Phosphorus-Solubilizing Capacity of Mortierella Species Isolated from Rhizosphere Soil of a Poplar Plantation
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Sampling and Isolation of Phosphate-Solubilizing Fungi
2.2. Morphological Characterization
2.3. Sequencing and Phylogenetic Analysis
2.4. Determination of Soluble Phosphorus under Different Culture Conditions
2.5. Determination of Organic Acids Using HPLC
2.6. Phosphatase Activity Assays
2.7. Statistical Analysis
3. Results
3.1. Phylogenetic Analyses
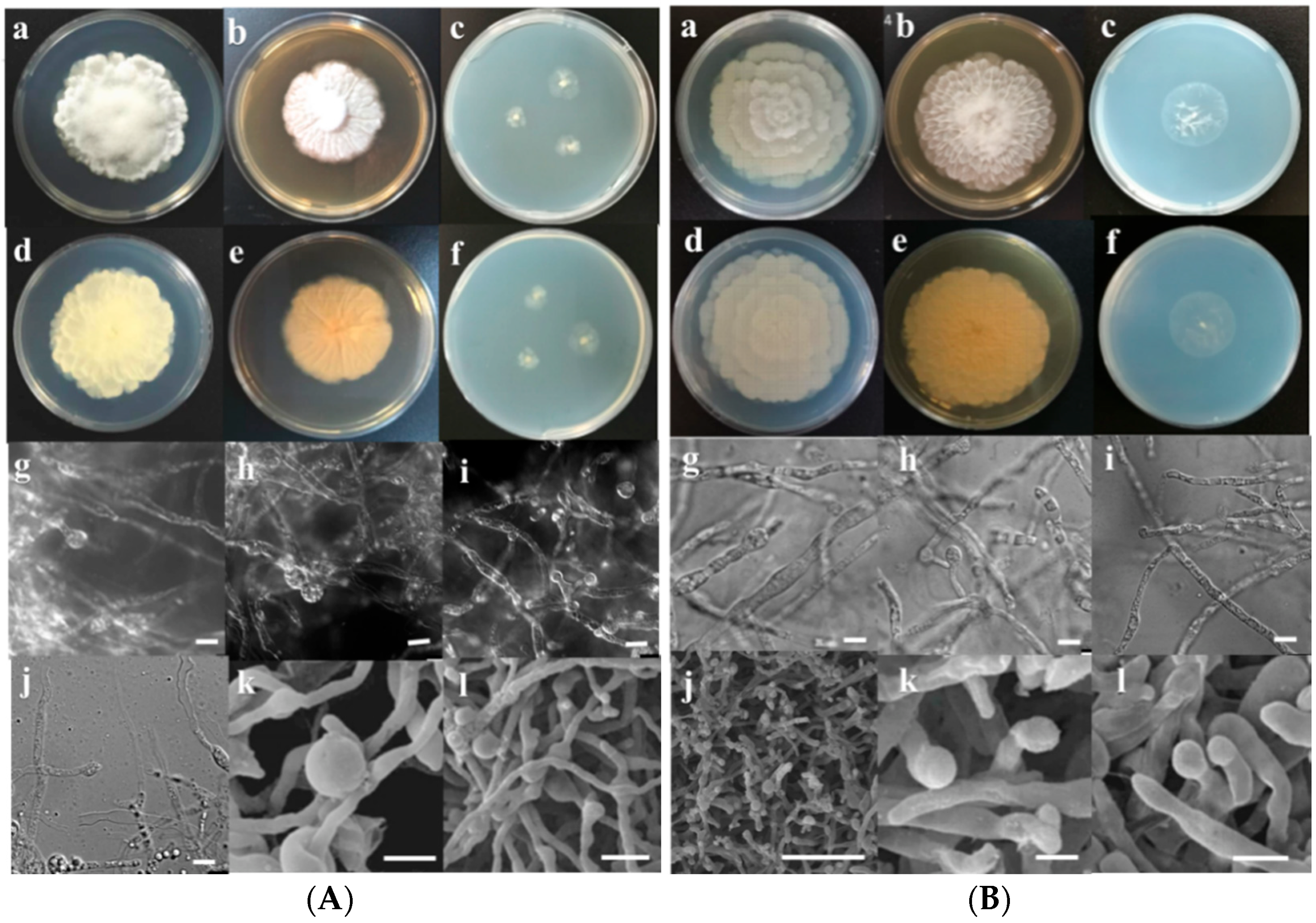
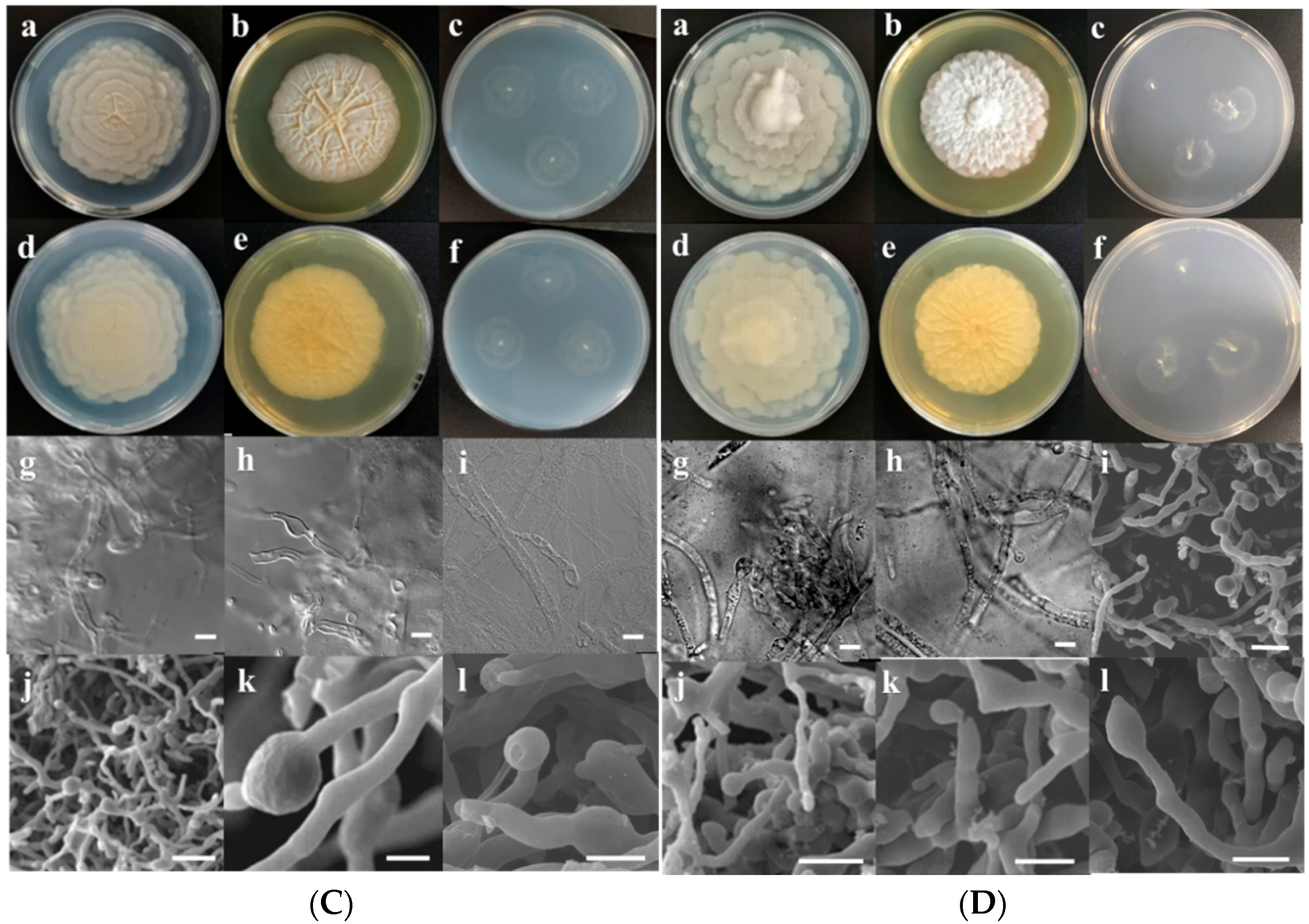
3.2. Morphological Characterization
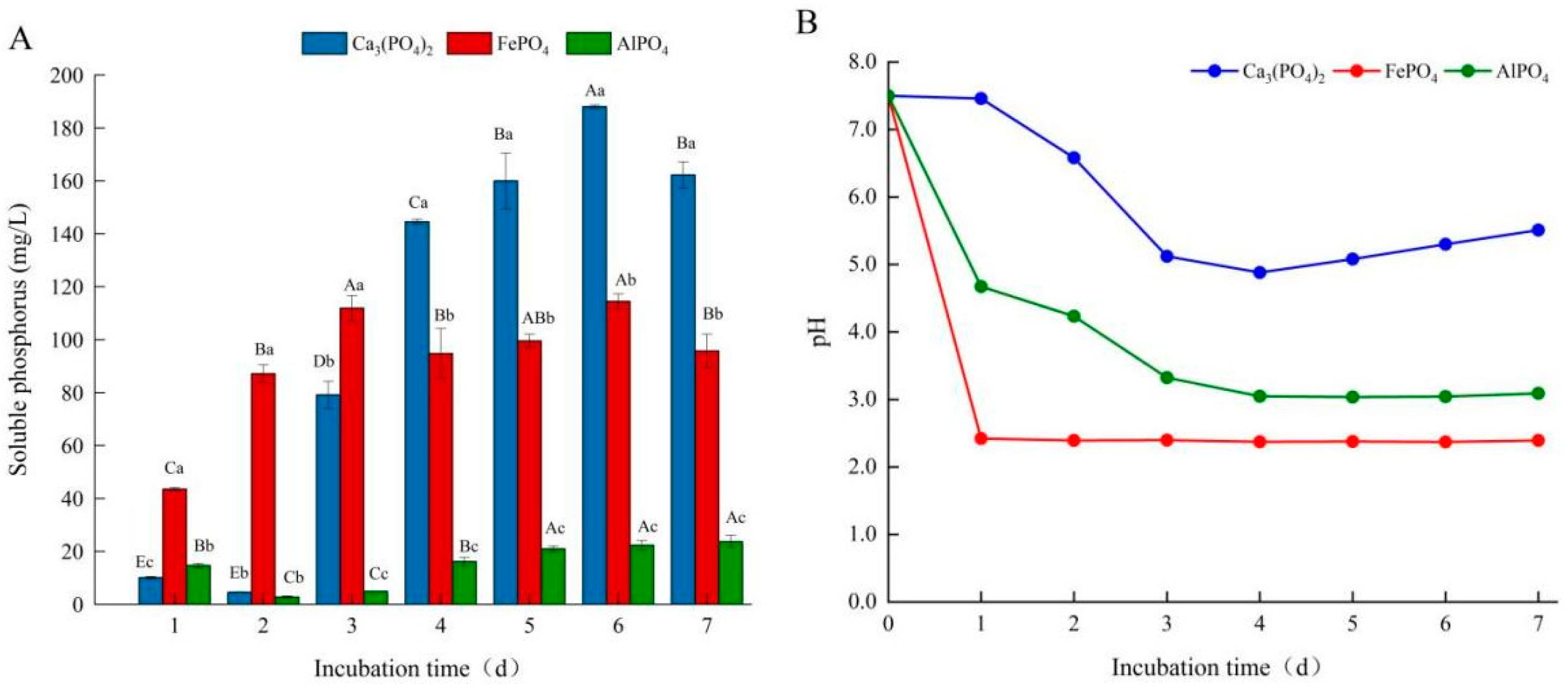
3.3. Effects of Different Phosphorus Sources on Phosphate-Solubilizing Capacity of Fungal Isolate L4
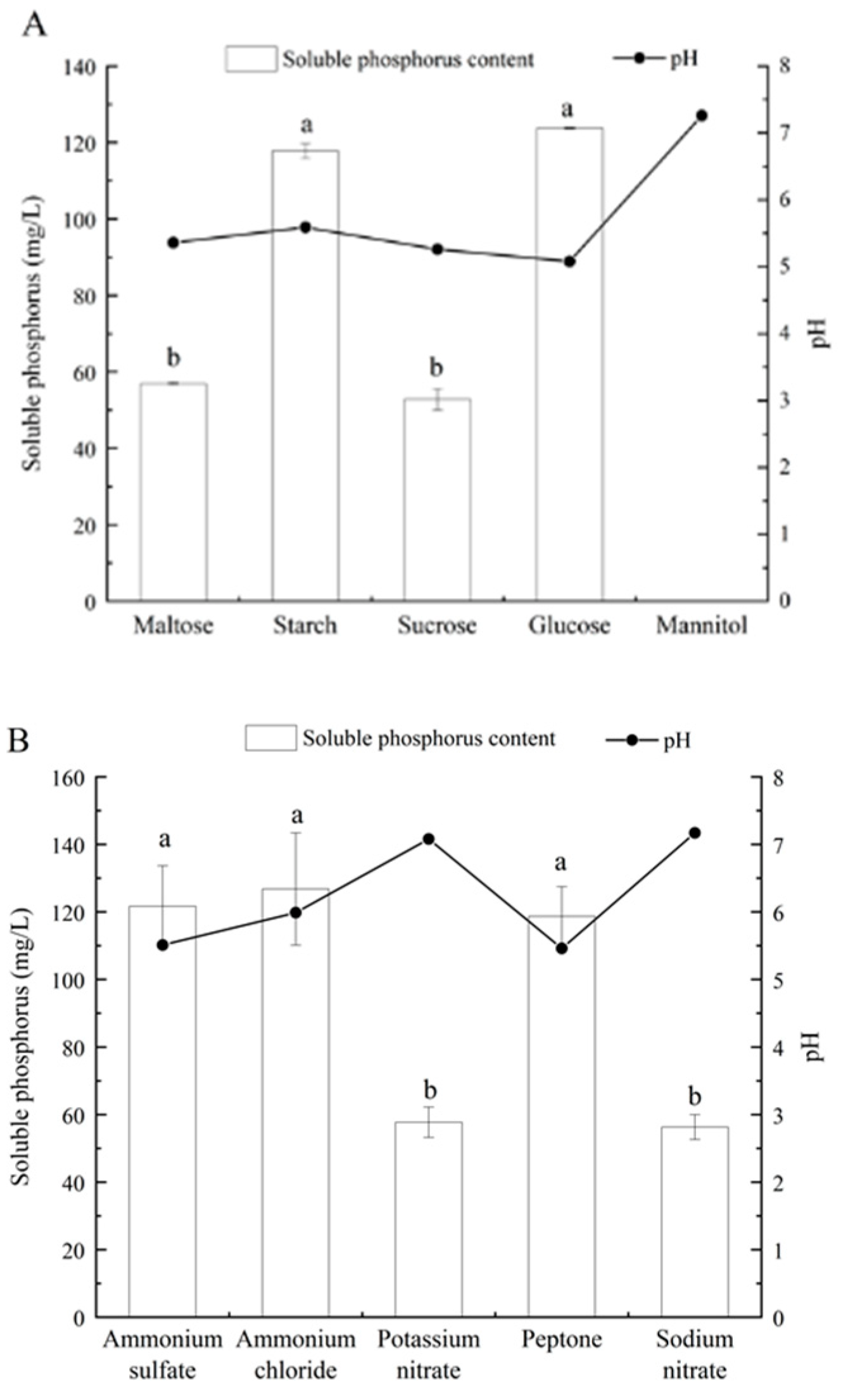
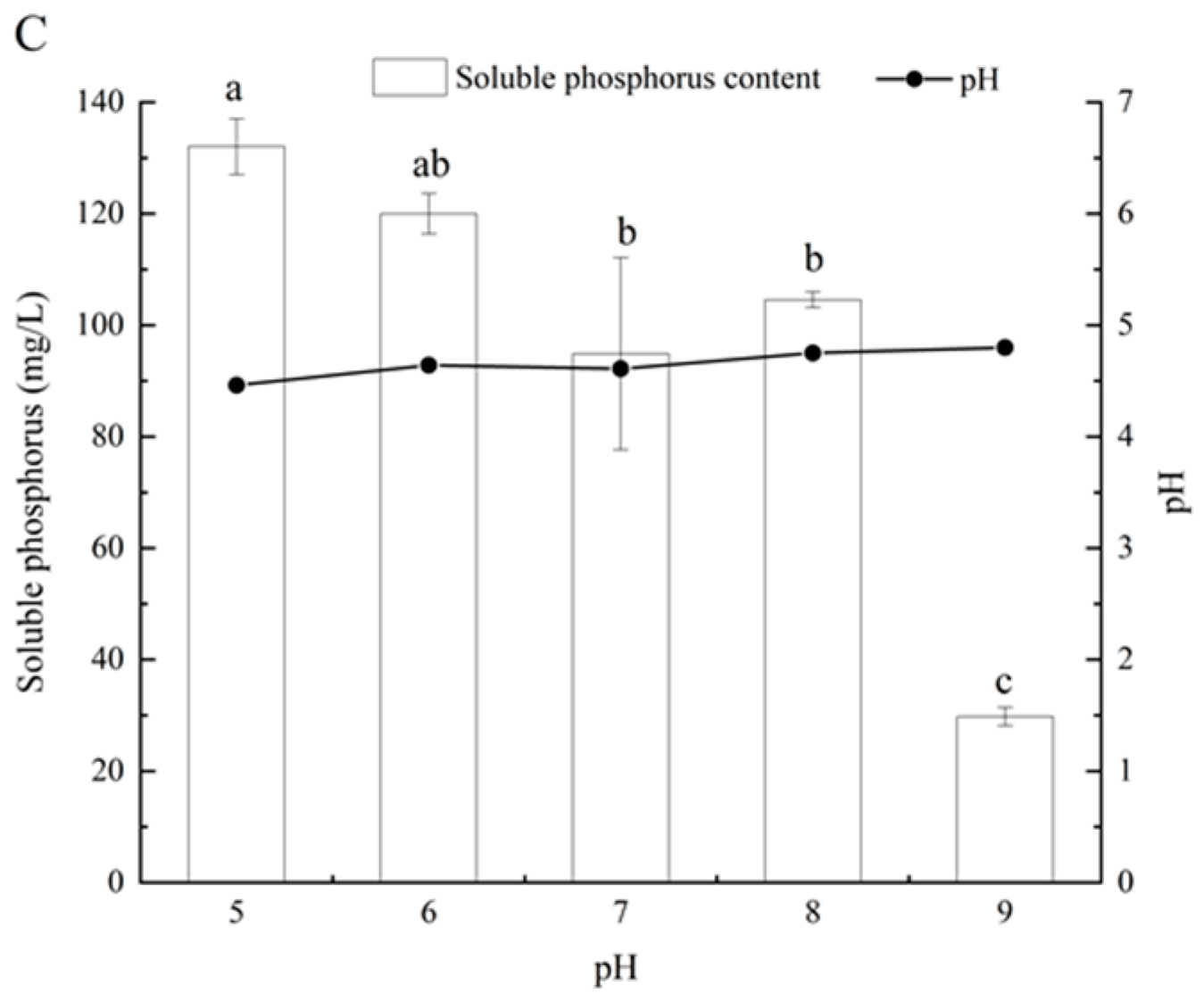
3.4. Effects of Different Carbon and Nitrogen Sources and Initial pH on Phosphate-Solubilizing Capacity of Fungal Isolate L4
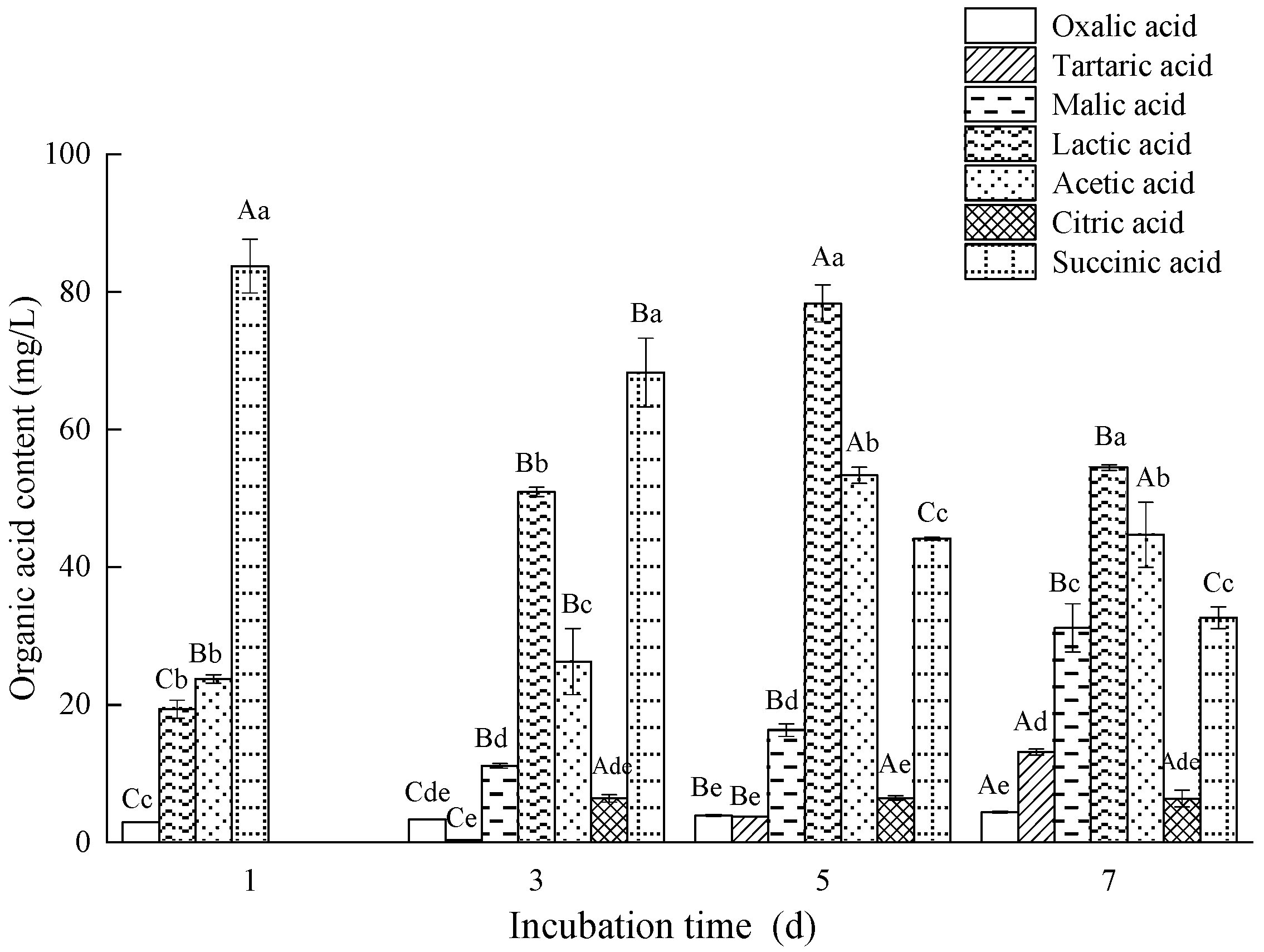
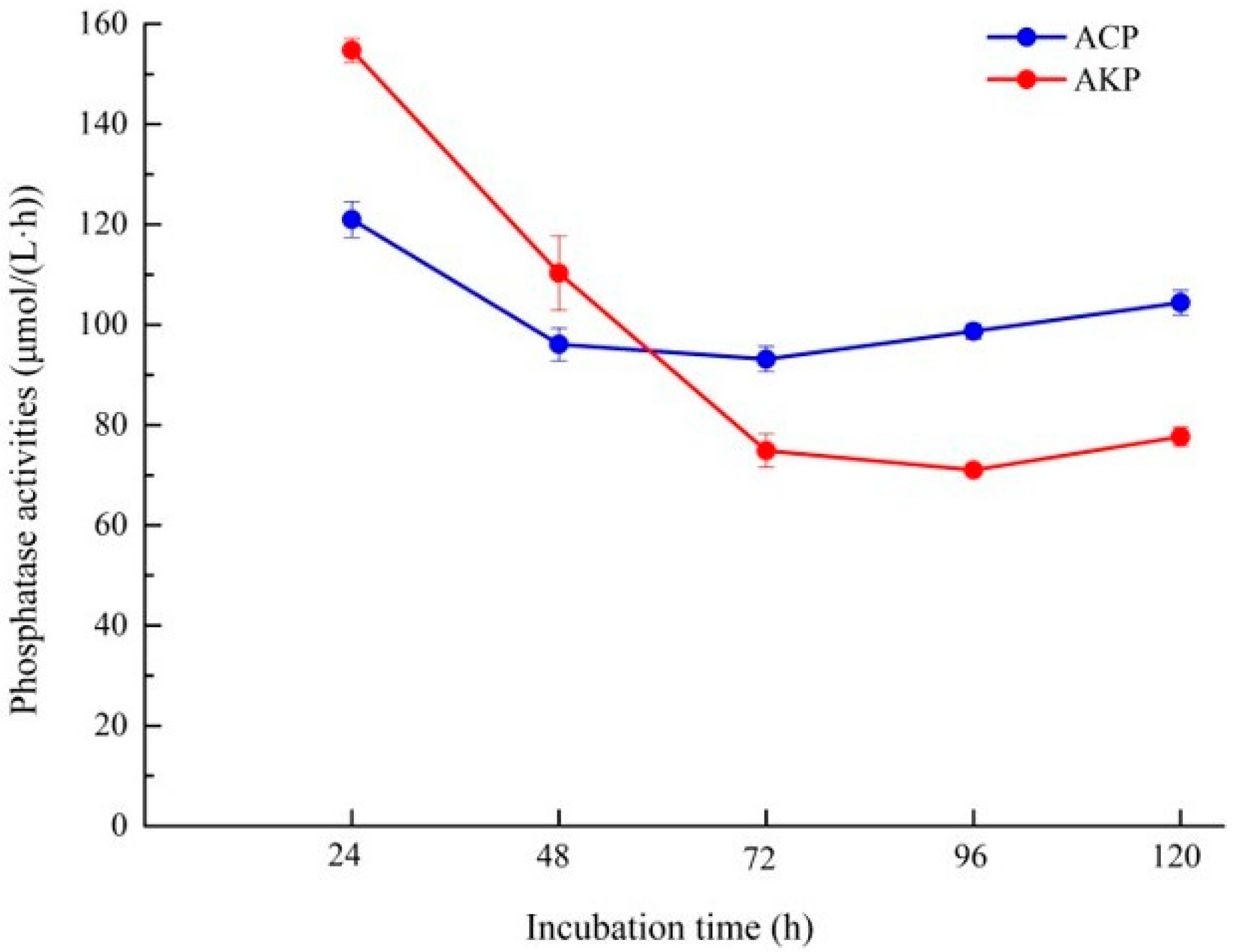
3.5. Analysis of Organic Acid and Phosphatase Activity of Fungal Isolate L4
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nath, D.; Maurya, B.R.; Meena, V.S. Documentation of five potassium- and phosphorus-solubilizing bacteria for their K and P-solubilization ability from various minerals. Biocatal. Agric. Biotechnol. 2017, 10, 174–181. [Google Scholar] [CrossRef]
- Balemi, T.; Negisho, K. Management of soil phosphorus and plant adaptation mechanisms to phosphorus stress for sustainable crop production: A review. J. Soil Sci. Plant Nutr. 2012, 12, 547–562. [Google Scholar] [CrossRef]
