Temperature Affects the Biological Control of Dinoflagellates by the Generalist Parasitoid Parvilucifera rostrata
Abstract
:1. Introduction
2. Methods
2.1. Origin of the Strains and Culture Conditions
2.2. Parasitoid–Host Dynamics
2.3. Zoospore Viability
2.4. Zoospore Yield
2.5. Host Growth and Parasitoid Functional Response
2.6. Modeling Approach
3. Results and Discussion
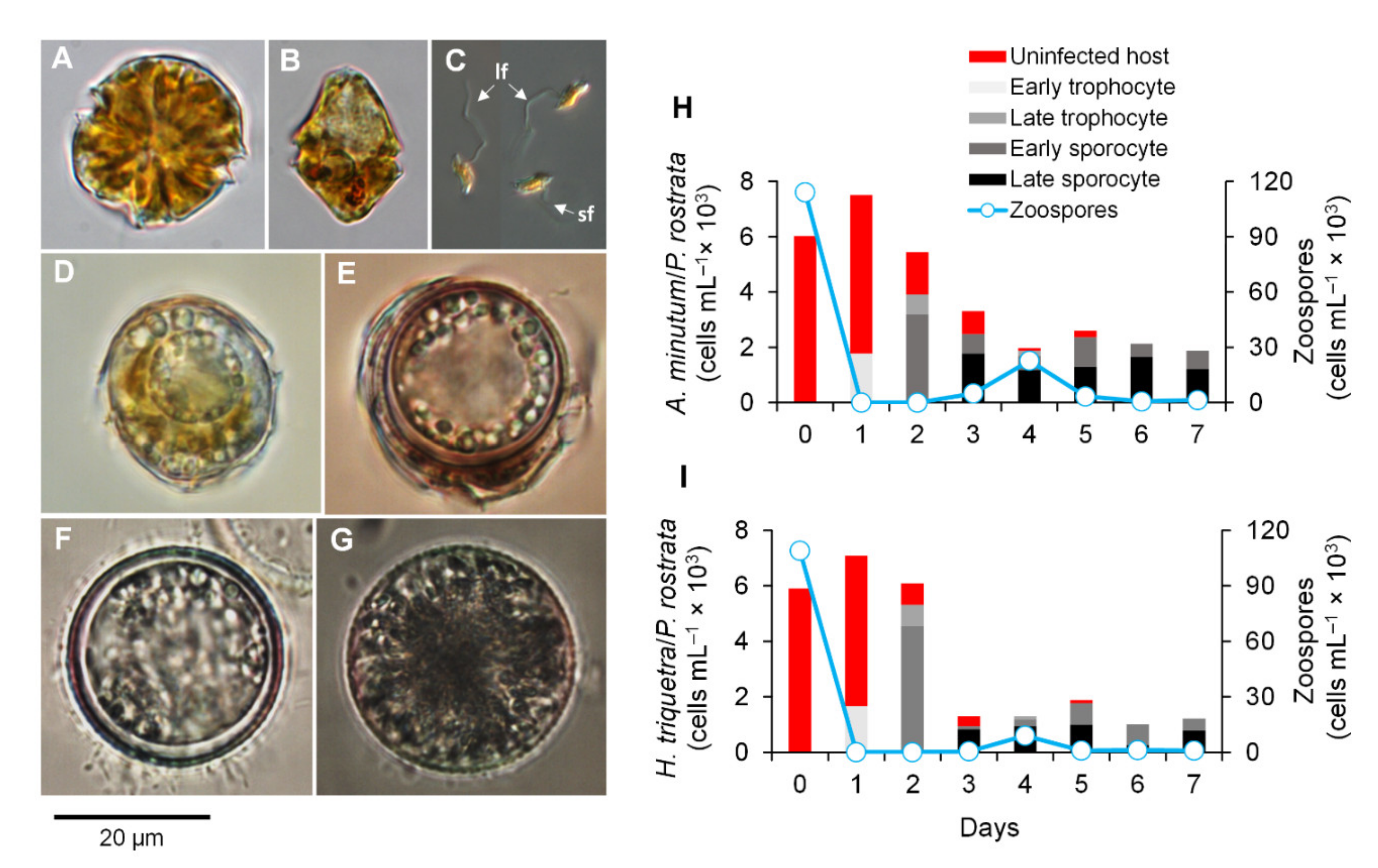
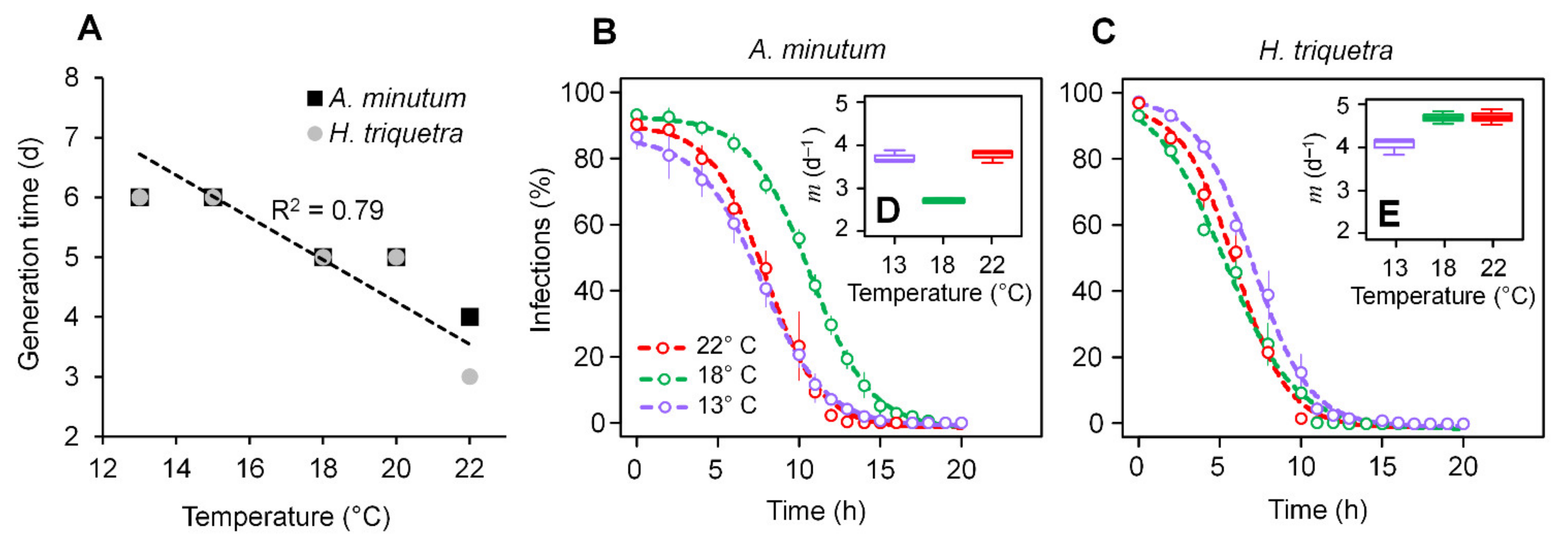
3.1. Parasitoid–Host Dynamics
3.2. Zoospore Infectivity and Viability
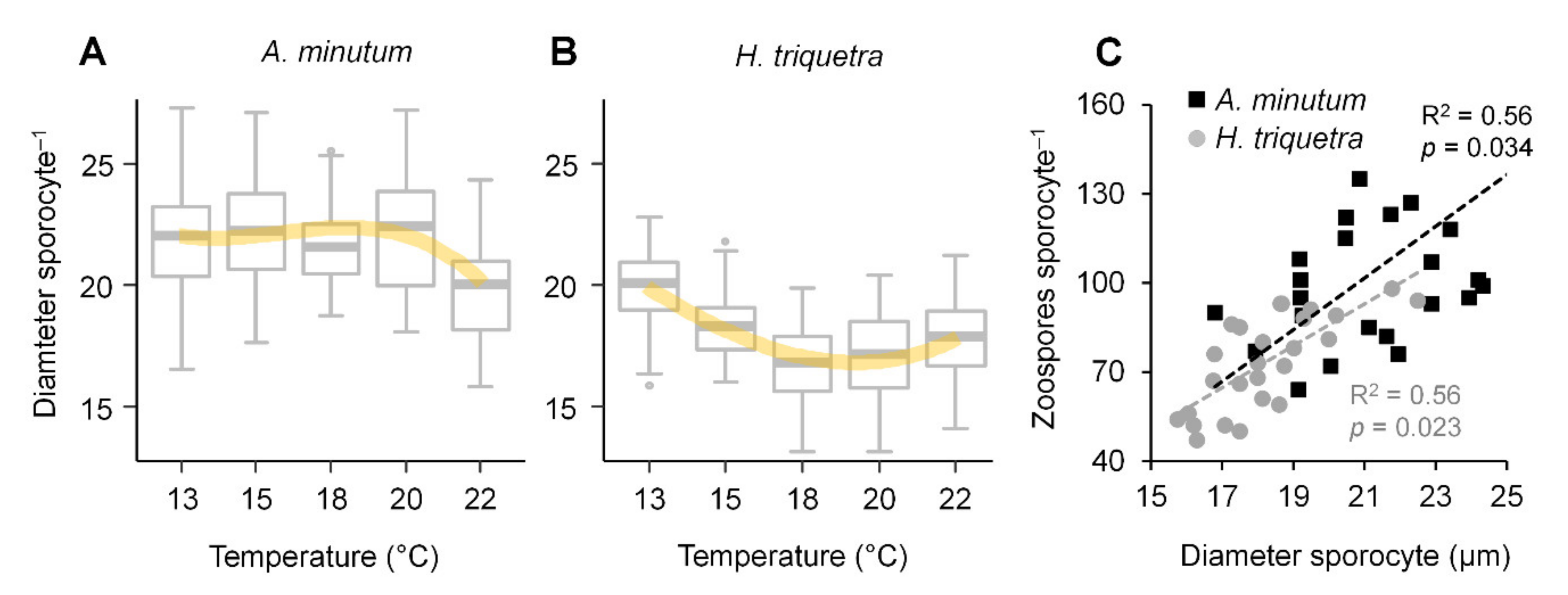
3.3. Zoospore Yield
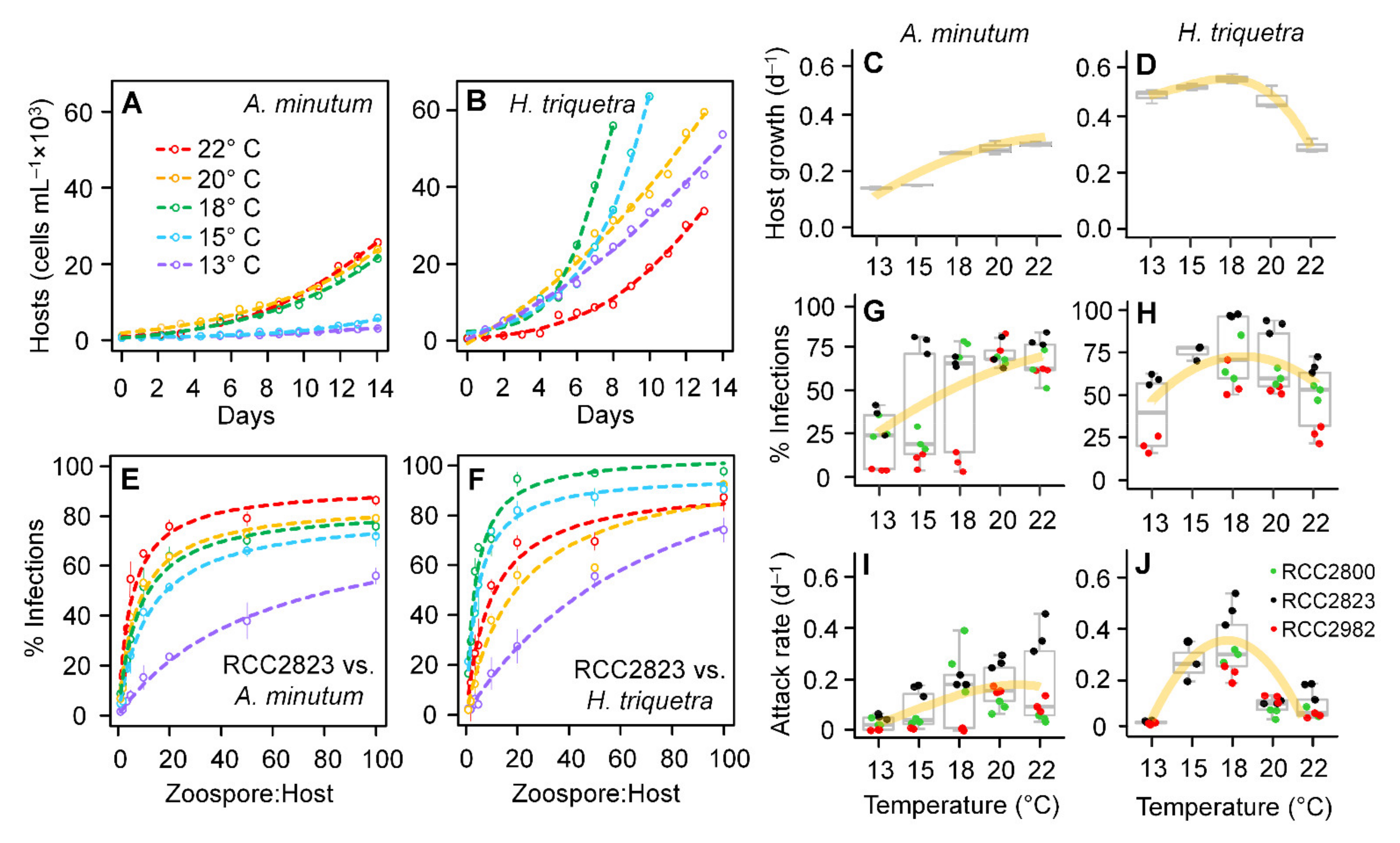
3.4. Host Growth and Parasitoid Functional Response
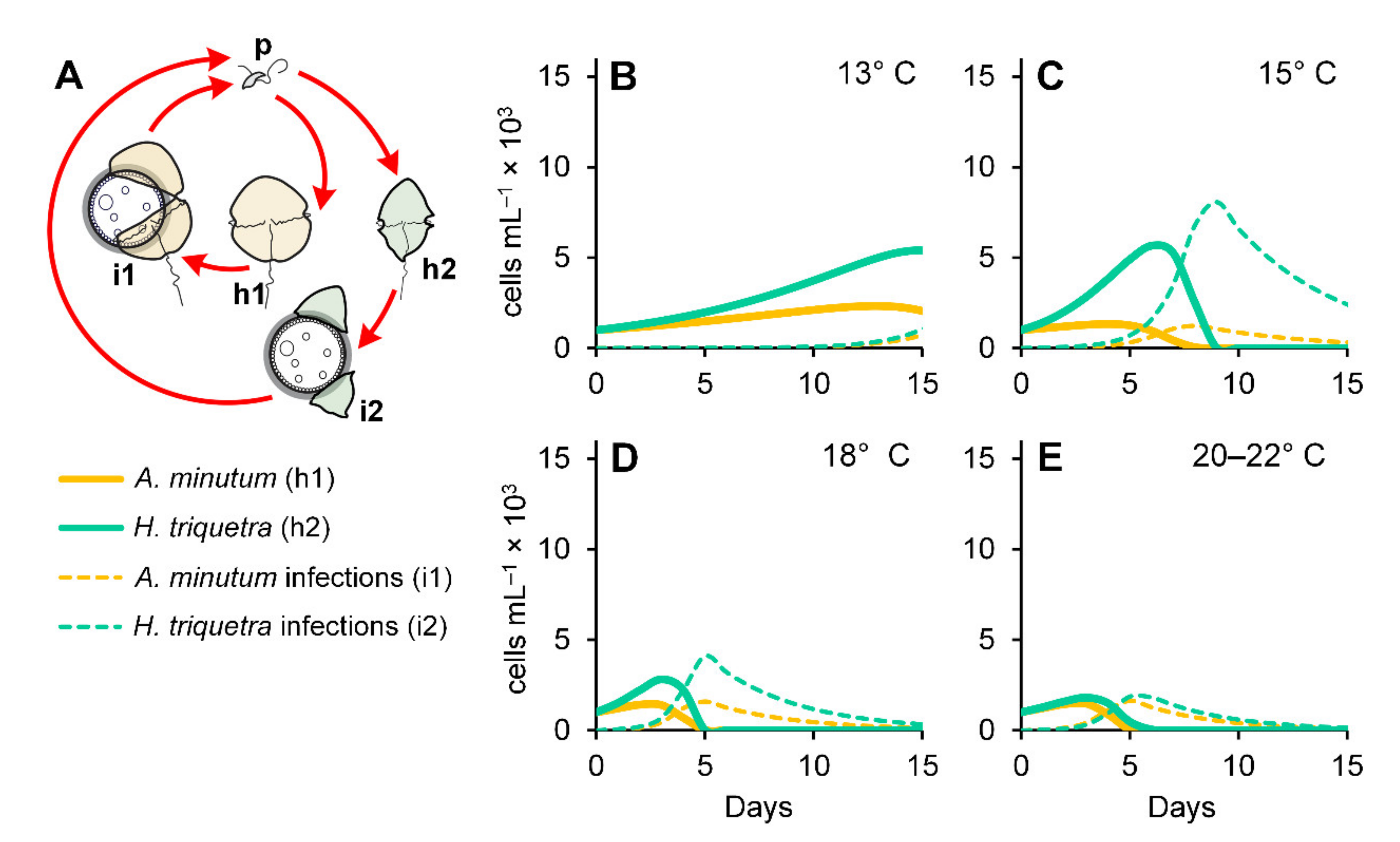
3.5. Numerical Simulations
4. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kagami, M.; Bruin, A.; Ibelings, B.W.; Donk, E. Parasitic chytrids: Their effects on phytoplankton communities and foodweb dynamics. Hydrobiologia 2007, 578, 113–129. [Google Scholar] [CrossRef] [Green Version]
- Coats, D.W.; Park, M.G. Parasitism of photosynthetic dinoflagellates by three strains of Amoebophrya (Dinophyta): Parasite survival, infectivity, generation time, and host specificity. J. Phycol. 2002, 38, 520–528. [Google Scholar] [CrossRef]
- Montagnes, D.J.S.; Chambouvet, A.; Guillou, L.; Fenton, A. Responsibility of microzooplankton and parasite pressure for the demise of toxic dinoflagellate blooms. Aquat. Microb. Ecol. 2008, 53, 211–225. [Google Scholar] [CrossRef]
