Borrelia burgdorferi Outer Membrane Vesicles Contain Antigenic Proteins, but Do Not Induce Cell Death in Human Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacteria Cultures
2.2. Human Cell Cultures
2.3. BbOMV Purification
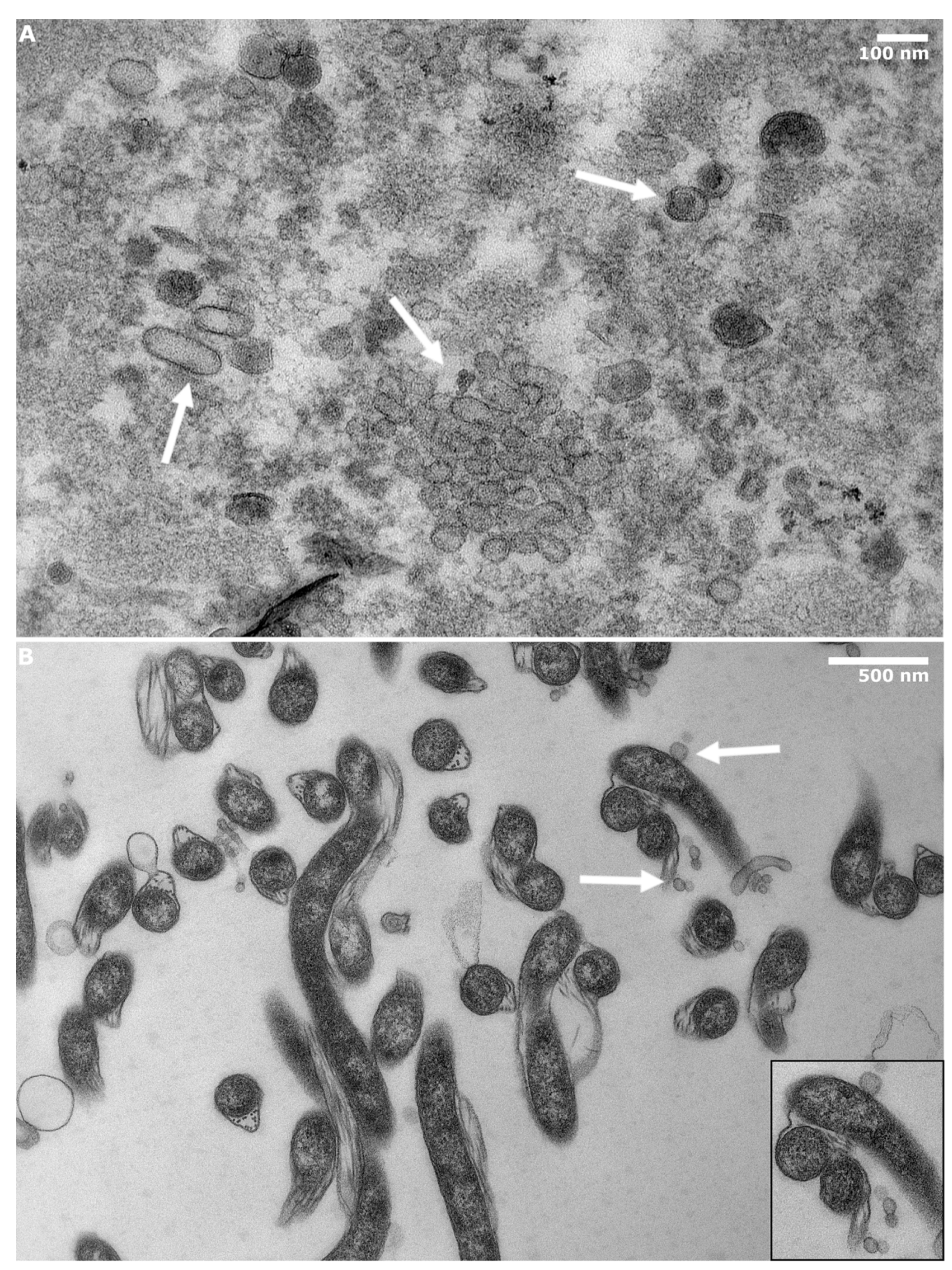
2.4. Transmission Electron Microscopy
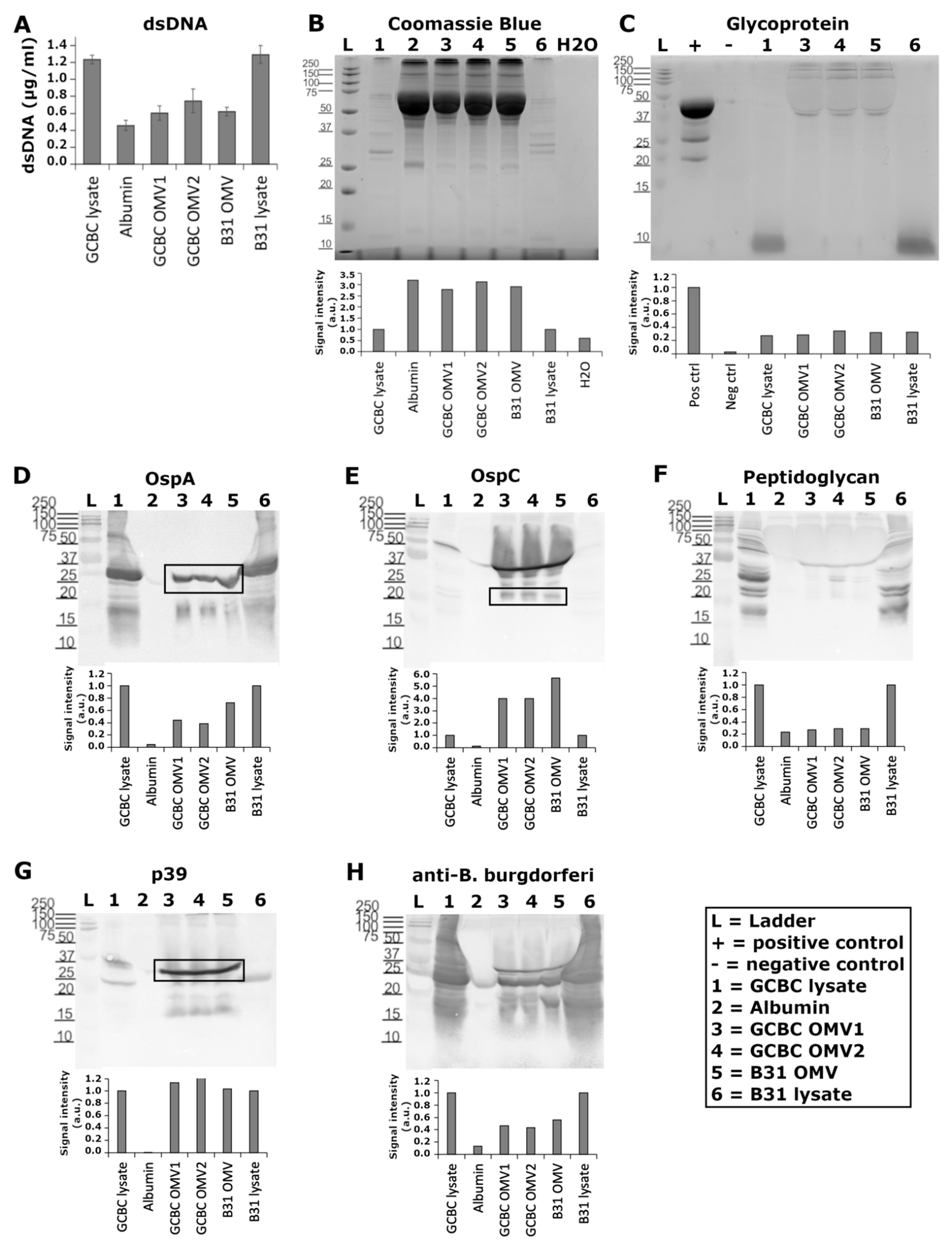
2.5. Characterization of BbOMVs
2.5.1. BbOMV Size Analysis
2.5.2. Qubit Analysis
2.5.3. SDS-PAGE
2.5.4. Western Blot
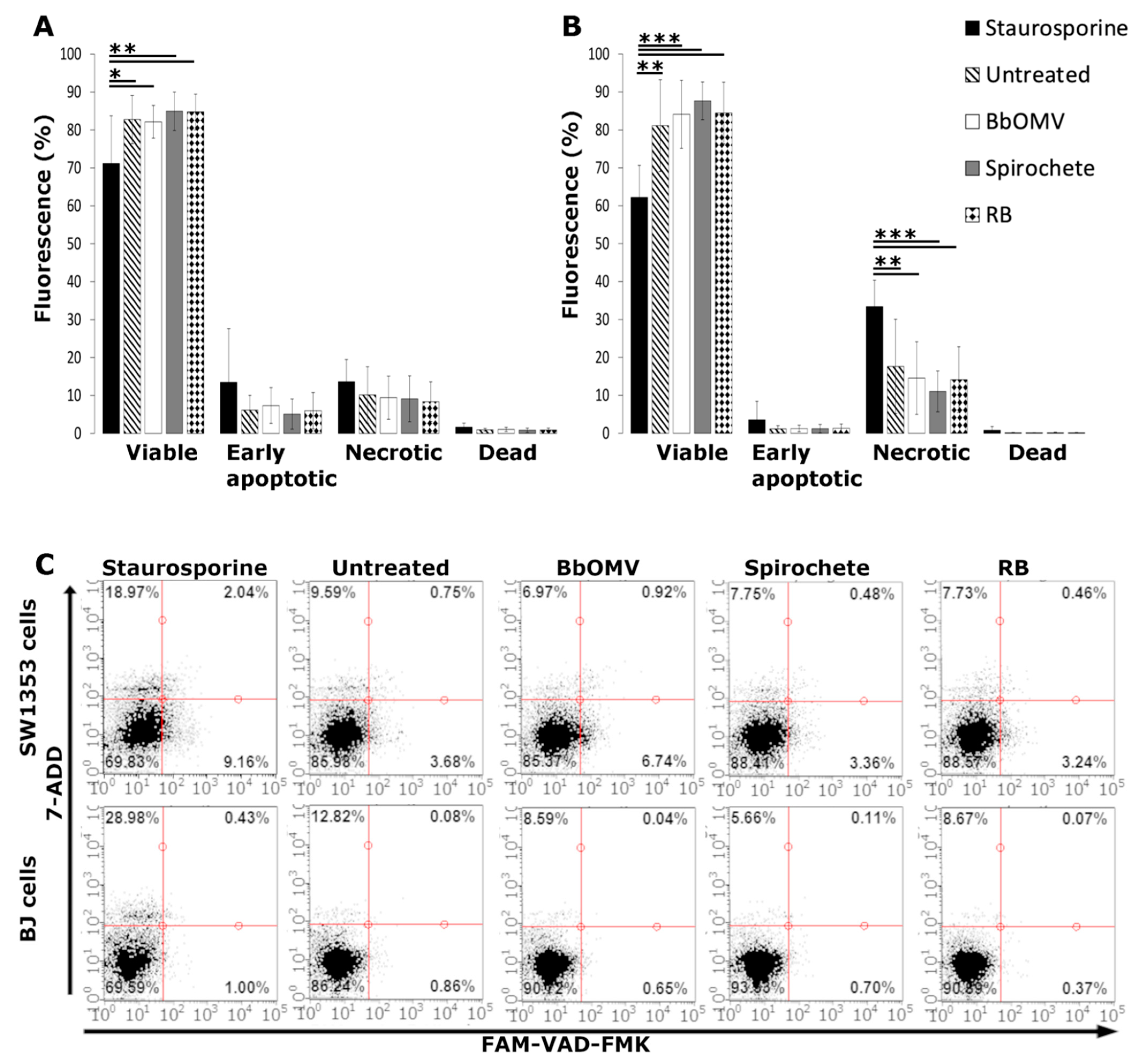
2.6. Caspase Activation Analysis
2.7. Statistical Analysis
3. Results
3.1. BbOMVs Were on Average 33 nm in Diameter
3.2. Known Antigenic Markers Were Located in BbOMVs
3.3. BbOMVs Did Not Induce Cell Death in Human Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mead, P.S. Epidemiology of Lyme Disease. Infect. Dis. Clin. N. Am. 2015, 29, 187–210. [Google Scholar] [CrossRef]
- Burgdorfer, W.; Barbour, A.G.; Hayes, S.F.; Benach, J.L.; Grunwaldt, E.; Davis, J.P. Lyme disease—A tick-borne spirochetosis? Science 1982, 216, 1317–1319. [Google Scholar] [CrossRef]
- Wormser, G.P.; Dattwyler, R.J.; Shapiro, E.D.; Halperin, J.J.; Steere, A.C.; Klempner, M.S.; Krause, P.J.; Bakken, J.S.; Strle, F.; Stanek, G.; et al. The clinical assessments treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: Clinical practice guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 2006, 43, 1089–1134. [Google Scholar] [CrossRef]
- Sehgal, V.N.; Khurana, A. Lyme disease/borreliosis as a systemic disease. Clin. Dermatol. 2015, 33, 542–550. [Google Scholar] [CrossRef]
- Nanagara, R.; Duray, P.H.; Schumacher, H.R. Ultrastructural demonstrational of spirochetal antigens in synovial membrane in chronic lyme disease: Possible factors contributing to persistence of organisms. Hum. Pathol. 1996, 27, 1025–1034. [Google Scholar] [CrossRef]
- Hulïnská, D.; Votýpka, J.; Valešová, M. Persistence of Borrelia garinii and Borrelia afzelii in patients with Lyme arthritis. Zent. Bakteriol. 1999, 289, 301–318. [Google Scholar] [CrossRef]