- Xu, R.; Li, T.; Shen, M.; Yang, Z.L.; Zhao, Z.W. Evidence for a Dark Septate Endophyte (Exophiala Pisciphila, H93) Enhancing Phosphorus Absorption by Maize Seedlings. Plant Soil 2020, 452, 249–266. [Google Scholar] [CrossRef]
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Gobi, T.A. Phosphate solubilizing microbes: Sustainable approach for managing phosphorus deficiency in agricultural soils. Springerplus 2013, 2, 587. [Google Scholar] [CrossRef] [PubMed]
- Baweja, P.; Kumar, S.; Kumar, G. Fertilizers and Pesticides: Their Impact on Soil Health and Environment. Soil Health 2020, 59, 265–285. [Google Scholar]
- Bouwman, A.F.; Beusen, A.H.W.; Lassaletta, L.; Apeldoorn, D.F.V.; Grinsven, H.J.M.V.; Zhang, J.; Van, M.K.I. Lessons from temporal and spatial patterns in global use of N and P fertilizer on cropland. Sci. Rep. 2017, 7, 40366. [Google Scholar] [CrossRef]
- Mohammed, N.; Chanai, E.; Alkhorayef, M. The impact of the extensive use of phosphate fertilizers on radioactivity levels in farm soil and vegetables in Tanzania. J. Radioanal. Nucl. Chem. 2016, 307, 2373–2379. [Google Scholar] [CrossRef]
- Cao, N.D. Phosphate and Potassium Solubilizing Bacteria from Weathered Materials of denatured Rock Mountain, Ha Tien, Kien Giang Province, Vietnam. Am. J. Life Sci. 2014, 1, 88–92. [Google Scholar]
- Moro, H.; Park, H.-D.; Kunito, T. Organic Phosphorus Substantially Contributes to Crop Plant Nutrition in Soils with Low Phosphorus Availability. Agronomy 2021, 11, 903. [Google Scholar] [CrossRef]
- Pandey, A.; Das, N.; Kumar, B.; Rinu, K.; Trivedi, P. Phosphate solubilization by Penicillium spp. isolated from soil samples of Indian Himalayan region. World J. Microbiol. Biotechnol. 2008, 24, 97–102. [Google Scholar] [CrossRef]
