Google Scholar] [CrossRef]
- Parthasarathy, R. Monitoring Microbial Communities Using Light Sheet Fluorescence Microscopy. Curr. Opin. Microbiol. 2018, 43, 31–37. [Google Scholar] [CrossRef]
- Schlomann, B.H.; Wiles, T.J.; Wall, E.S.; Guillemin, K.; Parthasarathy, R. Bacterial Cohesion Predicts Spatial Distribution in the Larval Zebrafish Intestine. Biophys. J. 2018, 115, 2271–2277. [Google Scholar] [CrossRef]
- Wiles, T.J.; Schlomann, B.H.; Wall, E.S.; Betancourt, R.; Parthasarathy, R.; Guillemin, K. Swimming Motility of a Gut Bacterial Symbiont Promotes Resistance to Intestinal Expulsion and Enhances Inflammation. PLoS Biol. 2020, 18, e3000661. [Google Scholar] [CrossRef]
- Taormina, M.J.; Jemielita, M.; Stephens, W.Z. Investigating Bacterial-Animal Symbioses with Light Sheet Microscopy. Biol. Bull. 2012, 223, 7–20. [Google Scholar] [CrossRef]
- Schlomann, B.H.; Wiles, T.J.; Wall, E.S.; Guillemin, K.; Parthasarathy, R. Sublethal Antibiotics Collapse Gut Bacterial Populations by Enhancing Aggregation and Expulsion. Proc. Natl. Acad. Sci. USA 2019, 116, 21392–21400. [Google Scholar] [CrossRef]
- House, B.; Kus, J.; Prayitno, N.; Mair, R.; Que, L.; Chingcuanco, F.; Gannon, V.; Cvitkovitch, D.G.; Foster, D.B. Acid-Stress-Induced Changes in Enterohaemorrhagic Escherichia Coli O157: H7 Virulence. Microbiology 2009, 155, 2907–2918. [Google Scholar] [CrossRef]
- Peano, C.; Chiaramonte, F.; Motta, S.; Pietrelli, A.; Jaillon, S.; Rossi, E.; Consolandi, C.; Champion, O.L.; Michell, S.L.; Freddi, L.; et al. Gene and Protein Expression in Response to Different Growth Temperatures and Oxygen Availability in Burkholderia thailandensis. PLoS ONE 2014, 9, e93009. [Google Scholar] [CrossRef]
- Berndt, A.; Müller, J.; Borsi, L.; Kosmehl, H.; Methner, U.; Berndt, A. Reorganisation of the Caecal Extracellular Matrix upon Salmonella Infection-Relation between Bacterial Invasiveness and Expression of Virulence Genes. Vet. Microbiol. 2009, 133, 123–137. [Google Scholar] [CrossRef]
- Tolman, J.S.; Valvano, M.A. Global Changes in Gene Expression by the Opportunistic Pathogen Burkholderia cenocepacia in Response to Internalization by Murine Macrophages. BMC Genom. 2012, 13, 63. [Google Scholar] [CrossRef]
- Jiang, L.; Ni, Z.; Wang, L.; Feng, L.; Liu, B. Loss of the lac operon contributes to Salmonella invasion of epithelial cells through derepression of flagellar synthesis. Curr. Microbiol. 2015, 70, 315–323. [Google Scholar] [CrossRef]
- Snyder, J.A.; Haugen, B.J.; Buckles, E.L.; Lockatell, C.V.; Johnson, D.E.; Donnenberg, M.S.; Welch, R.A.; Mobley, H.L.T. Transcriptome of Uropathogenic Escherichia coli during Urinary Tract Infection. Infect. Immun. 2004, 72, 6373–6381. [Google Scholar] [CrossRef]
- Lane, M.C.; Alteri, C.J.; Smith, S.N.; Mobley, H.L.T. Expression of Flagella Is Coincident with Uropathogenic Escherichia coli Ascension to the Upper Urinary Tract. Proc. Natl. Acad. Sci. USA 2007, 104, 16669–16674. [Google Scholar] [CrossRef]
- Tang, Y.; Xin, G.; Zhao, L.M.; Huang, L.X.; Qin, Y.X.; Su, Y.Q.; Zheng, W.Q.; Wu, B.; Lin, N.; Yan, Q.P. Novel Insights into Host-Pathogen Interactions of Large Yellow Croakers (Larimichthys crocea) and Pathogenic Bacterium Pseudomonas plecoglossicida Using Time-Resolved Dual RNA-Seq of Infected Spleens. Zool. Res. 2020, 41, 314–327. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, L.; Zhang, W. Tools for Genomic and Transcriptomic Analysis of Microbes at Single-Cell Level. Front. Microbiol. 2017, 8, 1831. [Google Scholar] [CrossRef]
- Kuchina, A.; Brettner, L.M.; Paleologu, L.; Roco, C.M.; Rosenberg, A.B.; Carignano, A.; Kibler, R.; Hirano, M.; DePaolo, R.W.; Seelig, G. Microbial single-cell RNA sequencing by split-pool barcoding. Science 2021, 371, eaba5257. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, M.; Yu, K.; Zeng, X.; Liu, X. Mass spectrometry-based proteomic approaches to study pathogenic bacteria-host interactions. Protein Cell 2015, 6, 265–274. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Y.; Fu, J.; Zhang, B.; Cheng, S.; Wu, M.; Wang, Z.; Jiang, J.; Chang, C.; Liu, X. Salmonella Proteomic Profiling during Infection Distinguishes the Intracellular Environment of Host Cells. mSystems 2019, 4, e00314-18. [Google Scholar] [CrossRef]
| Macroscopic Assay | Applications | References |
|---|---|---|
| Soft-agar tubes | Easily identification of motile and non-motile bacteria | [26] |
| Soft-agar plates | Quantification of motility level, and identification of a motility type (Table 2) or patterns at a population level | [5,9,26] |