- Jephcott, T.G.; Alves-De-Souza, C.; Gleason, F.H.; van Ogtrop, F.F.; Sime-Ngando, T.; Karpov, S.A.; Guillou, L. Ecological impacts of parasitic chytrids, syndiniales and perkinsids on populations of marine photosynthetic dinoflagellates. Fungal Ecol. 2016, 19, 47–58. [Google Scholar] [CrossRef]
- Coats, D.W.; Bockstahler, K.R. Occurrence of the parasitic dinoflagellate Amoebophrya ceratii in Chesapeake Bay populations of Gymnodinium sanguineum. J. Eukaryot. Microbiol. 1994, 41, 586–593. [Google Scholar] [CrossRef]
- Chambouvet, A.; Morin, P.; Marie, D.; Guillou, L. Control of toxic marine dinoflagellate blooms by serial parasitic killers. Science 2008, 322, 1254–1257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alacid, E.; Reñé, A.; Camp, J.; Garcés, E. In situ occurrence, prevalence and dynamics of Parvilucifera parasitoids during recurrent blooms of the toxic dinoflagellate Alexandrium minutum. Front. Microbiol. 2017, 8, 1624. [Google Scholar] [CrossRef] [PubMed]
- Chambouvet, A.; Alves-de-Souza, C.; Cueff, V.; Marie, D.; Karpov, S.; Guillou, L. Interplay between the parasite Amoebophrya sp. (Alveolata) and the cyst formation of the red tide dinoflagellate Scrippsiella trochoidea. Protist 2011, 162, 637–649. [Google Scholar] [CrossRef]
- Blanquart, F.; Valero, M.; Alves-de-Souza, C.; Dia, A.; Lepelletier, F.; Bigeard, E.; Jeanthon, C.; Destombe, C.; Guillou, L. Evidence for parasite-mediated selection during short-lasting toxic algal blooms. Proc. R. Soc. B 2016, 283, 20161870. [Google Scholar] [CrossRef]