- Jutras, B.L.; Lochhead, R.B.; Kloos, Z.A.; Biboy, J.; Strle, K.; Booth, C.J.; Govers, S.K.; Gray, J.; Schumann, P.; Vollmer, W.; et al. Borrelia burgdorferi peptidoglycan is a persistent antigen in patients with Lyme arthritis. Proc. Natl. Acad. Sci. USA 2019, 116, 13498–13507. [Google Scholar] [CrossRef] [PubMed]
- Embers, M.E.; Barthold, S.W.; Borda, J.T.; Bowers, L.; Doyle, L.; Hodzic, E.; Jacobs, M.B.; Hasenkampf, N.R.; Martin, D.S.; Narasimhan, S.; et al. Persistence of Borrelia burgdorferi in rhesus macaques following antibiotic treatment of disseminated infection. PLoS ONE 2012, 7, e29914. [Google Scholar] [CrossRef]
- Hodzic, E.; Imai, D.; Feng, S.; Barthold, S.W. Resurgence of persisting non-cultivable Borrelia burgdorferi following antibiotic treatment in mice. PLoS ONE 2014, 9, e86907. [Google Scholar] [CrossRef] [PubMed]
- Bockenstedt, L.K.; Gonzalez, D.G.; Haberman, A.M.; Belperron, A.A. Spirochete antigens persist near cartilage after murine Lyme borreliosis therapy. J. Clin. Invest. 2012, 122, 2652–2660. [Google Scholar] [CrossRef]
- McBroom, A.J.; Kuehn, M.J. Outer Membrane Vesicles. EcoSal Plus 2005, 1. [Google Scholar] [CrossRef]
- Toyofuku, M.; Nomura, N.; Eberl, L. Types and origins of bacterial membrane vesicles. Nat. Rev. Microbiol. 2019, 17, 13–24. [Google Scholar] [CrossRef]
- Orench-Rivera, N.; Kuehn, M.J. Environmentally controlled bacterial vesicle-mediated export. Cell. Microbiol. 2016, 18, 1525–1536. [Google Scholar] [CrossRef] [PubMed]
- Unal, C.M.; Schaar, V.; Riesbeck, K. Bacterial outer membrane vesicles in disease and preventive medicine. Semin. Immunopathol. 2011, 33, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Forsberg, C.W.; Beveridge, T.J.; Hellstrom, A. Cellulase and xylanase release from Bacteroides succinogenes and its importance in the rumen environment. Appl. Environ. Microbiol. 1981, 42, 886–896. [Google Scholar] [CrossRef] [PubMed]