- Linu, M.S.; Asok, A.K.; Thampi, M.; Sreekumar, J.; Jisha, M.S. Plant Growth Promoting Traits of Indigenous Phosphate Solubilizing Pseudomonas aeruginosa Isolates from Chilli (Capsicumannuum L.) Rhizosphere. Commun. Soil Sci. Plant Anal. 2019, 50, 444–457. [Google Scholar] [CrossRef]
- Nelofer, R.; Syed, Q.; Nadeem, M.; Bashir, F.; Mazhar, S.; Hassan, A. Isolation of Phosphorus-Solubilizing Fungus from Soil to Supplement Biofertilizer. Arab. J. Sci. Eng. 2016, 41, 2131–2138. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, F.S.; Wu, X.Q.; Luan, F.G.; Zhang, L.P.; Fang, X.M.; Wan, S.Z.; Hu, X.F.; Ye, J.R.; Daniel, C. Isolation and characterization of two phosphate-solubilizing fungi from rhizosphere soil of moso bamboo and their functional capacities when exposed to different phosphorus sources and pH environments. PLoS ONE 2018, 13, e0199625. [Google Scholar]
- Alam, S.; Khalil, A.; Ayub, N.; Rashid, M. In vitro Solubilization of Inorganic Phosphate by Phosphate Solubilizing Microorganisms (PSM) from Maize Rhizosphere. Int. J. Agric. Biol. 2002, 4, 454–458. [Google Scholar]
- Majeed, A.; Abbasi, M.K.; Hameed, S.; Imran, A.; Rahim, N. Isolation and characterization of plant growth-promoting rhizobacteria from wheat rhizosphere and their effect on plant growth promotion. Front. Microbiol. 2020, 6, 198. [Google Scholar]
- Pawar, V.C.; Thaker, V.S. Acid phosphatase and invertase activities of Aspergillus niger. Mycoscience 2009, 50, 323–330. [Google Scholar] [CrossRef]
- Bononi, L.; Chiaramonte, J.B.; Pansa, C.C.; Moitinho, M.A.; Melo, S. Phosphorus-solubilizing Trichoderma spp. from Amazon soils improve soybean plant growth. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Lokesh, S.T.; Kumar, K.; Thippeswamy, B. Screening of efficient phosphate solubilizing fungi from mine soil and effect of phosphofungi on seed germination and vigour index of ground nut (Arachis hypogaea L.) and green gram (Vigna radiata L.). Int. J. Biol. Res. 2016, 4, 288–294. [Google Scholar] [CrossRef]