- Reñé, A.; Timoneda, N.; Sampedro, N.; Alacid, E.; Gallisai, R.; Gordi, J.; Fernández-Valero, A.D.; Pernice, M.C.; Flo, E.; Garcés, E. Host preferences of coexisting Perkinsea parasitoids during coastal dinoflagellate blooms. Mol. Ecol. 2021, 30, 2417–2433. [Google Scholar] [CrossRef] [PubMed]
- Alacid, E.; Reñé, A.; Garcés, E. New insights into the parasitoid Parvilucifera sinerae life cycle: The development and kinetics of infection of a bloom-forming dinoflagellate host. Protist 2015, 166, 677–699. [Google Scholar] [CrossRef] [PubMed]
- Garcés, E.; Alacid, E.; René, A.; Petrou, K.; Simo, R. Host-released dimethylsulphide activates the dinoflagellate parasitoid Parvilucifera sinerae. ISME J. 2013, 7, 1065–1068. [Google Scholar] [CrossRef] [Green Version]
- Alacid, E.; Reñé, A.; Gallisai, R.; Paloheimo, A.; Garcés, E.; Kremp, A. Description of two new coexisting parasitoids of blooming dinoflagellates in the Baltic sea: Parvilucifera catillosa sp. nov. and Parvilucifera sp. (Perkinsea, Alveolata). Harmful Algae 2020, 100, 101944. [Google Scholar] [CrossRef]
- Llaveria, G.; Garcés, E.; Ross, O.N.; Figueroa, R.I.; Sampedro, N.; Berdalet, E. Small-scale turbulence can reduce parasite infectivity to dinoflagellates. Mar. Ecol. Prog. Ser. 2010, 412, 45–56. [Google Scholar] [CrossRef]
- Holling, C.S. Some characteristics of simple types of predation and parasitism. Can. Entomol. 1959, 91, 385–398. [Google Scholar] [CrossRef]