- Dorward, D.W.; Schwan, T.G.; Garon, C.F. Immune capture and detection of Borrelia burgdorferi antigens in urine, blood, or tissues from infected ticks, mice, dogs, and humans. J. Clin. Microbiol. 1991, 29, 1162–1170. [Google Scholar] [CrossRef] [PubMed]
- Brandtzaeg, P.; Bryn, K.; Kierulf, P.; Øvstebø, R.; Namork, E.; Aase, B.; Jantzen, E. Meningococcal endotoxin in lethal septic shock plasma studied by gas chromatography, mass-spectrometry, ultracentrifugation, and electron microscopy. J. Clin. Investig. 1992, 89, 816–823. [Google Scholar] [CrossRef][Green Version]
- Hellman, J.; Loiselle, P.M.; Zanzot, E.M.; Allaire, J.E.; Tehan, M.M.; Boyle, L.A.; Kurnick, J.T.; Warren, H.S. Release of gram-negative outer-membrane proteins into human serum and septic rat blood and their interactions with immunoglobulin in antiserum to Escherichia coli J5. J. Infect. Dis. 2000, 181, 1034–1043. [Google Scholar] [CrossRef]
- Hynes, S.O.; Keenan, J.I.; Ferris, J.A.; Annuk, H.; Moran, A.P. Lewis epitopes on outer membrane vesicles of relevance to Helicobacter pylori pathogenesis. Helicobacter 2005, 10, 146–156. [Google Scholar] [CrossRef]
- Perez Vidakovics, M.L.A.; Jendholm, J.; Mörgelin, M.; Månsson, A.; Larsson, C.; Cardell, L.O.; Riesbeck, K. B cell activation by outer membrane vesicles—A novel virulence mechanism. PLoS Pathog. 2010, 6, e1000724. [Google Scholar] [CrossRef]
- Avila-Calderón, E.D.; Araiza-Villanueva, M.G.; Cancino-Diaz, J.C.; López-Villegas, E.O.; Sriranganathan, N.; Boyle, S.M.; Contreras-Rodríguez, A. Roles of bacterial membrane vesicles. Arch. Microbiol. 2015, 197, 1–10. [Google Scholar] [CrossRef]
- Ellis, T.N.; Kuehn, M.J. Virulence and Immunomodulatory Roles of Bacterial Outer Membrane Vesicles. Microbiol. Mol. Biol. Rev. 2010, 74, 81–94. [Google Scholar] [CrossRef]
- Dorward, D.W.; Garon, C.F.; Judd, R.C. Export and intercellular transfer of DNA via membrane blebs of Neisseria gonorrhoeae. J. Bacteriol. 1989, 171, 2499–2505. [Google Scholar] [CrossRef]
- Shoberg, R.J.; Thomas, D.D. Specific adherence of Borrelia burgdorferi extracellular vesicles to human endothelial cells in culture. Infect. Immun. 1993, 61, 3892–3900. [Google Scholar] [CrossRef]