- Vandepol, N.; Liber, J.; Desirò, A.; Na, H.; Kennedy, M.; Barry, K.; Grigoriev, I.V.; Miller, A.N.; O’Donnell, K.; Stajich, J.E. Resolving the Mortierellaceae phylogeny through synthesis of multi-gene phylogenetics and phylogenomics. Fungal Divers. 2020, 104, 267–289. [Google Scholar] [CrossRef]
- Coemans, E. Quelques hyphomycetes nouveaux.1.Mortierella polycephala et Martensella pectinata. Bull. De L’académie R. De Bot. Belg. Sér. 2 1863, 15, 536–544. [Google Scholar]
- Ozimek, E.; Hanaka, A. Mortierella Species as the Plant Growth-Promoting Fungi Present in the Agricultural Soils. Agriculture 2020, 11, 7. [Google Scholar] [CrossRef]
- Botha, A.; Paul, I.; Roux, C.; Kock, J.; Coetzee, D.J.; Strauss, T.; Maree, C. An isolation procedure for arachidonic acid producing Mortierella species. Antonie Van Leeuwenhoek 1999, 75, 253–256. [Google Scholar] [CrossRef] [PubMed]
- DiLegge, M.J.; Manter, D.K.; Vivanco, J.M. A novel approach to determine generalist nematophagous microbes reveals Mortierella globalpina as a new biocontrol agent against Meloidogyne spp. nematodes. Sci. Rep. 2019, 9, 7521. [Google Scholar] [CrossRef] [PubMed]
- de León, Y.M.-P.; Muñoz-Castellanos, L.N.; Ruiz-Cisneros, M.F.; Pérez-Corral, D.A.; Ornelas-Paz, J.d.J.; Acosta-Muñiz, C.H.; Berlanga-Reyes, D.I.; Rios-Velasco, C. Identificación morfológica y molecular de especies de Mortierella asociados a rizosfera de manzanos con síntomas de enfermedades radiculares. Rev. Mex. De Fitopatol. 2018, 36, 184–195. [Google Scholar] [CrossRef]
