First Outbreak of NDM-1-Producing Klebsiella pneumoniae ST11 in a Portuguese Hospital Centre during the COVID-19 Pandemic
Abstract
1. Introduction
2. Materials and Methods
2.1. Hospital Setting and Outbreak Description
2.2. Bacterial Strains
2.3. Antimicrobial Susceptibility Testing
2.4. Molecular Methods and Detection of Carbapenemase Genes
2.5. Whole Genome Sequencing (WGS)
2.6. Drug Resistance Associated Genes, Virulence Genes, Capsular Types and Plasmid Replicons
2.7. Ethical Approval
3. Results
3.1. Outbreak Description of Clinical Strains
3.2. Antimicrobial Susceptibility
3.3. Identification of Carbapenemase Genes
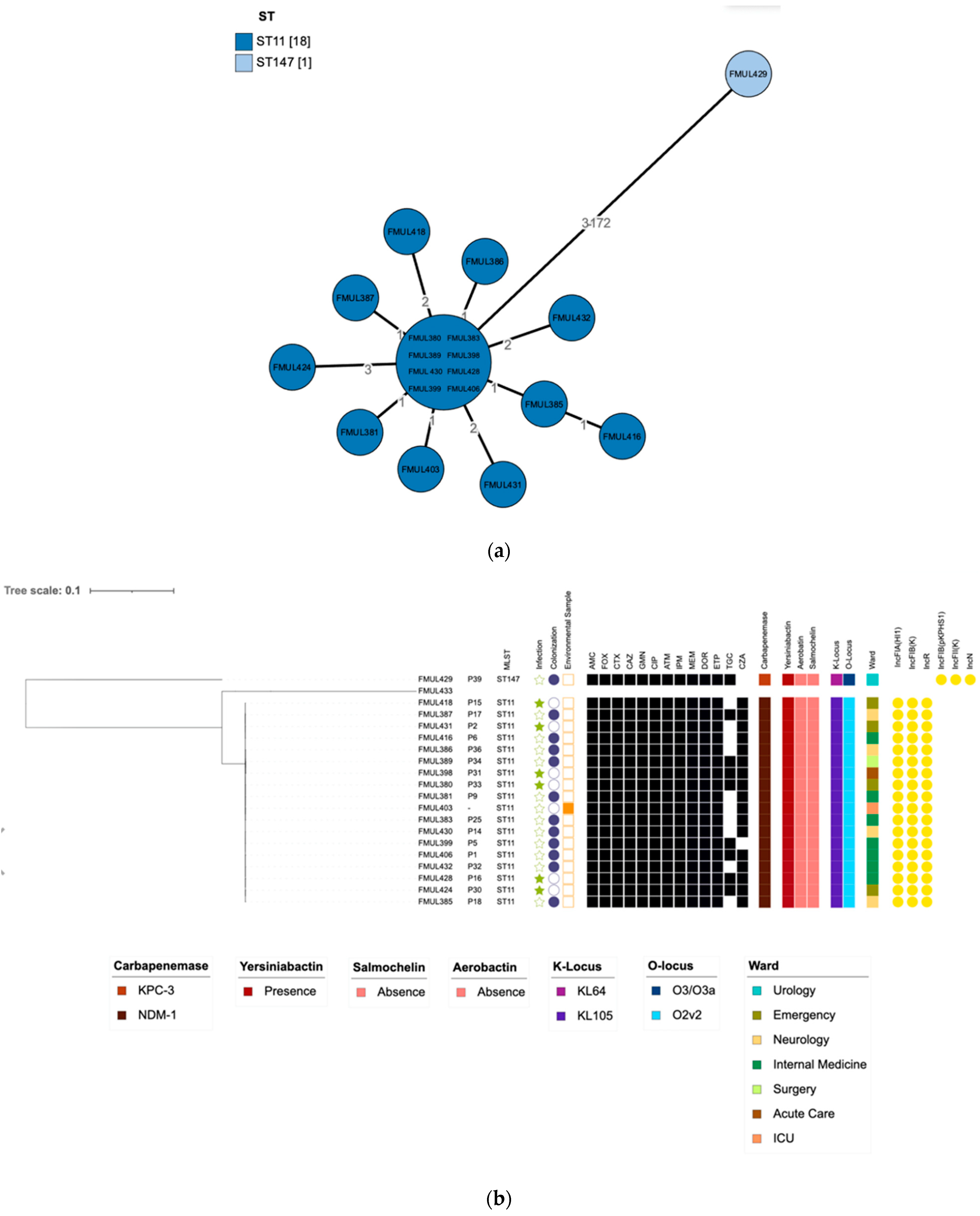
3.4. WGS Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Temkin, E.; Adler, A.; Lerner, A.; Carmeli, Y. Carbapenem-resistant Enterobacteriaceae: Biology, epidemiology, and management. Ann. N. Y. Acad. Sci. 2014, 1323, 22–42. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. Available online: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 13 December 2021).