- Andersen, R.A. Algal Culturing Techniques; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Starr, R.; Zeikus, J. UTEX-The culture collection of algae at the University of Texas at Austin. J. Phycol. 1993, 29, 1–106. [Google Scholar] [CrossRef]
- Lepelletier, F.; Karpov, S.A.; le Panse, S.; Bigeard, E.; Skovgaard, A.; Jeanthon, C.; Guillou, L. Parvilucifera rostrata sp. nov. (Perkinsozoa), a novel parasitoid that infects planktonic dinoflagellates. Protist 2014, 165, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Ogle, D.H.; Doll, J.; Wheeler, P.; Dinno, A. FSA: Simple Fisheries Stock Assessment Methods v. R Package Version. 2019. Available online: https://cran.r-project.org/web/packages/FSA/FSA.pdf (accessed on 29 September 2019).
- CRAN. The Comprehensive R Archive Network. Available online: https://cran.r-project.org/ (accessed on 25 May 2018).
- Guillard, R. Methods for microflagellates and nannoplankton. In Handbook of Phycological Methods. Culture Methods and Growth Measurements; Stein, J., Ed.; Cambridge University Press: Cambridge, UK, 1973; pp. 69–85. [Google Scholar]
- Salomon, P.S.; Stolte, W. Predicting the population dynamics in Amoebophrya parasitoids and their dinoflagellate hosts using a mathematical model. Mar. Ecol. Prog. Ser. 2010, 419, 1–10. [Google Scholar] [CrossRef]