- Skare, J.T.; Shang, E.S.; Foley, D.M.; Blanco, D.R.; Champion, C.I.; Mirzabekov, T.; Sokolov, Y.; Kagan, B.L.; Miller, J.N.; Lovett, M.A. Virulent strain associated outer membrane proteins of Borrelia burgdorferi. J. Clin. Investig. 1995, 96, 2380–2392. [Google Scholar] [CrossRef]
- Whitmire, W.M.; Garon, C.F. Specific and nonspecific responses of murine B cells to membrane blebs of Borrelia burgdorferi. Infect. Immun. 1993, 61, 1460–1467. [Google Scholar] [CrossRef]
- Shoberg, R.J.; Thomas, D.D. Borrelia burgdorferi vesicle production occurs via a mechanism independent of immunoglobulin M involvement. Infect. Immun. 1995, 63, 4857–4861. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Promnares, K.; Qin, J.; He, M.; Shroder, D.Y.; Kariu, T.; Wang, Y.; Pal, U. Characterization of multiprotein complexes of the Borrelia burgdorferi outer membrane vesicles. J. Proteome Res. 2011, 10, 4556–4566. [Google Scholar] [CrossRef]
- Dorward, D.W.; Garon, C.F. DNA is packaged within membrane-derived vesicles of gram-negative but not gram-positive bacteria. Appl. Environ. Microbiol. 1990, 56, 1960–1962. [Google Scholar] [CrossRef] [PubMed]
- Malge, A.; Ghai, V.; Reddy, P.J.; Baxter, D.; Kim, T.-K.K.; Moritz, R.L.; Wang, K. mRNA transcript distribution bias between Borrelia burgdorferi bacteria and their outer membrane vesicles. FEMS Microbiol. Lett. 2018, 365, 135. [Google Scholar] [CrossRef] [PubMed]
- Beermann, C.; Groscurth, P.; Filgueira, L.; Wunderli-Allenspach, H. Lipoproteins from Borrelia burgdorferi applied in liposomes and presented by dendritic cells induce CD8+ T-lymphocytes in vitro. Cell. Immunol. 2000, 201, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Toledo, A.; Coleman, L.; Kuhlow, C.J.; Crowley, J.T.; Benach, J.L. The enolase of Borrelia burgdorferi is a plasminogen receptor released in outer membrane vesicles. Infect. Immun. 2012, 80, 359–368. [Google Scholar] [CrossRef]