- Rawlings, D.E. Biomining: Theory, Microbes and Industrial Processes; Springer: New York, NY, USA, 1997. [Google Scholar]
- Kataoka, R.; Takagi, K.; Sakakibara, F. A new endosulfan-degrading fungus, Mortierella species, isolated from a soil contaminated with organochlorine pesticides. J. Pestic. Sci. 2010, 35, 326–332. [Google Scholar] [CrossRef]
- White, T.J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols, a Guide to Methods and Applications; Academic Press: London, UK, 1990. [Google Scholar]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_X Windows Interface: Flexible Strategies for Multiple Sequence Alignment Aided by Quality Analysis Tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Kucey, R.; Janzen, H.H.; Leggett, M.E. Microbially Mediated Increases in Plant-Available Phosphorus. Adv. Agron. 1989, 42, 199–228. [Google Scholar]
- Wang, J.; Zhao, Y.G.; Maqbool, F. Capability of Penicillium oxalicum y2 to release phosphate from different insoluble phosphorus sources and soil. Folia Microbiol. 2021, 66, 69–77. [Google Scholar] [CrossRef]
- Zuniga-Silgado, D.; Rivera-Leyva, J.C.; Coleman, J.J.; Sanchez-Reyez, A.; Valencia-Diaz, S.; Serrano, M.; de-Bashan, L.E.; Folch-Mallol, J.L. Soil Type Affects Organic Acid Production and Phosphorus Solubilization Efficiency Mediated by Several Native Fungal Strains from Mexico. Microorganisms 2020, 8, 1337. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Sperber, J.I. Solution of apatite by soil microorganisms producing organic acids. Aust. J. Agric. Res. 1958, 9, 782–787. [Google Scholar] [CrossRef]
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial Phosphorus Solubilization and Its Potential for Use in Sustainable Agriculture. Front. Microbiol. 2017, 8, 971. [Google Scholar] [CrossRef]