- Kumarasamy, K.K.; Toleman, M.A.; Walsh, T.R.; Bagaria, J.; Butt, F.; Balakrishnan, R.; Chaudhary, U.; Doumith, M.; Giske, C.G.; Irfan, S.; et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: A molecular, biological, and epidemiological study. Lancet Infect. Dis. 2010, 10, 597–602. [Google Scholar] [CrossRef]
- Wilson, M.E.; Chen, L.H. NDM-1 and the Role of Travel in Its Dissemination. Curr. Infect. Dis. Rep. 2012, 14, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Yong, D.; Toleman, M.A.; Giske, C.G.; Cho, H.S.; Sundman, K.; Lee, K.; Walsh, T.R. Characterization of a new metallo-beta-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob. Agents Chemother. 2009, 53, 5046–5054. [Google Scholar] [CrossRef] [PubMed]
- Ruppe, E.; Armand-Lefevre, L.; Estellat, C.; El-Mniai, A.; Boussadia, Y.; Consigny, P.H.; Girard, P.M.; Vittecoq, D.; Bouchaud, O.; Pialoux, G.; et al. Acquisition of carbapenemase-producing Enterobacteriaceae by healthy travellers to India, France, February 2012 to March 2013. Euro Surveill. 2014, 19, 20768. [Google Scholar] [CrossRef]
- Grundmann, H.; Glasner, C.; Albiger, B.; Aanensen, D.M.; Tomlinson, C.T.; Andrasevic, A.T.; Canton, R.; Carmeli, Y.; Friedrich, A.W.; Giske, C.G.; et al. Occurrence of carbapenemase-producing Klebsiella pneumoniae and Escherichia coli in the European survey of carbapenemase-producing Enterobacteriaceae (EuSCAPE): A prospective, multinational study. Lancet Infect. Dis. 2017, 17, 153–163. [Google Scholar] [CrossRef]
- Fuster, B.; Tormo, N.; Salvador, C.; Gimeno, C. Detection of two simultaneous outbreaks of Klebsiella pneumoniae coproducing OXA-48 and NDM-1 carbapenemases in a tertiary-care hospital in Valencia, Spain. New Microbes New Infect. 2020, 34, 100660. [Google Scholar] [CrossRef] [PubMed]
- Perez-Vazquez, M.; Sola Campoy, P.J.; Ortega, A.; Bautista, V.; Monzon, S.; Ruiz-Carrascoso, G.; Mingorance, J.; Gonzalez-Barbera, E.M.; Gimeno, C.; Aracil, B.; et al. Emergence of NDM-producing Klebsiella pneumoniae and Escherichia coli in Spain: Phylogeny, resistome, virulence and plasmids encoding blaNDM-like genes as determined by WGS. J. Antimicrob. Chemother. 2019, 74, 3489–3496. [Google Scholar] [CrossRef]
- Sampere, A.; Garcia Martinez de Artola, D.; Alcoba Florez, J.; Perez Roth, E. Emergence of carbapenem-resistant NDM-1-producing Klebsiella pneumoniae high-risk sequence type 147 in a tertiary care hospital in Tenerife, Spain. J. Glob. Antimicrob. Resist. 2019, 17, 240–241. [Google Scholar] [CrossRef]
- Manageiro, V.; Sampaio, D.A.; Pereira, P.; Rodrigues, P.; Vieira, L.; Palos, C.; Canica, M. Draft Genome Sequence of the First NDM-1-Producing Providencia stuartii Strain Isolated in Portugal. Genome Announc. 2015, 3, e01077-15. [Google Scholar] [CrossRef]
