Malolactic Fermentation: New Approaches to Old Problems
Abstract
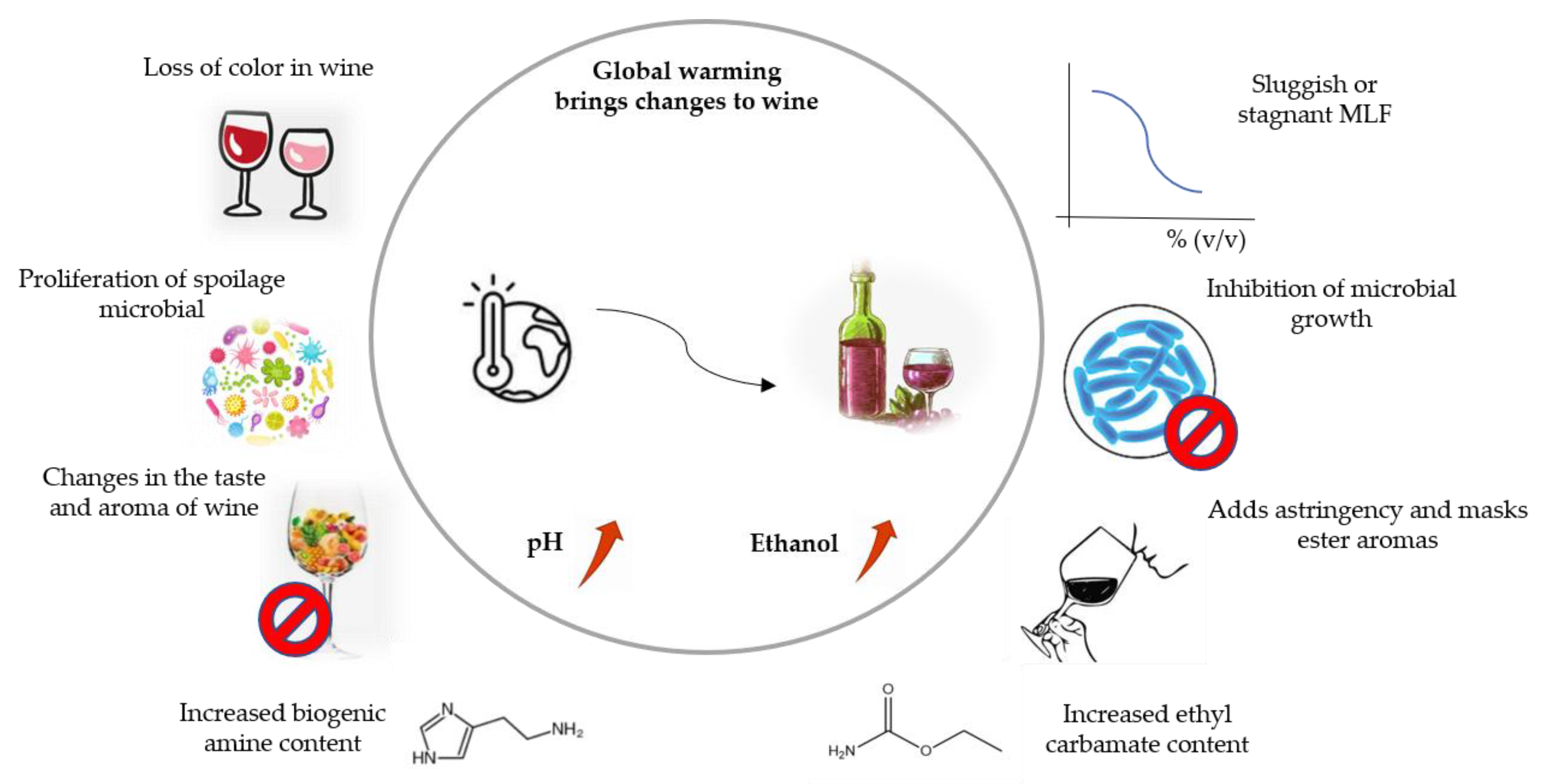
1. Introduction
2. Effects of Different Inoculation Methods on MLF and Wine Quality
2.1. Co-Inoculation vs. Sequential Inoculation
2.2. Mixed Co-Inoculation
2.3. Influence of Bacteria-Yeast Interactions on MLF
3. New MLF Approaches to Handle Environmental Challenges in the Wine Industry
3.1. Selection of MLF New Strains
3.1.1. The Performance of Lactobacillus spp. in MLF
Lactiplantibacillus plantarum
Lentilactobacillus hilgardii
3.1.2. Pediococcus spp. Has MLF Potential Strains
3.2. Research on the Adaptation of Wine Strains to Environmental Variations
3.3. Other Strategies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cappello, M.S.; Zapparoli, G.; Logrieco, A.; Bartowsky, E.J. Linking wine lactic acid bacteria diversity with wine aroma and flavour. Int. J. Food Microbiol. 2017, 243, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Bartowsky, E.J.; Costello, P.J.; Chambers, P.J. Emerging trends in the application of malolactic fermentation. Aust. J. Grape Wine Res. 2015, 21, 663–669. [Google Scholar] [CrossRef]
- Berbegal, C.; Fragasso, M.; Russo, P.; Bimbo, F.; Grieco, F.; Spano, G.; Capozzi, V. Climate Changes and Food Quality: The Potential of Microbial Activities as Mitigating Strategies in the Wine Sector. Fermentation 2019, 5, 85. [Google Scholar] [CrossRef]
- Berbegal, C.; Pena, N.; Russo, P.; Grieco, F.; Pardo, I.; Ferrer, S.; Spano, G.; Capozzi, V. Technological properties of Lactobacillus plantarum strains isolated from grape must fermentation. Food Microbiol. 2016, 57, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Onetto, C.A.; Bordeu, E. Pre-alcoholic fermentation acidification of red grape must using Lactobacillus plantarum. Antonie. Van. Leeuwenhoek 2015, 108, 1469–1475. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, V.; Tufariello, M.; De Simone, N.; Fragasso, M.; Grieco, F. Biodiversity of Oenological Lactic Acid Bacteria: Species and Strain-Dependent Plus/Minus Effects on Wine Quality and Safety. Fermentation 2021, 7, 24. [Google Scholar] [CrossRef]
- Virdis, C.; Sumby, K.; Bartowsky, E.; Jiranek, V. Lactic Acid Bacteria in Wine: Technological Advances and Evaluation of Their Functional Role. Front. Microbiol. 2021, 11, 16. [Google Scholar] [CrossRef]
- La Hens, D.V.; Bravo-Ferrada, B.M.; Delfederico, L.; Caballero, A.C.; Semorile, L.C. Prevalence of Lactobacillus plantarum and Oenococcus oeni during spontaneous malolactic fermentation in Patagonian red wines revealed by polymerase chain reaction-denaturing gradient gel electrophoresis with two targeted genes. Aust. J. Grape Wine Res. 2015, 21, 49–56. [Google Scholar] [CrossRef]
- Mendoza, L.M.; Merin, M.G.; Morata, V.I.; Farias, M.E. Characterization of wines produced by mixed culture of autochthonous yeasts and Oenococcus oeni from the northwest region of Argentina. J. Ind. Microbiol. Biotechnol. 2011, 38, 1777–1785. [Google Scholar] [CrossRef]
- Munoz, V.; Beccaria, B.; Abreo, E. Simultaneous and successive inoculations of yeasts and lactic acid bacteria on the fermentation of an unsulfited Tannat grape must. Braz. J. Microbiol. 2014, 45, 59–66. [Google Scholar] [CrossRef]
- Azzolini, M.; Tosi, E.; Vagnoli, P.; Krieger, S.; Zapparoli, G. Evaluation of technological effects of yeast-bacterial co-inoculation in red table wine. Ital. J. Food Sci. 2010, 22, 257–263. [Google Scholar]
- Bartle, L.; Sumby, K.; Sundstrom, J.; Jiranek, V. The microbial challenge of winemaking: Yeast-bacteria compatibility. FEMS Yeast Res. 2019, 19, 16. [Google Scholar] [CrossRef] [PubMed]
- Krieger-Weber, S.; Heras, J.M.; Suarez, C. Lactobacillus plantarum, a New Biological Tool to Control Malolactic Fermentation: A Review and an Outlook. Beverages 2020, 6, 23. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Rousseaux, S.; Tourdot-Marechal, R.; Sadoudi, M.; Gougeon, R.; Schmitt-Kopplin, P.; Alexandre, H. Wine microbiome: A dynamic world of microbial interactions. Crit. Rev. Food Sci. Nutr. 2017, 57, 856–873. [Google Scholar] [CrossRef] [PubMed]