- Osorno-Bedoya, L.; Osorio-Veja, N.W. Evaluación de factores que afectan la bioacidulación de roca fosfórica bajo condiciones in vitro. Rev. Colomb. De Biotecnol. 2017, 19, 55–62. [Google Scholar] [CrossRef]
- Osorno, L.; Osorio, N.W. Effect of Carbon and Nitrogen Source and Concentration on Rock Phosphate Dissolution Induced by Fungi. J. Appl. Biotechnol. 2014, 2, 32–42. [Google Scholar] [CrossRef]
- Osorio, N.W.; Habte, M. Synergistic influence of an arbuscular mycorrhizal fungus and a P solubilizing fungus on growth and P uptake of Leucaena leucocephala in an oxisol. Arid. Land Res. Manag. 2001, 15, 263–274. [Google Scholar] [CrossRef]
- Di Simine, C.D.; Sayer, J.A.; Gadd, G.M. Solubilization of zinc phosphate by a strain of Pseudomonas fluorescens isolated from a forest soil. Biol. Fert. Soils 1998, 28, 87–94. [Google Scholar] [CrossRef]
- Kim, K.Y.; Mcdonald, G.A.; Jordan, D. Solubilization of hydroxyapatite by Enterobacter agglomerans and cloned Escherichia coli in culture medium. Biol. Fertil. Soils 1997, 24, 347–352. [Google Scholar] [CrossRef]
- Asea, P.; Kucey, R.; Stewart, J. Inorganic phosphate solubilization by two Penicillium species in solution culture and soil. Soil Biol. Biochem. 1988, 20, 459–464. [Google Scholar] [CrossRef]
- Kucey, R. Phosphate solubilising bacteria and fungi in various cultivated and virgin Alberta soils. Can J Soil Sci. Can. J. Soil Sci. 1983, 63, 671–678. [Google Scholar] [CrossRef]
- de Oliveira, G.; André, M.; Moreira, L.; Olinto, d.; Pereira, L.; Ribeiro, I.; Nikolay, d.; Vassilev, B.; Costa, M.D. Mechanisms of phosphate solubilization by fungal isolates when exposed to different P sources. Ann. Microbiol. 2013, 64, 239–249. [Google Scholar] [CrossRef]