- Teixeira, P.; Tacao, M.; Pureza, L.; Goncalves, J.; Silva, A.; Cruz-Schneider, M.P.; Henriques, I. Occurrence of carbapenemase-producing Enterobacteriaceae in a Portuguese river: blaNDM, blaKPC and blaGES among the detected genes. Environ. Pollut. 2020, 260, 113913. [Google Scholar] [CrossRef] [PubMed]
- Aires-de-Sousa, M.; Ortiz de la Rosa, J.M.; Goncalves, M.L.; Costa, A.; Nordmann, P.; Poirel, L. Occurrence of NDM-1-producing Morganella morganii and Proteus mirabilis in a single patient in Portugal: Probable in vivo transfer by conjugation. J. Antimicrob. Chemother. 2020, 75, 903–906. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Yigit, H.; Queenan, A.M.; Anderson, G.J.; Domenech-Sanchez, A.; Biddle, J.W.; Steward, C.D.; Alberti, S.; Bush, K.; Tenover, F.C. Novel carbapenem-hydrolyzing beta-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2001, 45, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Naas, T.; Nicolas, D.; Collet, L.; Bellais, S.; Cavallo, J.D.; Nordmann, P. Characterization of VIM-2, a carbapenem-hydrolyzing metallo-beta-lactamase and its plasmid- and integron-borne gene from a Pseudomonas aeruginosa clinical isolate in France. Antimicrob. Agents Chemother. 2000, 44, 891–897. [Google Scholar] [CrossRef]
- Poirel, L.; Le Thomas, I.; Naas, T.; Karim, A.; Nordmann, P. Biochemical sequence analyses of GES-1, a novel class A extended-spectrum beta-lactamase, and the class 1 integron In52 from Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2000, 44, 622–632. [Google Scholar] [CrossRef]
- Dolejska, M.; Duskova, E.; Rybarikova, J.; Janoszowska, D.; Roubalova, E.; Dibdakova, K.; Maceckova, G.; Kohoutova, L.; Literak, I.; Smola, J.; et al. Plasmids carrying blaCTX-M-1 and qnr genes in Escherichia coli isolates from an equine clinic and a horseback riding centre. J. Antimicrob. Chemother. 2011, 66, 757–764. [Google Scholar] [CrossRef]
- Feng, Y.; Zou, S.; Chen, H.; Yu, Y.; Ruan, Z. BacWGSTdb 2.0: A one-stop repository for bacterial whole-genome sequence typing and source tracking. Nucleic Acids Res. 2021, 49, D644–D650. [Google Scholar] [CrossRef]
- Ruan, Z.; Feng, Y. BacWGSTdb, a database for genotyping and source tracking bacterial pathogens. Nucleic Acids Res. 2016, 44, D682–D687. [Google Scholar] [CrossRef]
- Ruan, Z.; Yu, Y.; Feng, Y. The global dissemination of bacterial infections necessitates the study of reverse genomic epidemiology. Brief. Bioinform. 2020, 21, 741–750. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v4: Recent updates and new developments. Nucleic Acids Res. 2019, 47, W256–W259. [Google Scholar] [CrossRef] [PubMed]
- Bosch, T.; Lutgens, S.P.M.; Hermans, M.H.A.; Wever, P.C.; Schneeberger, P.M.; Renders, N.H.M.; Leenders, A.; Kluytmans, J.; Schoffelen, A.; Notermans, D.; et al. Outbreak of NDM-1-Producing Klebsiella pneumoniae in a Dutch Hospital, with Interspecies Transfer of the Resistance Plasmid and Unexpected Occurrence in Unrelated Health Care Centers. J. Clin. Microbiol. 2017, 55, 2380–2390. [Google Scholar] [CrossRef] [PubMed]