- Moriarty, T.J.; Norman, M.U.; Colarusso, P.; Bankhead, T.; Kubes, P.; Chaconas, G. Real-time high resolution 3D imaging of the lyme disease spirochete adhering to and escaping from the vasculature of a living host. PLoS Pathog. 2008, 4, e1000090. [Google Scholar] [CrossRef] [PubMed]
- Barbour, A.G. Isolation and cultivation of Lyme disease spirochetes. Yale J. Biol. Med. 1984, 57, 521–525. [Google Scholar] [PubMed]
- Meriläinen, L.; Herranen, A.; Schwarzbach, A.; Gilbert, L. Morphological and biochemical features of Borrelia burgdorferi pleomorphic forms. Microbiology 2015, 161, 516–527. [Google Scholar] [CrossRef]
- Georgilis, K.; Peacocke, M.; Klempner, M.S. Fibroblasts Protect the Lyme Disease Spirochete, Borrelia burgdorferi, from Ceftriaxone In Vitro. J. Infect. Dis. 1992, 166, 440–444. [Google Scholar] [CrossRef]
- Chen, L.; Li, Q.; Wang, J.; Jin, S.; Zheng, H.; Lin, J.; He, F.; Zhang, H.; Ma, S.; Mei, J.; et al. MiR-29b-3p promotes chondrocyte apoptosis and facilitates the occurrence and development of osteoarthritis by targeting PGRN. J. Cell. Mol. Med. 2017, 21, 3347–3359. [Google Scholar] [CrossRef]
- Garon, C.F.; Dorward, D.W.; Corwin, M.D. Structural features of Borrelia burgdorferi—The lyme disease spirochete: Silver staining for nucleic acids. Scanning Microsc. 1989, 3, 109–115. [Google Scholar]
- Huttunen, M.; Waris, M.; Kajander, R.; Hyypia, T.; Marjomaki, V. Coxsackievirus A9 Infects Cells via Nonacidic Multivesicular Bodies. J. Virol. 2014, 88, 5138–5151. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Karvonen, K.; Nykky, J.; Marjomäki, V.; Gilbert, L. Distinctive Evasion Mechanisms to Allow Persistence of Borrelia burgdorferi in Different Human Cell Lines. Front. Microbiol. 2021, 12, 3004. [Google Scholar] [CrossRef] [PubMed]
- Sapi, E.; Kaur, N.; Anyanwu, S.; Luecke, D.F.; Datar, A.; Patel, S.; Rossi, M.; Stricker, R.B. Evaluation of in-vitro antibiotic susceptibility of different morphological forms of Borrelia burgdorferi. Infect. Drug Resist. 2011, 4, 97–113. [Google Scholar] [CrossRef]