- Guzzon, R.; Villega, T.R.; Pedron, M.; Malacarne, M.; Nicolini, G.; Larcher, R. Simultaneous yeast-bacteria inoculum. A feasible solution for the management of oenological fermentation in red must with low nitrogen content. Ann. Microbiol. 2013, 63, 805–808. [Google Scholar] [CrossRef]
- Du Plessis, H.W.; du Toit, M.; Hoff, J.W.; Hart, R.S.; Ndimba, B.K.; Jolly, N.P. Characterisation of Non-Saccharomyces Yeasts Using Different Methodologies and Evaluation of their Compatibility with Malolactic Fermentation. S. Afr. J. Enol. Vitic. 2017, 38, 46–63. [Google Scholar] [CrossRef]
- Canas, P.M.I.; Perez-Martin, F.; Romero, E.G.; Prieto, S.S.; Herreros, M.D.P. Influence of inoculation time of an autochthonous selected malolactic bacterium on volatile and sensory profile of Tempranillo and Merlot wines. Int. J. Food Microbiol. 2012, 156, 245–254. [Google Scholar] [CrossRef]
- Berbegal, C.; Garofalo, C.; Russo, P.; Pati, S.; Capozzi, V.; Spano, G. Use of Autochthonous Yeasts and Bacteria in Order to Control Brettanomyces bruxellensis in Wine. Fermentation 2017, 3, 65. [Google Scholar] [CrossRef]
- Du Plessis, H.; du Toit, M.; Nieuwoudt, H.; van der Rijst, M.; Kidd, M.; Jolly, N. Effect of Saccharomyces, Non-Saccharomyces Yeasts and Malolactic Fermentation Strategies on Fermentation Kinetics and Flavor of Shiraz Wines. Fermentation 2017, 3, 64. [Google Scholar] [CrossRef]
- Wells, A.; Osborne, J.P. Impact of acetaldehyde and pyruvic acid-bound sulphur dioxide on wine lactic acid bacteria. Lett. Appl. Microbiol. 2012, 54, 187–194. [Google Scholar] [CrossRef]
- Sumby, K.M.; Grbin, P.R.; Jiranek, V. Implications of new research and technologies for malolactic fermentation in wine. Appl. Microbiol. Biotechnol. 2014, 98, 8111–8132. [Google Scholar] [CrossRef] [PubMed]
- Ferrando, N.; Araque, I.; Ortis, A.; Thornes, G.; Bautista-Gallego, J.; Bordons, A.; Reguant, C. Evaluating the effect of using non-Saccharomyces on Oenococcus oeni and wine malolactic fermentation. Food Res. Int. 2020, 138, 109779. [Google Scholar] [CrossRef] [PubMed]
- Lasik-Kurdys, M.; Majcher, M.; Nowak, J. Effects of Different Techniques of Malolactic Fermentation Induction on Diacetyl Metabolism and Biosynthesis of Selected Aromatic Esters in Cool-Climate Grape Wines. Molecules 2018, 23, 2549. [Google Scholar] [CrossRef]
- Abrahamse, C.E.; Bartowsky, E.J. Timing of malolactic fermentation inoculation in Shiraz grape must and wine: Influence on chemical composition. World J. Microbiol. Biotechnol. 2012, 28, 255–265. [Google Scholar] [CrossRef]
- Antalick, G.; Perello, M.C.; de Revel, G. Co-inoculation with Yeast and LAB Under Winery Conditions: Modification of the Aromatic Profile of Merlot Wines. S. Afr. J. Enol. Vitic. 2013, 34, 223–232. [Google Scholar] [CrossRef]
- Diez-Ozaeta, I.; Lavilla, M.; Amarita, F. Effect of inoculation strategy with autochthonous Oenococcus oeni strains on aroma development in Rioja Alavesa Tempranillo wines. LWT Food Sci. Technol. 2022, 162, 113399. [Google Scholar] [CrossRef]
- Canas, P.M.I.; Romero, E.G.; Alonso, S.G.; Herreros, M. Changes in the aromatic composition of Tempranillo wines during spontaneous malolactic fermentation. J. Food Compos. Anal. 2008, 21, 724–730. [Google Scholar] [CrossRef]
- Devi, A.; Archana, K.M.; Bhavya, P.K.; Anu-Appaiah, K.A. Non-anthocyanin polyphenolic transformation by native yeast and bacteria co-inoculation strategy during vinification. J. Sci. Food Agric. 2018, 98, 1162–1170. [Google Scholar] [CrossRef]
- Devi, A.; Anu-Appaiah, K.A. Yeast-Bacterial Interactions during Malolactic Inoculations Affecting Anthocyanin Adsorption and Content in Shiraz Wine. Am. J. Enol. Vitic. 2020, 71, 105–113. [Google Scholar] [CrossRef]