- Escobar Perez, J.A.; Olarte Escobar, N.M.; Castro-Cardozo, B.; Valderrama Marquez, I.A.; Garzon Aguilar, M.I.; Martinez de la Barrera, L.; Barrero Barreto, E.R.; Marquez-Ortiz, R.A.; Moncada Guayazan, M.V.; Vanegas Gomez, N. Outbreak of NDM-1-producing Klebsiella pneumoniae in a neonatal unit in Colombia. Antimicrob. Agents Chemother. 2013, 57, 1957–1960. [Google Scholar] [CrossRef] [PubMed]
- Hamzaoui, Z.; Ocampo-Sosa, A.; Maamar, E.; Fernandez Martinez, M.; Ferjani, S.; Hammami, S.; Harbaoui, S.; Genel, N.; Arlet, G.; Saidani, M.; et al. An Outbreak of NDM-1-Producing Klebsiella pneumoniae, Associated with OmpK35 and OmpK36 Porin Loss in Tunisia. Microb. Drug Resist. 2018, 24, 1137–1147. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Garcia, M.; Perez-Viso, B.; Leon-Sampedro, R.; Navarro-San Francisco, C.; Lopez-Fresnena, N.; Diaz-Agero, C.; Morosini, M.I.; Ruiz-Garbajosa, P.; Canton, R. Outbreak of NDM-1+CTX-M-15+DHA-1-producing Klebsiella pneumoniae high-risk clone in Spain owing to an undetectable colonised patient from Pakistan. Int. J. Antimicrob. Agents 2019, 54, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Izdebski, R.; Sitkiewicz, M.; Urbanowicz, P.; Krawczyk, M.; Brisse, S.; Gniadkowski, M. Genomic background of the Klebsiella pneumoniae NDM-1 outbreak in Poland, 2012–2018. J. Antimicrob. Chemother. 2020, 75, 3156–3162. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, J.; Inoue, F.M.; Lobo, A.P.T.; Ibanes, A.S.; Tufik, S.; Kiffer, C.R.V. A major monoclonal hospital outbreak of NDM-1-producing Klebsiella pneumoniae ST340 and the first report of ST2570 in Brazil. Infect. Control Hosp. Epidemiol. 2019, 40, 492–494. [Google Scholar] [CrossRef] [PubMed]
- Savov, E.; Politi, L.; Spanakis, N.; Trifonova, A.; Kioseva, E.; Tsakris, A. NDM-1 Hazard in the Balkan States: Evidence of the First Outbreak of NDM-1-Producing Klebsiella pneumoniae in Bulgaria. Microb. Drug Resist. 2018, 24, 253–259. [Google Scholar] [CrossRef]
- Zheng, R.; Zhang, Q.; Guo, Y.; Feng, Y.; Liu, L.; Zhang, A.; Zhao, Y.; Yang, X.; Xia, X. Outbreak of plasmid-mediated NDM-1-producing Klebsiella pneumoniae ST105 among neonatal patients in Yunnan, China. Ann. Clin. Microbiol. Antimicrob. 2016, 15, 10. [Google Scholar] [CrossRef]
- Caneiras, C.; Calisto, F.; Jorge da Silva, G.; Lito, L.; Melo-Cristino, J.; Duarte, A. First Description of Colistin and Tigecycline-Resistant Acinetobacter baumannii Producing KPC-3 Carbapenemase in Portugal. Antibiotics 2018, 7, 96. [Google Scholar] [CrossRef]