- Spagnoletti, F.N.; Tobar, N.E.; Fernández, D.; Chiocchio, V.M.; Lavado, R.S. Dark septate endophytes present different potential to solubilize calcium, iron and aluminum phosphates. Appl. Soil Ecol. 2017, 111, 25–32. [Google Scholar] [CrossRef]
- Narsian, V.; Patel, H.H. Aspergillus aculeatus as a rock phosphate solubilizer. Soil Biol. Biochem. 2000, 32, 559–565. [Google Scholar] [CrossRef]
- Qiao, Z. Screening and Optimization of Culture Conditions for Phosphate Solubilizing Penicillium in Yellow Soil. J. Henan Agric. Sci. 2019, 48, 56–61. [Google Scholar]
- Kanse, O.S.; Whitelaw-Weckert, M.; Kadam, T.A.; Bhosale, H.J. Phosphate solubilization by stress-tolerant soil fungus Talaromyces funiculosus SLS8 isolated from the Neem rhizosphere. Ann. Microbiol. 2015, 65, 85–93. [Google Scholar] [CrossRef]
- Tallapragada, P. Solubilization of Different Inorganic Phosphates by Aspergillus niger and Penicilium oxalicum. Adv. Bioresearch 2015, 6, 113–119. [Google Scholar]
- Tian, D.; Wang, L.; Hu, J.; Zhang, L.; Zhou, N.; Xia, J.; Xu, M.; Yusef, K.K.; Wang, S.; Li, Z.; et al. A study of P release from Fe-P and Ca-P via the organic acids secreted by Aspergillus niger. J. Microbiol. 2021, 59, 819–826. [Google Scholar] [CrossRef]
- Xiao, C.; Chi, R.; He, H.; Qiu, G.; Wang, D.; Zhang, W. Isolation of Phosphate-Solubilizing Fungi from Phosphate Mines and Their Effect on Wheat Seedling Growth. Appl. Biochem. Biotechnol. 2009, 159, 330–342. [Google Scholar] [CrossRef]
- Bakri, M.M. Tri-Calcium and Zinc Phosphates Solubilization by Aspergillus niger and Its Relation to Organic Acids Production. BioNanoScience 2019, 9, 238–244. [Google Scholar] [CrossRef]