- Arancio, M. Etude Théorique des Interactions entre des Dinoflagellés et des Parasitoïdes Eucaryotes en Environnement Mélangé: Persistance du Système et Succession Phytoplanctonique/Theoretical study of Dinoflagellates and Eukaryot Parasitoids Interactions in Mixed Environment: System Persistence and Phytoplanktonic Succession. Ph.D. Thesis, Université de Lille, Lille, France, 2014. [Google Scholar]
- Norén, F.; Moestrup, Ø.; Rehnstam-Holm, A.S. Parvilucifera infectans Norén et Moestrup gen. et sp. nov. (Perkinsozoa phylum nov.): A parasitic flagellate capable of killing toxic microalgae. Eur. J. Protistol. 1999, 35, 233–254. [Google Scholar] [CrossRef]
- Frenken, T.; Velthuis, M.; de Senerpont Domis, L.N.; Stephan, S.; Aben, R.; Kosten, S.; van Donk, E.; van de Waal, D.B. Warming accelerates termination of a phytoplankton spring bloom by fungal parasites. Glob. Change Biol. 2016, 22, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Velthuis, M.; de Senerpont Domis, L.N.; Frenken, T.; Stephan, S.; Kazanjian, G.; Aben, R.; Hilt, S.; Kosten, S.; van Donk, E.; van de Waal, D.B. Warming advances top-down control and reduces producer biomass in a freshwater plankton community. Ecosphere 2017, 8, e01651. [Google Scholar] [CrossRef]
- Lepelletier, F. Identification de Parasites Impliqués dans la Régulation des Efflorescences de la Microalgue Toxique Alexandrium minutum/Identification of Parasites Involved in the Bloom Control of the Toxic Microalgae Alexandrium minutum. Ph.D. Thesis, Université Pierre et Marie Curie, Paris, France, 2013. [Google Scholar]