- Guzzon, R.; Moser, S.; Davide, S.; Villegas, T.R.; Malacarne, M.; Larcher, R.; Nardi, T.; Vagnoli, P.; Krieger-Weber, S. Exploitation of Simultaneous Alcoholic and Malolactic Fermentation of Incrocio Manzoni, a Traditional Italian White Wine. S. Afr. J. Enol. Vitic. 2016, 37, 124–131. [Google Scholar] [CrossRef][Green Version]
- Smit, A.Y.; Engelbrecht, L.; du Toit, M. Managing your wine fermentation to reduce the risk of biogenic amine formation. Front. Microbiol. 2012, 3, 76. [Google Scholar] [CrossRef] [PubMed]
- Balmaseda, A.; Rozes, N.; Leal, M.A.; Bordons, A.; Reguant, C. Impact of changes in wine composition produced by non-Saccharomyces on malolactic fermentation. Int. J. Food Microbiol. 2021, 337, 108954. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, Y.; Liu, L.; Zheng, M.; Feng, Z.; Hu, K.; Tao, Y. Effects of inoculation timing and mixed fermentation with Pichia fermentans on Oenococcus oeni viability, fermentation duration and aroma production during wine malolactic fermentation. Food Res. Int. 2022, 159, 111604. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Li, S.Y.; Zhao, H.F.; Gu, P.; Chen, Y.Q.; Zhang, B.L.; Zhu, B.Q. Acetaldehyde released by Lactobacillus plantarum enhances accumulation of pyranoanthocyanins in wine during malolactic fermentation. Food Res. Int. 2018, 108, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Devi, A.; Anu-Appaiah, K.A. Mixed malolactic co-culture (Lactobacillus plantarum and Oenococcus oeni) with compatible Saccharomyces influences the polyphenolic, volatile and sensory profile of Shiraz wine. LWT-Food Sci. Technol. 2021, 135, 110246. [Google Scholar] [CrossRef]
- Nardi, T.; Panero, L.; Petrozziello, M.; Guaita, M.; Tsolakis, C.; Cassino, C.; Vagnoli, P.; Bosso, A. Managing wine quality using Torulaspora delbrueckii and Oenococcus oeni starters in mixed fermentations of a red Barbera wine. Eur. Food Res. Technol. 2019, 245, 293–307. [Google Scholar] [CrossRef]
- Tufariello, M.; Capozzi, V.; Spano, G.; Cantele, G.; Venerito, P.; Mita, G.; Grieco, F. Effect of Co-Inoculation of Candida zemplinina, Saccharomyces cerevisiae and Lactobacillus plantarum for the Industrial Production of Negroamaro Wine in Apulia (Southern Italy). Microorganisms 2020, 8, 726. [Google Scholar] [CrossRef]
- Tristezza, M.; di Feo, L.; Tufariello, M.; Grieco, F.; Capozzi, V.; Spano, G.; Mita, G.; Grieco, F. Simultaneous inoculation of yeasts and lactic acid bacteria: Effects on fermentation dynamics and chemical composition of Negroamaro wine. LWT-Food Sci. Technol. 2016, 66, 406–412. [Google Scholar] [CrossRef]
- Russo, P.; Englezos, V.; Capozzi, V.; Pollon, M.; Segade, S.R.; Rantsiou, K.; Spano, G.; Cocolin, L. Effect of mixed fermentations with Starmerella bacillaris and Saccharomyces cerevisiae on management of malolactic fermentation. Food Res. Int. 2020, 134, 109246. [Google Scholar] [CrossRef] [PubMed]
- Englezos, V.; Jolly, N.P.; Di Gianvito, P.; Rantsiou, K.; Cocolin, L. Microbial interactions in winemaking: Ecological aspects and effect on wine quality. Trends Food Sci. Technol. 2022, 127, 99–113. [Google Scholar] [CrossRef]
- Du Plessis, H.; Du Toit, M.; Nieuwoudt, H.; Van der Rijst, M.; Hoff, J.; Jolly, N. Modulation of Wine Flavor using Hanseniaspora uvarum in Combination with Different Saccharomyces cerevisiae, Lactic Acid Bacteria Strains and Malolactic Fermentation Strategies. Fermentation 2019, 5, 64. [Google Scholar] [CrossRef]
- Berbegal, C.; Borruso, L.; Fragasso, M.; Tufariello, M.; Russo, P.; Brusetti, L.; Spano, G.; Capozzi, V. A Metagenomic-Based Approach for the Characterization of Bacterial Diversity Associated with Spontaneous Malolactic Fermentations in Wine. Int. J. Mol. Sci. 2019, 20, 3980. [Google Scholar] [CrossRef] [PubMed]