- Sharma, B.; Brown, A.V.; Matluck, N.E.; Hu, L.T.; Lewis, K. Borrelia burgdorferi, the causative agent of Lyme disease, forms drug-tolerant persister cells. Antimicrob. Agents Chemother. 2015, 59, 4616–4624. [Google Scholar] [CrossRef]
- Rudenko, N.; Golovchenko, M.; Kybicova, K.; Vancova, M. Metamorphoses of Lyme disease spirochetes: Phenomenon of Borrelia persisters. Parasites Vectors 2019, 12, 237. [Google Scholar] [CrossRef]
- Gankema, H.; Wensink, J.; Guinee, P.A.M.; Jansen, W.H.; Witholt, B. Some characteristics of the outer membrane material released by growing enterotoxigenic Escherichia coli. Infect. Immun. 1980, 29, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Renelli, M.; Matias, V.; Lo, R.Y.; Beveridge, T.J. DNA-containing membrane vesicles of Pseudomonas aeruginosa PAO1 and their genetic transformation potential. Microbiology 2004, 150, 2161–2169. [Google Scholar] [CrossRef]
- Kaparakis-Liaskos, M.; Ferrero, R.L. Immune modulation by bacterial outer membrane vesicles. Nat. Rev. Immunol. 2015, 15, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Schwan, T.G.; Piesman, J.; Golde, W.T.; Dolan, M.C.; Rosa, P.A. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc. Natl. Acad. Sci. USA 1995, 92, 2909–2913. [Google Scholar] [CrossRef]
- Grimm, D.; Tilly, K.; Byram, R.; Stewart, P.E.; Krum, J.G.; Bueschel, D.M.; Schwan, T.G.; Policastro, P.F.; Elias, A.F.; Rosa, P.A. Outer-surface protein C of the Lyme disease spirochete: A protein induced in ticks for infection of mammals. Proc. Natl. Acad. Sci. USA 2004, 101, 3142–3147. [Google Scholar] [CrossRef]
- Carrasco, S.E.; Troxell, B.; Yang, Y.; Brandt, S.L.; Li, H.; Sandusky, G.E.; Condon, K.W.; Serezani, C.H.; Yang, X.F. Outer surface protein OspC is an antiphagocytic factor that protects Borrelia burgdorferi from phagocytosis by macrophages. Infect. Immun. 2015, 83, 4848–4860. [Google Scholar] [CrossRef] [PubMed]