- Caneiras, C.; Lito, L.; Mayoralas-Alises, S.; Diaz-Lobato, S.; Melo-Cristino, J.; Duarte, A. Virulence and resistance determinants of Klebsiella pneumoniae isolated from a Portuguese tertiary university hospital centre over a 31-year period. Enferm. Infecc. Microbiol. Clin. 2019, 37, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Caneiras, C.; Lito, L.; Melo-Cristino, J.; Duarte, A. Community- and Hospital-Acquired Klebsiella pneumoniae Urinary Tract Infections in Portugal: Virulence and Antibiotic Resistance. Microorganisms 2019, 7, 138. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, E.; Ribeiro, R.; Silva, C.J.C.; Alves, R.; Baptista, R.; Condinho, S.; Rosa, M.J.; Perdigao, J.; Caneiras, C.; Duarte, A. An Update on Wastewater Multi-Resistant Bacteria: Identification of Clinical Pathogens Such as Escherichia coli O25b:H4-B2-ST131-Producing CTX-M-15 ESBL and KPC-3 Carbapenemase-Producing Klebsiella oxytoca. Microorganisms 2021, 9, 576. [Google Scholar] [CrossRef] [PubMed]
- Perdigao, J.; Caneiras, C.; Elias, R.; Modesto, A.; Spadar, A.; Phelan, J.; Campino, S.; Clark, T.G.; Costa, E.; Saavedra, M.J.; et al. Genomic Epidemiology of Carbapenemase Producing Klebsiella pneumoniae Strains at a Northern Portuguese Hospital Enables the Detection of a Misidentified Klebsiella variicola KPC-3 Producing Strain. Microorganisms 2020, 8, 1986. [Google Scholar] [CrossRef] [PubMed]
- Pitout, J.D.; Nordmann, P.; Poirel, L. Carbapenemase-Producing Klebsiella pneumoniae, a Key Pathogen Set for Global Nosocomial Dominance. Antimicrob. Agents Chemother. 2015, 59, 5873–5884. [Google Scholar] [CrossRef]
- Wyres, K.L.; Lam, M.M.C.; Holt, K.E. Population genomics of Klebsiella pneumoniae. Nat. Rev. Microbiol. 2020, 18, 344–359. [Google Scholar] [CrossRef]
- Bonnin, R.A.; Jousset, A.B.; Chiarelli, A.; Emeraud, C.; Glaser, P.; Naas, T.; Dortet, L. Emergence of New Non-Clonal Group 258 High-Risk Clones among Klebsiella pneumoniae Carbapenemase-Producing K. pneumoniae Isolates, France. Emerg. Infect. Dis. 2020, 26, 1212–1220. [Google Scholar] [CrossRef]
- Ribeiro, T.G.; Novais, A.; Rodrigues, C.; Nascimento, R.; Freitas, F.; Machado, E.; Peixe, L. Dynamics of clonal and plasmid backgrounds of Enterobacteriaceae producing acquired AmpC in Portuguese clinical settings over time. Int. J. Antimicrob. Agents 2019, 53, 650–656. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, Z.; Ge, Y.; He, F. Unravelling the genome sequence of NDM-1 and KPC-2 co-producing Klebsiella pneumoniae ST11 isolated from a bloodstream infection. J. Glob. Antimicrob. Resist. 2020, 20, 339–341. [Google Scholar] [CrossRef]
- Zheng, B.; Xu, H.; Lv, T.; Guo, L.; Xiao, Y.; Huang, C.; Zhang, S.; Chen, Y.; Han, H.; Shen, P.; et al. Stool Samples of Acute Diarrhea Inpatients as a Reservoir of ST11 Hypervirulent KPC-2-Producing Klebsiella pneumoniae. mSystems 2020, 5, e00498-20. [Google Scholar] [CrossRef]