- Englezos, V.; Cachon, D.C.; Rantsiou, K.; Blanco, P.; Petrozziello, M.; Pollon, M.; Giacosa, S.; Segade, S.R.; Rolle, L.; Cocolin, L. Effect of mixed species alcoholic fermentation on growth and malolactic activity of lactic acid bacteria. Appl. Microbiol. Biotechnol. 2019, 103, 7687–7702. [Google Scholar] [CrossRef]
- Snyder, E.C.; Jiranek, V.; Hranilovic, A. Impact of Lachancea thermotolerans strain and lactic acid concentration on Oenococcus oeni and malolactic fermentation in wine. OENO One 2021, 55, 365–380. [Google Scholar] [CrossRef]
- Iorizzo, M.; Testa, B.; Lombardi, S.J.; Garcia-Ruiz, A.; Munoz-Gonzalez, C.; Bartolome, B.; Moreno-Arribas, M.V. Selection and technological potential of Lactobacillus plantarum bacteria suitable for wine malolactic fermentation and grape aroma release. LWT-Food Sci. Technol. 2016, 73, 557–566. [Google Scholar] [CrossRef]
- Brizuela, N.; Tymczyszyn, E.E.; Semorile, L.C.; La Hens, D.V.; Delfederico, L.; Hollmann, A.; Bravo-Ferrada, B. Lactobacillus plantarum as a malolactic starter culture in winemaking: A new (old) player? Electron. J. Biotechnol. 2019, 38, 10–18. [Google Scholar] [CrossRef]
- Vicente, J.; Baran, Y.; Navascues, E.; Santos, A.; Calderon, F.; Marquina, D.; Rauhut, D.; Benito, S. Biological management of acidity in wine industry: A review. Int. J. Food Microbiol. 2022, 375, 109726. [Google Scholar] [CrossRef]
- Sun, J.; Ge, Y.; Gu, X.; Li, R.; Ma, W.; Jin, G. Identification and Characterization of Malolactic Bacteria Isolated from the Eastern Foothills of Helan Mountain in China. Foods 2022, 11, 2455. [Google Scholar] [CrossRef]
- Mazzoli, R.; Lamberti, C.; Coisson, J.D.; Purrotti, M.; Arlorio, M.; Giuffrida, M.G.; Giunta, C.; Pessione, E. Influence of ethanol, malate and arginine on histamine production of Lactobacillus hilgardii isolated from an Italian red wine. Amino Acids 2009, 36, 81–89. [Google Scholar] [CrossRef]
- Zhuravleva, D.E.; Iskhakova, Z.I.; Ozhegov, G.D.; Gogoleva, N.E.; Khusnutdinova, D.R.; Shagimardanova, E.I.; Forchhammer, K.; Kayumov, A.R. Complete Genome Sequence of Lactobacillus hilgardii LMG 7934, Carrying the Gene Encoding for the Novel PII-Like Protein PotN. Curr. Microbiol. 2020, 77, 3538–3545. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Jiranek, V.; Hayes, A.M.; Grbin, P.R. Isolation and Characterization of High-Ethanol-Tolerance Lactic Acid Bacteria from Australian Wine. Foods 2022, 11, 1231. [Google Scholar] [CrossRef] [PubMed]
- Du Toit, M.; Engelbrecht, L.; Lerm, E.; Krieger-Weber, S. Lactobacillus: The Next Generation of Malolactic Fermentation Starter Cultures-an Overview. Food Bioprocess Technol. 2011, 4, 876–906. [Google Scholar] [CrossRef]
- Coulon, J.; Houles, A.; Dimopoulou, M.; Maupeu, J.; Dols-Lafargue, M. Lysozyme resistance of the ropy strain Pediococcus parvulus IOEB 8801 is correlated with beta-glucan accumulation around the cell. Int. J. Food Microbiol. 2012, 159, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Escamilla, M.L.; Valdes, S.E.; Soriano, J.; Tomasini, A. Effect of some nutritional and environmental parameters on the production of diacetyl and on starch consumption by Pediococcus pentosaceus and Lactobacillus acidophilus in submerged cultures. J. Appl. Microbiol. 2000, 88, 142–153. [Google Scholar] [CrossRef]
- Coton, M.; Romano, A.; Spano, G.; Ziegler, K.; Vetrana, C.; Desmarais, C.; Lonvaud-Funel, A.; Lucas, P.; Coton, E. Occurrence of biogenic amine-forming lactic acid bacteria in wine and cider. Food Microbiol. 2010, 27, 1078–1085. [Google Scholar] [CrossRef]
- Wade, M.E.; Strickland, M.T.; Osborne, J.P.; Edwards, C.G. Role of Pediococcus in winemaking. Aust. J. Grape Wine Res. 2019, 25, 7–24. [Google Scholar] [CrossRef]
- Strickland, M.T.; Schopp, L.M.; Edwards, C.G.; Osborne, J.P. Impact of Pediococcus spp. on Pinot noir Wine Quality and Growth of Brettanomyces. Am. J. Enol. Vitic. 2016, 67, 188–198. [Google Scholar] [CrossRef]