- Akin, E.; McHugh, G.L.; Flavell, R.A.; Fikrig, E.; Steere, A.C. The immunoglobulin (IgG) antibody response to OspA and OspB correlates with severe and prolonged lyme arthritis and the IgG response to P35 correlates with mild and brief arthritis. Infect. Immun. 1999, 67, 173–181. [Google Scholar] [CrossRef]
- Kesty, N.C.; Mason, K.M.; Reedy, M.; Miller, S.E.; Kuehn, M.J. Enterotoxigenic Escherichia coli vesicles target toxin delivery into mammalian cells. EMBO J. 2004, 23, 4538–4549. [Google Scholar] [CrossRef]
- Bauman, S.J.; Kuehn, M.J. Pseudomonas aeruginosa vesicles associate with and are internalized by human lung epithelial cells. BMC Microbiol. 2009, 9, 26. [Google Scholar] [CrossRef]
- Pal, U.; Wang, P.; Bao, F.; Yang, X.; Samanta, S.; Schoen, R.; Wormser, G.P.; Schwartz, I.; Fikrig, E. Borrelia burgdorferi basic membrane proteins A and B participate in the genesis of Lyme arthritis. J. Exp. Med. 2008, 205, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Cardenas-de la Garza, J.A.; De la Cruz-Valadez, E.; Ocampo-Candiani, J.; Welsh, O. Clinical spectrum of Lyme disease. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 201–208. [Google Scholar] [CrossRef]
- Ismail, S.; Hampton, M.B.; Keenan, J.I. Helicobacter pylori outer membrane vesicles modulate proliferation and interleukin-8 production by gastric epithelial cells. Infect. Immun. 2003, 71, 5670–5675. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, K.; Horii, T.; Yamashino, T.; Hashikawa, S.; Barua, S.; Hasegawa, T.; Watanabe, H.; Ohta, M. Production of Shiga toxin by Escherichia coli measured with reference to the membrane vesicle-associated toxins. FEMS Microbiol. Lett. 2006, 192, 139–144. [Google Scholar] [CrossRef]
- Kim, K.M.; Zamaleeva, A.I.; Lee, Y.W.; Ahmed, M.R.; Kim, E.; Lee, H.R.; Pothineni, V.R.; Tao, J.; Rhee, S.; Jayakumar, M.; et al. Characterization of brain dysfunction induced by bacterial lipopeptides that alter neuronal activity and network in rodent brains. J. Neurosci. 2018, 38, 10672–10691. [Google Scholar] [CrossRef] [PubMed]