- Qi, Y.; Wei, Z.; Ji, S.; Du, X.; Shen, P.; Yu, Y. ST11, the dominant clone of KPC-producing Klebsiella pneumoniae in China. J. Antimicrob. Chemother. 2011, 66, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Khalid, S.; Ali, S.M.; Khan, A.U. Occurrence of blaNDM Variants Among Enterobacteriaceae From a Neonatal Intensive Care Unit in a Northern India Hospital. Front. Microbiol. 2018, 9, 407. [Google Scholar] [CrossRef]
- Ho, P.L.; Lo, W.U.; Yeung, M.K.; Lin, C.H.; Chow, K.H.; Ang, I.; Tong, A.H.; Bao, J.Y.; Lok, S.; Lo, J.Y. Complete sequencing of pNDM-HK encoding NDM-1 carbapenemase from a multidrug-resistant Escherichia coli strain isolated in Hong Kong. PLoS ONE 2011, 6, e17989. [Google Scholar] [CrossRef][Green Version]
- Mathers, A.J.; Cox, H.L.; Kitchel, B.; Bonatti, H.; Brassinga, A.K.; Carroll, J.; Scheld, W.M.; Hazen, K.C.; Sifri, C.D. Molecular dissection of an outbreak of carbapenem-resistant Enterobacteriaceae reveals Intergenus KPC carbapenemase transmission through a promiscuous plasmid. mBio 2011, 2, e00204-11. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.M.C.; Wick, R.R.; Watts, S.C.; Cerdeira, L.T.; Wyres, K.L.; Holt, K.E. A genomic surveillance framework and genotyping tool for Klebsiella pneumoniae and its related species complex. Nat. Commun. 2021, 12, 4188. [Google Scholar] [CrossRef] [PubMed]
- Bentivegna, E.; Alessio, G.; Spuntarelli, V.; Luciani, M.; Santino, I.; Simmaco, M.; Martelletti, P. Impact of COVID-19 prevention measures on risk of health care-associated Clostridium difficile infection. Am. J. Infect. Control 2021, 49, 640–642. [Google Scholar] [CrossRef]
- Bentivegna, E.; Luciani, M.; Arcari, L.; Santino, I.; Simmaco, M.; Martelletti, P. Reduction of Multidrug-Resistant (MDR) Bacterial Infections during the COVID-19 Pandemic: A Retrospective Study. Int. J. Environ. Res. Public Health 2021, 18, 1003. [Google Scholar] [CrossRef]
- Silva, A.R.O.; Salgado, D.R.; Lopes, L.P.N.; Castanheira, D.; Emmerick, I.C.M.; Lima, E.C. Increased Use of Antibiotics in the Intensive Care Unit During Coronavirus Disease (COVID-19) Pandemic in a Brazilian Hospital. Front. Pharmacol. 2021, 12, 778386. [Google Scholar] [CrossRef]
| Patient | Organism | Age (Gender) | Hospital Ward | Date of Isolation | Biological Product | Colonisation Site |
|---|---|---|---|---|---|---|
| P1 | K. pneumoniae | 47 (M) | Internal Medicine | 18/09/2020 | - | Rectal swab |
| P2 | K. pneumoniae | 55 (F) | Emergency | 05/12/2020 | Ascitic fluid | - |
| P3 | K. pneumoniae | 62 (M) | Surgery | 29/08/2020 | - | Rectal swab |
| P4 | K. pneumoniae | 64 (F) | Urology | 28/09/2020 | Urine | - |
| P5 | K. pneumoniae | 65 (M) | Internal Medicine | 10/09/2020 | - | Rectal swab |
| P6 | K. pneumoniae | 93 (M) | Internal Medicine | 09/10/2020 | - | Rectal swab |
| P7 | E. coli | 66 (M) | Internal Medicine | 26/09/2020 | - | Rectal swab |
| K. pneumoniae | 26/09/2020 | - | Rectal swab | |||
| P8 | K. pneumoniae | 92 (F) | Internal Medicine | 31/08/2020 | - | Rectal swab |