- Brizuela, N.S.; Bravo-Ferrada, B.M.; Pozo-Bayon, M.A.; Pozo-Bayon, M.A.; Tymczyszyn, E.E. Changes in the volatile profile of Pinot noir wines caused by Patagonian Lactobacillus plantarum and Oenococcus oeni strains. Food Res. Int. 2018, 106, 22–28. [Google Scholar] [CrossRef]
- Wang, S.Y.; Zhu, H.Z.; Lan, Y.B.; Liu, R.J.; Liu, Y.R.; Zhang, B.L.; Zhu, B.Q. Modifications of Phenolic Compounds, Biogenic Amines, and Volatile Compounds in Cabernet Gernishct Wine through Malolactic Fermentation by Lactobacillus plantarum and Oenococcus oeni. Fermentation 2020, 6, 15. [Google Scholar] [CrossRef]
- De Orduna, R.M. Climate change associated effects on grape and wine quality and production. Food Res. Int. 2010, 43, 1844–1855. [Google Scholar] [CrossRef]
- Lucio, O.; Pardo, I.; Krieger-Weber, S.; Heras, J.M.; Ferrer, S. Selection of Lactobacillus strains to induce biological acidification in low acidity wines. LWT-Food Sci. Technol. 2016, 73, 334–341. [Google Scholar] [CrossRef]
- Lucio, O.; Pardo, I.; Heras, J.M.; Krieger, S.; Ferrer, S. Influence of yeast strains on managing wine acidity using Lactobacillus plantarum. Food Control. 2018, 92, 471–478. [Google Scholar] [CrossRef]
- Urbina, A.; Calderon, F.; Benito, S. The Combined Use of Lachancea thermotolerans and Lactiplantibacillus plantarum (former Lactobacillus plantarum) in Wine Technology. Foods 2021, 10, 1356. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zhang, W.; Wu, Y.; Shi, X.; Yang, X.; Song, Y.; Qin, Y.; Ye, D.; Liu, Y. Pilot-Scale Vinification of Cabernet Sauvignon Using Combined Lactiplantibacillus plantarum and Saccharomyces cerevisiae to Achieve Wine Acidification. Foods 2022, 11, 2511. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, V.; Russo, P.; Ladero, V.; Fernandez, M.; Fiocco, D.; Alvarez, M.A.; Grieco, F.; Spano, G. Biogenic amines degradation by Lactobacillus plantarum: Toward a potential application in wine. Front. Microbiol. 2012, 3, 122. [Google Scholar] [CrossRef]
- Sun, S.; Jiang, D.; Fan, M.; Li, H.; Jin, C.; Liu, W. Selection of a versatile Lactobacillus plantarum for wine production and identification and preliminary characterisation of a novel histamine-degrading enzyme. Int. J. Food Sci. Tech. 2020, 55, 2608–2618. [Google Scholar] [CrossRef]
- Jiang, D.; Li, H.; Sun, S. Verification of a novel glyceraldehyde-3-phosphate dehydrogenase capable of histamine degradation and its preliminary application in wine production. Food Sci. Biotechnol. 2020, 29, 1719–1726. [Google Scholar] [CrossRef]
- Lerm, E.; Engelbrecht, L.; du Toit, M. Selection and Characterisation of Oenococcus oeni and Lactobacillus plantarum South African Wine Isolates for Use as Malolactic Fermentation Starter Cultures. S. Afr. J. Enol. Vitic. 2011, 32, 280–295. [Google Scholar] [CrossRef]
- Bravo-Ferrada, B.M.; Hollmann, A.; Delfederico, L.; La Hens, D.V.; Caballero, A.; Semorile, L. Patagonian red wines: Selection of Lactobacillus plantarum isolates as potential starter cultures for malolactic fermentation. World J. Microbiol. Biotechnol. 2013, 29, 1537–1549. [Google Scholar] [CrossRef]
- Brizuela, N.S.; Bravo-Ferrada, B.M.; la Hens, D.V.; Hollmann, A.; Delfederico, L.; Caballero, A.; Tymczyszyn, E.E.; Semorile, L. Comparative vinification assays with selected Patagonian strains of Oenococcus oeni and Lactobacillus plantarum. LWT Food Sci. Technol. 2017, 77, 348–355. [Google Scholar] [CrossRef]