- Figueroa, R.I.; Garcés, E.; Massana, R.; Camp, J. Description, host-specificity, and strain selectivity of the dinoflagellate parasite Parvilucifera sinerae sp. nov. (Perkinsozoa). Protist 2008, 159, 563–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcés, E.; Alacid, E.; Bravo, I.; Fraga, S.; Figueroa, R.I. Parvilucifera sinerae (Alveolata, Myzozoa) is a generalist parasitoid of dinoflagellates. Protist 2013, 164, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Millette, N.; Pierson, J.; Aceves, A.; Stoecker, D. Mixotrophy in Heterocapsa rotundata: A mechanism for dominating the winter phytoplankton. Limnol. Oceanogr. 2017, 62, 836–845. [Google Scholar] [CrossRef]
| State Variable/Parameters | Unit | Values | |||||
|---|---|---|---|---|---|---|---|
| State variables | |||||||
| K | Carrying capacity | cells mL−1 | 40,000 | ||||
| H1 | Alexandrium minutum | cells mL−1 | 1000 | ||||
| H2 | Heterocapsa triquetra | cells mL−1 | 1000 | ||||
| P | Parvilucifera rostrata zoospores | cells mL−1 | 2000 | ||||
| I1 | Infected A. minutum | cells mL−1 | 0 | ||||
| I2 | Infected H. triquetra | cells mL−1 | 0 | ||||
| Parameters | 13 °C | 15 °C | 18 °C | 20 °C | 22 °C | ||
| r1 | A. minutum growth rate | d−1 | 0.16 | 0.23 | 0.30 | 0.37 | 0.31 |
| r2 | H. triquetra growth rate | d−1 | 0.50 | 0.53 | 0.56 | 0.48 | 0.31 |
| a1 | P. rostrata attack rate on A. minutum a | d−1 × 103 | 0.03 | 0.08 | 0.15 | 0.17 | 0.17 |
| a2 | P. rostrata attack rate on H. triquetra a | d−1 × 103 | 0.02 | 0.40 | 0.32 | 0.09 | 0.09 |