| P9 | K. pneumoniae | 62 (M) | Internal Medicine | 26/08/2020 | - | Rectal swab |
| 07/10/2020 | Rectal swab | |||||
| P10 | K. pneumoniae | 94 (F) | Internal Medicine | 03/10/2020 | - | Rectal swab |
| P11 | K. pneumoniae | 38 (M) | ICU | 19/09/2020 | - | Rectal swab |
| P12 | K. pneumoniae | 85 (F) | Internal Medicine | 26/08/2020 | - | Rectal swab |
| 03/11/2020 | Urine | - | ||||
| P13 | K. pneumoniae | 79 (M) | Internal Medicine | 01/10/2020 | - | Rectal swab |
| P14 | K. pneumoniae | 59 (M) | Neurology | 16/11/2020 | - | Rectal swab |
| P15 | K. pneumoniae | 80 (M) | Internal Medicine | 02/10/2020 | - | Rectal swab |
| Emergency | 11/10/2020 | Urine | - | |||
| Emergency | 11/10/2020 | Blood | - | |||
| P16 | K. pneumoniae | 77 (M) | Internal Medicine | 09/11/2020 | Urine | - |
| P17 | K. pneumoniae | 56 (M) | Pulmonology | 28/08/2020 | - | Rectal swab |
| P18 | K. pneumoniae | 65 (M) | Neurology | 27/08/2020 | - | Rectal swab |
| P19 | K. pneumoniae | 87 (F) | Internal Medicine | 12/10/2020 | Urine | - |
| P20 | K. pneumoniae | 64 (M) | ICU | 17/09/2020 | - | Rectal swab |
| Surgery | 06/10/2020 | Urine | - | |||
| P21 | K. pneumoniae | 81 (F) | Neurology | 14/10/2020 | - | Rectal swab |
| P22 | K. pneumoniae | 76 (F) | Cardiology | 31/08/2020 | - | Rectal swab |
| P23 | K. pneumoniae | 84 (M) | Orthopedics | 29/08/2020 | - | Rectal swab |
| P24 | K. pneumoniae | 79 (M) | Neurology | 04/11/2020 | Urine | - |
| P25 | K. pneumoniae | 61 (M) | Internal Medicine | 26/08/2020 | - | Rectal swab |
| P26 | K. pneumoniae | 81 (F) | Internal Medicine | 15/10/2020 | - | Rectal swab |
| P27 | K. pneumoniae | 83 (F) | Internal Medicine | 28/10/2020 | - | Rectal swab |
| P28 | K. pneumoniae | 55 (M) | Pulmonology | 26/08/2020 | - | Rectal swab |
| P29 | K. pneumoniae | 77 (M) | ICU | 12/10/2020 | - | Rectal swab |
| 12/10/2020 | Ascitic fluid | - | ||||
| P30 | K. pneumoniae | 89 (M) | Internal Medicine | 30/08/2020 | - | Rectal swab |
| Emergency | 26/10/2020 | Urine | - | |||
| P31 | K. pneumoniae | 77 (M) | Acute Care | 28/08/2020 | - | Rectal swab |
| 01/09/2020 | Urine | - | ||||
| P32 | K. pneumoniae | 63 (F) | Internal Medicine | 12/09/2020 | - | Rectal swab |
| 15/12/2020 | Rectal swab | |||||
| P33 | K. pneumoniae | 56 (F) | Emergency | 25/08/2020 | Urine | - |
| P34 | K. pneumoniae | 81 (M) | Surgery | 28/08/2020 | - | Rectal swab |
| P35 | K. pneumoniae | 56 (M) | Surgery | 13/09/2020 | Ascitic fluid | - |
| 17/09/2020 | - | Rectal swab | ||||
| P36 | K. pneumoniae | 62 (M) | Neurology | 27/08/2020 | - | Rectal swab |
| P37 | K. pneumoniae | 81 (M) | Surgery | 28/08/2020 | - | Rectal swab |
| P38 | K. pneumoniae | 74 (M) | Surgery | 29/08/2020 | - | Rectal swab |
| P39 | K. pneumoniae | 59 (M) | Urology | 11/11/2020 | - | Rectal swab |