- Mtshali, P.S.; Divol, B.; van Rensburg, P.; du Toit, M. Genetic screening of wine-related enzymes in Lactobacillus species isolated from South African wines. J. Appl. Microbiol. 2010, 108, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Takase, H.; Sasaki, K.; Kiyomichi, D.; Kobayashi, H.; Matsuo, H.; Takata, R. Impact of Lactobacillus plantarum on thiolprecursor biotransformation leading to production of 3-sulfanylhexan-1-ol. Food Chem. 2018, 259, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Araque, I.; Reguant, C.; Rozes, N.; Bordons, A. Influence of wine-like conditions on arginine utilization by lactic acid bacteria. Int. Microbiol. 2011, 14, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Seijas, J.; Garcia-Fraga, B.; da Silva, A.F.; Zas-Garcia, X.; Lois, L.C.; Gago-Martinez, A.; Leao-Martins, J.M.; Sieiro, C. Evaluation of Malolactic Bacteria Associated with Wines from Albarino Variety as Potential Starters: Screening for Quality and Safety. Foods 2020, 9, 99. [Google Scholar] [CrossRef] [PubMed]
- Aredes Fernandez, P.A.; Farias, M.E.; de Nadra, M.C. Interaction between Oenococcus oeni and Lactobacillus hilgardii isolated from wine. Modification of available nitrogen and biogenic amine production. Biotechnol. Lett. 2010, 32, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Gustaw, K.; Koper, P.; Polak-Berecka, M.; Rachwal, K.; Skrzypczak, K.; Wasko, A. Genome and Pangenome Analysis of Lactobacillus hilgardii FLUB-A New Strain Isolated from Mead. Int. J. Mol. Sci. 2021, 22, 3780. [Google Scholar] [CrossRef]
- Sumby, K.M.; Grbin, P.R.; Jiranek, V. Characterization of EstCOo8 and EstC34, intracellular esterases, from the wine-as-sociated lactic acid bacteria Oenococcus oeni and Lactobacillus hilgardii. J. Appl. Microbiol. 2013, 114, 413–422. [Google Scholar] [CrossRef]
- Zhu, F.M.; Du, B.; Li, J. Aroma enhancement and enzymolysis regulation of grape wine using β-glycosidase. Food Sci. Nutr. 2014, 2, 139–145. [Google Scholar] [CrossRef]
- Landete, J.M.; Ferrer, S.; Pardo, I. Which lactic acid bacteria are responsible for histamine production in wine? J. Appl. Microbiol. 2005, 99, 580–586. [Google Scholar] [CrossRef]
- Llauberes, R.M.; Richard, B.; Lonvaud, A.; Dubourdieu, D.; Fournet, B. Structure of an exocellular β-D-glucan from Pediococcus sp., a wine lactic bacteria. Carbohydr. Res. 1990, 203, 103–107. [Google Scholar] [CrossRef]
- Walling, E.; Gindreau, E.; Lonvaud-Funel, A. A putative glucan synthase gene dos detected in exopolysaccharide-producing Pediococcus damnosus and Oenococcus oeni strains isolated from wine and cider. Int. J. Food Microbiol. 2005, 98, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Bauer, R.; Cowan, D.A.; Crouch, A. Acrolein in Wine: Importance of 3-Hydroxypropionaldehyde and Derivatives in Production and Detection. J. Agric. Food Chem. 2010, 58, 3243–3250. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.G.; Peterson, J.C. Sorbent Extraction and Analysis of Volatile Metabolites Synthesized by Lactic Acid Bacteria in a Synthetic Medium. J. Food. Sci. 1994, 59, 192–196. [Google Scholar] [CrossRef]
- Garcia-Ruiz, A.; Gonzalez-Rompinelli, E.M.; Bartolome, B.; Moreno-Arribas, M.V. Potential of wine-associated lactic acid bacteria to degrade biogenic amines. Int. J. Food Microbiol. 2011, 148, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Callejon, S.; Sendra, R.; Ferrer, S.; Pardo, I. Identification of a novel enzymatic activity from lactic acid bacteria able to degrade biogenic amines in wine. Appl. Microbiol. Biotechnol. 2014, 98, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Juega, M.; Costantini, A.; Bonello, F.; Cravero, M.C.; Martinez-Rodriguez, A.J.; Carrascosa, A.V.; Garcia-Moruno, E. Effect of malolactic fermentation by Pediococcus damnosus on the composition and sensory profile of Albarino and Caino white wines. J. Appl. Microbiol. 2014, 116, 586–595. [Google Scholar] [CrossRef]
- Kaur, B.; Kumar, B.; Kaur, G.; Chakraborty, D.; Kaur, K. Application of recombinant Pediococcus acidilactici BD16 (fcs(+)/ech(+)) in malolactic fermentation. Appl. Microbiol. Biotechnol. 2015, 99, 3015–3028. [Google Scholar] [CrossRef]
- Li, N.; Duan, J.Y.; Gao, D.W.; Luo, J.H.; Zheng, R.Y.; Bian, Y.H.; Zhang, X.W.; Ji, B.S. Mutation and selection of Oenococcus oeni for controlling wine malolactic fermentation. Eur. Food Res. Technol. 2015, 240, 93–100. [Google Scholar] [CrossRef]