| h | P. rostrata handling time | d | 5 | 5 | 4 | 4 | 3 |
| ε1 | Zoospores per infected A. minutum b | zoospores host−1 | 105 | 105 | 105 | 105 | 105 |
| ε2 | Zoospores per infected H. triquetra b | zoospores host−1 | 7494 | 74 | 74 | 74 | 74 |
| m | Zoospore mortality c | d−1 | 3 | 3 | 3 | 3 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmitt, M.; Telusma, A.; Bigeard, E.; Guillou, L.; Alves-de-Souza, C. Temperature Affects the Biological Control of Dinoflagellates by the Generalist Parasitoid Parvilucifera rostrata. Microorganisms 2022, 10, 385. https://doi.org/10.3390/microorganisms10020385
Schmitt M, Telusma A, Bigeard E, Guillou L, Alves-de-Souza C. Temperature Affects the Biological Control of Dinoflagellates by the Generalist Parasitoid Parvilucifera rostrata. Microorganisms. 2022; 10(2):385. https://doi.org/10.3390/microorganisms10020385
Chicago/Turabian StyleSchmitt, Matthew, Aaron Telusma, Estelle Bigeard, Laure Guillou, and Catharina Alves-de-Souza. 2022. "Temperature Affects the Biological Control of Dinoflagellates by the Generalist Parasitoid Parvilucifera rostrata" Microorganisms 10, no. 2: 385. https://doi.org/10.3390/microorganisms10020385
APA StyleSchmitt, M., Telusma, A., Bigeard, E., Guillou, L., & Alves-de-Souza, C. (2022). Temperature Affects the Biological Control of Dinoflagellates by the Generalist Parasitoid Parvilucifera rostrata. Microorganisms, 10(2), 385. https://doi.org/10.3390/microorganisms10020385