| Using low concentrations of a metabolizable chemoattractant | Assessing chemotactic motility | [27,28] |
| Using fluorescent labelling | Identification of more than two bacteria in co-swarming experiments, increasing contrast with the media, and studying of motility-related compounds | [31] |
| Motility Type | Agar Concentration | References |
|---|---|---|
| Swimming | ̴ 0.3% | [34] |
| Swarming (temperate) | 0.5–0.8% | [35] |
| Swarming (robust) | >1.5% | [35] |
| Twitching 1 | 1% | [36] |
| Sliding | 0.3–0.4%, or 1–2% has also been used | [37,38] |
| Gliding | ≤7% in Myxococcus xanthus | [39] |
| Microscopic Techniques | Advantages | Disadvantages | Applications |
|---|---|---|---|
| Bright field microscopy | Simplest, cheapest, and highly accessible | Resolution limited by the wavelength of light, low contrast | Rapidly identification of a motile bacteria |
| Dark field microscopy | Contrast enhancement of unstained samples | Resolution limited by the wavelength of light | Visualization of motile bacteria, flagella |
| Phase contrast microscopy | Contrast enhancement of unstained samples | Resolution limited by the wavelength of light | Visualization of motile bacteria, and bacterial orientation |
| Differential interference contrast microscopy (DIC) | Contrast enhancement of unstained samples, edges of the object are highlighted | Resolution limited by the wavelength of light | Visualization of motile bacteria, and bacterial orientation |
| Confocal microscopy or laser scanning confocal microscopy (LSCM) | High resolution imaging due to reduction of background fluorescence; to collect serial optical sections from thick samples. Contrast and definition are improved | May not be fast enough to capture relevant dynamics; limited to the number of excitation wavelengths available from common lasers; imaging depth limited | Visualization of motile bacteria in thin tissues |
| Spinning disk confocal microscopy | Image acquisition speed is higher than LSCM improving the observation of dynamic processes and reducing photodamage | Imaging depth limited; sensitive camera is needed | Visualization of motile bacteria in thin tissues |
| Multiphoton confocal microscopy | Deeper penetration in tissue (>100 μm) compared to LSCM | Higher phototoxicity and photobleaching in the focal plane compared to LSCM | Visualization of motile bacteria in thick living tissue |
| Light-sheet fluorescent microscopy (LSFM) or selective plane illumination microscopy (SPIM) | High 3D resolution images | Sample mounting may be challenging; reduced resolution in depth compared to confocal microscopy | Visualization of motile bacteria in thick living tissue |
| Light-field-based selective volume illumination microscopy (SVIM) | Captures a 3D volume in a single snapshot | Requires specialized hardware; smaller spatial range than SPIM | Visualization of motile bacteria in thick living tissue in a single snapshot |
| Digital holographic microscopy (DHM) | High imaging speed; high resolution; adjust focus after the image is recorded, since all focus planes are recorded simultaneously by the hologram | Low scattering efficiency of bacteria | Visualization of several free-swimming bacteria |
| Differential dynamic microscopy (DDM) | Great number of bacteria can be processed simultaneously | Unsuited for obtaining specific motility parameters | Quick evaluation of motility responses at a whole-population level |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palma, V.; Gutiérrez, M.S.; Vargas, O.; Parthasarathy, R.; Navarrete, P. Methods to Evaluate Bacterial Motility and Its Role in Bacterial–Host Interactions. Microorganisms 2022, 10, 563. https://doi.org/10.3390/microorganisms10030563
Palma V, Gutiérrez MS, Vargas O, Parthasarathy R, Navarrete P. Methods to Evaluate Bacterial Motility and Its Role in Bacterial–Host Interactions. Microorganisms. 2022; 10(3):563. https://doi.org/10.3390/microorganisms10030563
Chicago/Turabian StylePalma, Victoria, María Soledad Gutiérrez, Orlando Vargas, Raghuveer Parthasarathy, and Paola Navarrete. 2022. "Methods to Evaluate Bacterial Motility and Its Role in Bacterial–Host Interactions" Microorganisms 10, no. 3: 563. https://doi.org/10.3390/microorganisms10030563
APA StylePalma, V., Gutiérrez, M. S., Vargas, O., Parthasarathy, R., & Navarrete, P. (2022). Methods to Evaluate Bacterial Motility and Its Role in Bacterial–Host Interactions. Microorganisms, 10(3), 563. https://doi.org/10.3390/microorganisms10030563