- Betteridge, A.; Grbin, P.; Jiranek, V. Improving Oenococcus Oeni to overcome challenges of wine malolactic fermentation. Trends Biotechnol. 2015, 33, 547–553. [Google Scholar] [CrossRef]
- Betteridge, A.L.; Sumby, K.M.; Sundstrom, J.F.; Grbin, P.R.; Jiranek, V. Application of directed evolution to develop ethanol tolerant Oenococcus oeni for more efficient malolactic fermentation. Appl. Microbiol. Biotechnol. 2018, 102, 921–932. [Google Scholar] [CrossRef]
- Jiang, J.; Sumby, K.M.; Sundstrom, J.F.; Grbin, P.R.; Jiranek, V. Directed evolution of Oenococcus oeni strains for more efficient malolactic fermentation in a multi-stressor wine environment. Food Microbiol. 2018, 73, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Pophaly, S.D.; Singh, R.; Pophaly, S.D.; Kaushik, J.K.; Tomar, S.K. Current status and emerging role of glutathione in food grade lactic acid bacteria. Microb. Cell Fact. 2012, 11, 114. [Google Scholar] [CrossRef] [PubMed]
- Cecconi, D.; Milli, A.; Rinalducci, S.; Zolla, L.; Zapparoli, G. Proteomic analysis of Oenococcus oeni freeze-dried culture to assess the importance of cell acclimation to conduct malolactic fermentation in wine. Electrophoresis 2009, 30, 2988–2995. [Google Scholar] [CrossRef] [PubMed]
- Jara, C.; Romero, J. Genome Sequences of Three Oenococcus oeni Strains Isolated from Maipo Valley, Chile. Genome Announc. 2015, 3, e00866-15. [Google Scholar] [CrossRef] [PubMed]
- Margalef-Catala, M.; Araque, I.; Bordons, A.; Reguant, C. Genetic and transcriptional study of glutathione metabolism in Oenococcus oeni. Int. J. Food Microbiol. 2017, 242, 61–69. [Google Scholar] [CrossRef]
- Jiang, S. Screening of Excellent Anti Oxidative Oenococcus oeni Strains and Research on Promoting the Quality of Wine. Ph.D. Thesis, Northwest A & F University, Yangling, China, 2016. [Google Scholar]
- Di Martino, C.; Testa, B.; Letizia, F.; Iorizzo, M.; Lombardi, S.J.; Ianiro, M.; Di Renzo, M.; Strollo, D.; Coppola, R. Effect of exogenous proline on the ethanolic tolerance and malolactic performance of Oenococcus oeni. J. Food Sci. Technol.-Mysore 2020, 57, 3973–3979. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Arenzana, L.; Portu, J.; Lopez, R.; Lopez, N.; Santamaria, P.; Garde-Cerdan, T.; Lopez-Alfaro, I. Inactivation of wine-associated microbiota by continuous pulsed electric field treatments. Innov. Food Sci. Emerg. Technol. 2015, 29, 187–192. [Google Scholar] [CrossRef]
- Puertolas, E.; Lopez, N.; Condon, S.; Alvarez, I.; Raso, J. Potential applications of PEF to improve red wine quality. Trends Food Sci. Technol. 2010, 21, 247–255. [Google Scholar] [CrossRef]
- Gonzalez-Arenzana, L.; Lopez-Alfaro, I.; Garde-Cerdan, T.; Portu, J.; Lopez, R.; Santamaria, P. Microbial inactivation and MLF performances of Tempranillo Rioja wines treated with PEF after alcoholic fermentation. Int. J. Food Microbiol. 2018, 269, 19–26. [Google Scholar] [CrossRef]
- Gonzalez-Arenzana, L.; Portu, J.; Lopez, N.; Santamaria, P.; Gutierrez, A.R.; Lopez, R.; Lopez-Alfaro, I. Pulsed Electric Field treatment after malolactic fermentation of Tempranillo Rioja wines: Influence on microbial, physicochemical and sensorial quality. Innov. Food Sci. Emerg. Technol. 2019, 51, 57–63. [Google Scholar] [CrossRef]
- Genisheva, Z.; Mussatto, S.I.; Oliveira, J.M.; Teixeira, J.A. Malolactic fermentation of wines with immobilised lactic acid bacteria Influence of concentration, type of support material and storage conditions. Food Chem. 2013, 138, 1510–1514. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Nogales, J.M.; Vila-Crespo, J.; Fernandez-Fernandez, E. Immobilization of Oenococcus oeni in lentikats (R) to develop malolactic fermentation in wines. Biotechnol. Prog. 2013, 29, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Simo, G.; Vila-Crespo, J.; Fernandez-Fernandez, E.; Ruiperez, V.; Rodriguez-Nogales, J.M. Highly Efficient Malolactic Fermentation of Red Wine Using Encapsulated Bacteria in a Robust Biocomposite of Silica-Alginate. J. Agric. Food Chem. 2017, 65, 5188–5197. [Google Scholar] [CrossRef]