- Crowley, J.T.; Toledo, A.M.; LaRocca, T.J.; Coleman, J.L.; London, E.; Benach, J.L. Lipid Exchange between Borrelia burgdorferi and Host Cells. PLoS Pathog. 2013, 9, e1003109. [Google Scholar] [CrossRef][Green Version]

| Marker | Molecular Mass (kDa) | Reference |
|---|---|---|
| OspA | 29/31 | [16,24,25,26,28] |
| OspB | 32/34 | [16,24,25,26,28] |
| OspC | 18 | [28] |
| OspD | 28/29 | [24,25,28] |
| Lp6.6/La7/p66 | 8/22/68 | [28] |
| p13 | 19 | [28,32] |
| p39 (BmpA) | 37 | [28,32] |
| Lack of flagella | 37.5/41 | [16,24,26] |
| Other unidentified proteins | 110/50 | [27] |
| 64/30/28/21/19/15/14 | [16] | |
| 23 | [26] | |
| 19.5 | [25] | |
| Enolase | 47 | [32] |
| BSA | 66 | [27] |
| Nucleotide form and Origin | ||
| DNA | Linear/circular | [23] |
| Linear/circular plasmids | [29] | |
| Linear chromosomal | [29] | |
| RNA transcripts | Mostly from plasmids | [30] |
| Other Markers | ||
| Porins | 0.6/12–13 nS | [25] |
| Diameter (chemically produced) | 300–1000 nm | [25] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karvonen, K.; Tammisto, H.; Nykky, J.; Gilbert, L. Borrelia burgdorferi Outer Membrane Vesicles Contain Antigenic Proteins, but Do Not Induce Cell Death in Human Cells. Microorganisms 2022, 10, 212. https://doi.org/10.3390/microorganisms10020212
Karvonen K, Tammisto H, Nykky J, Gilbert L. Borrelia burgdorferi Outer Membrane Vesicles Contain Antigenic Proteins, but Do Not Induce Cell Death in Human Cells. Microorganisms. 2022; 10(2):212. https://doi.org/10.3390/microorganisms10020212
Chicago/Turabian StyleKarvonen, Kati, Hanna Tammisto, Jonna Nykky, and Leona Gilbert. 2022. "Borrelia burgdorferi Outer Membrane Vesicles Contain Antigenic Proteins, but Do Not Induce Cell Death in Human Cells" Microorganisms 10, no. 2: 212. https://doi.org/10.3390/microorganisms10020212
APA StyleKarvonen, K., Tammisto, H., Nykky, J., & Gilbert, L. (2022). Borrelia burgdorferi Outer Membrane Vesicles Contain Antigenic Proteins, but Do Not Induce Cell Death in Human Cells. Microorganisms, 10(2), 212. https://doi.org/10.3390/microorganisms10020212