| P40 | K. pneumoniae | 83 (M) | Orthopedics | 30/08/2020 | - | Rectal swab |
| - | K. pneumoniae | - | Operation room * | 14/09/2020 | - | - |
| Species | MLST | Patients | Environmental Sample | Resistance Profile (Number of Strains) | Virulence Profile | Capsular Locus (KL) Antigen Locus (OL) | Plasmid Replicons | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| bla_Carb | β-Lactams | Other Resistance Genes | Fimbriae | ICEKp | Iron Uptake | K_Locus | O_Locus | |||||
| Klebsiella pneumoniae | ST11 | 17 | 1 | NDM-1 (n = 18) | CTX-M-15; SHV-11; OXA-1; TEM-1 (n = 18) | aac(3)-IId; aac(6′)-Ib-cr; strA; strB; qnrB1; tet(D); sul2; dfrA14; gyrA-83I; parC-80I (n = 18); catB4 (n = 1) | fimA-fimK; mrkA-mrkJ | ybt 10; ICEKp4 (n = 18) | enterobactin (entA-fes), aerobactin (iucA-iutA), salmochelin (iroE-iroN), yersiniabactin(fyuA-ybtX) | KL105 (n = 18) | O2v2 (n = 18) | IncFIA(HI1); IncFIB(K); IncR (n = 18) |
| Klebsiella pneumoniae | ST147 | 1 | - | KPC-3 | SHV-11 | gyrA-83I; parC-80I; fosA | fimA-fimK; mrkA-mrkJ | ybt 16; ICEKp12 | enterobactin (entA-fes), aerobactin (iutA), salmochelin (iroE-iroN), yersiniabactin (fyuA-ybtX) | KL64 | O2v1 | IncFIB(pKPHS1);IncFII(K);IncN |
| Escherichia coli | ST58 | 1 | - | NDM-1 | CTX-M-15; AmpC1; OXA-1; TEM-1 | aac(3)-IId; aac(6’)-Ib-cr; aadA; strA; strB; qnrB1; qnrS1; mphB; cmlA1; sul2; sul3; tet(A); tet(D); dfrA14 | fimA-fimI cfaA-cfaE, ecpA-ecpG | - | aerobactin (iucA-iutA); salmochelin (iroB-iroN) yersiniabactin (fyuA-ybtX), iron/manganese (sitA-sitD) | - | - | Col440II, IncFIA(HI1), IncFIB(AP001918), IncFIC(FII), IncI1, IncR, IncX4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendes, G.; Ramalho, J.F.; Duarte, A.; Pedrosa, A.; Silva, A.C.; Méndez, L.; Caneiras, C. First Outbreak of NDM-1-Producing Klebsiella pneumoniae ST11 in a Portuguese Hospital Centre during the COVID-19 Pandemic. Microorganisms 2022, 10, 251. https://doi.org/10.3390/microorganisms10020251
Mendes G, Ramalho JF, Duarte A, Pedrosa A, Silva AC, Méndez L, Caneiras C. First Outbreak of NDM-1-Producing Klebsiella pneumoniae ST11 in a Portuguese Hospital Centre during the COVID-19 Pandemic. Microorganisms. 2022; 10(2):251. https://doi.org/10.3390/microorganisms10020251
Chicago/Turabian StyleMendes, Gabriel, João F. Ramalho, Aida Duarte, Adriana Pedrosa, Ana Cristina Silva, Lucía Méndez, and Cátia Caneiras. 2022. "First Outbreak of NDM-1-Producing Klebsiella pneumoniae ST11 in a Portuguese Hospital Centre during the COVID-19 Pandemic" Microorganisms 10, no. 2: 251. https://doi.org/10.3390/microorganisms10020251
APA StyleMendes, G., Ramalho, J. F., Duarte, A., Pedrosa, A., Silva, A. C., Méndez, L., & Caneiras, C. (2022). First Outbreak of NDM-1-Producing Klebsiella pneumoniae ST11 in a Portuguese Hospital Centre during the COVID-19 Pandemic. Microorganisms, 10(2), 251. https://doi.org/10.3390/microorganisms10020251