- Di Gennaro, S.F.; Matese, A.; Primicerio, J.; Genesio, L.; Sabatini, F.; Di Blasi, S.; Vaccari, F.P. Wireless real-time monitoring of malolactic fermentation in wine barrels: The Wireless Sensor Bung system. Aust. J. Grape Wine Res. 2013, 19, 20–24. [Google Scholar] [CrossRef]
- Ojha, K.S.; Mason, T.J.; O’Donnell, C.P.; Kerry, J.P.; Tiwari, B.K. Ultrasound technology for food fermentation applications. Ultrason. Sonochem. 2017, 34, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Gimenez-Gomez, P.; Gutierrez-Capitan, M.; Capdevila, F.; Puig-Pujol, A.; Fernandez-Sanchez, C.; Jimenez-Jorquera, C. Robust L-malate bienzymatic biosensor to enable the on-site monitoring of malolactic fermentation of red wines. Anal. Chim. Acta 2017, 954, 105–113. [Google Scholar] [CrossRef] [PubMed]
| Method | Sequential Inoculation | Co-Inoculation |
|---|---|---|
| Positive effects | Yeast produces nutrients available for LAB [12] Avoid the production of acetic acid and D-lactic acid [13] Prevents adverse interactions between LAB and yeast [14] | Improve the efficiency of MLF [15] Enhance wine aroma complexity [16] Reduce the concentration of BAs in wine [17] Reduces color loss as well as bitterness and astringency perception in wines [12] Prevents the growth of Brettanomyces and produces phenolic off-odors [18] Metabolites produced by yeast strains promote LAB growth and improve MLF performance (Strain dependence) [19] |
| Negative effects | Prolong or delay of MLF [20] Increase the risk of microbial spoilage [7] Produces undesirable compounds such as BAs and Ethyl carbamate (EC) [7] | Inhibition of MLF [21] Sluggish or stagnant AF [14] Increased acetic acid content, which adversely affects the quality of the wine [22] |
| Strain | Past | Present |
|---|---|---|
| L. plantarum | Causes wine spoilage, including mousy off-flavours and excess acetic acid [2] | BAs degradation [45] Involved in improving wine color [34] With the most diverse range of plantaricins [46] Induced biological acidification of wine [47] More complex enzyme profile (Glycosidase/Protease/ Esterase/Lipase/Citrate lyase) [48] |
| L. hilgardii | Can produce undesirable me-tabolites such as BAs and EC (strain dependence) [49] | Have a wider range of environmental adapt-ability [50] MLF starter cultures with high tolerance to ethanol [51] Can be used as a source of new esterases to improve wine aroma (strain dependence) [52] |
| Pediococcus spp. | Causes wine spoilage, including ropiness and high level of diacetyl [53,54] Produce BAs and EC (strain dependence) [55] | Provide enzymes that degrade BAs [6] Induction of MLF in high pH red and white wines [56] May offer the opportunity to develop novel wine styles [57] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, J.; Wang, L.; Sun, J.; Ju, N.; Jin, G. Malolactic Fermentation: New Approaches to Old Problems. Microorganisms 2022, 10, 2363. https://doi.org/10.3390/microorganisms10122363
Fu J, Wang L, Sun J, Ju N, Jin G. Malolactic Fermentation: New Approaches to Old Problems. Microorganisms. 2022; 10(12):2363. https://doi.org/10.3390/microorganisms10122363
Chicago/Turabian StyleFu, Junwei, Ling Wang, Jingxian Sun, Ning Ju, and Gang Jin. 2022. "Malolactic Fermentation: New Approaches to Old Problems" Microorganisms 10, no. 12: 2363. https://doi.org/10.3390/microorganisms10122363
APA StyleFu, J., Wang, L., Sun, J., Ju, N., & Jin, G. (2022). Malolactic Fermentation: New Approaches to Old Problems. Microorganisms, 10(12), 2363. https://doi.org/10.3390/microorganisms10122363