- Achal, V.; Savant, V.V.; Reddy, M.S. Phosphate solubilization by a wild type strain and UV-induced mutants of Aspergillus tubingensis. Soil Biol. Biochem. 2007, 39, 695–699. [Google Scholar] [CrossRef]
- Jorquera, M.A.; Hernández, M.; Rengel, Z.; Marschner, P.; Mora, M. Isolation of culturable phosphobacteria with both phytate-mineralization and phosphate-solubilization activity from the rhizosphere of plants grown in a volcanic soil. Biol. Fertil. Soils 2008, 44, 1025. [Google Scholar] [CrossRef]
- Huang, R.; McGrath, S.P.; Hirsch, P.R.; Clark, I.M.; Storkey, J.; Wu, L.; Zhou, J.; Liang, Y. Plant-microbe networks in soil are weakened by century-long use of inorganic fertilizers. Microb. Biotechnol. Nov. 2019, 12, 1464–1475. [Google Scholar] [CrossRef]
- Mendes, G.O.; Murta, H.M.; Valadares, R.V.; da Silveira, W.B.; da Silva, I.R.; Costa, M.D. Oxalic acid is more efficient than sulfuric acid for rock phosphate solubilization. Miner. Eng. 2020, 155, 106458. [Google Scholar] [CrossRef]
- Vassileva, M.; Mendes, G.d.O.; Deriu, M.A.; di Benedetto, G.; Flor-Peregrin, E.; Mocali, S.; Martos, V.; Vassilev, N.; Fungi, P. Solubilization, and Plant Nutrition. Microorganisms 2022, 10, 1716. [Google Scholar] [CrossRef]

| Species | Collection Isolate | GenBank Accession Number | |
|---|---|---|---|
| Number | 28S | ITS | |
| L3 | CGMCC 3.26004 | ON045511 | ON038715 |
| L4 | CGMCC 3.26005 | ON045513 | ON038743 |
| L5 | CGMCC 3.26006 | ON045526 | ON038747 |
| L12 | CGMCC 3.26007 | ON045523 | ON038748 |
| M. antarctica | CBS 609.70T | NG_042563.1 | NR_111580.1 |
| M. antarctica | 24_1_3 | MT521860.1 | MT521806.1 |
| M. yunnanensis | KUMCC20-0009 | NG_075333.1 | NR_172421.1 |
| M. yunnanensis | KUMCC20-0013 | MT032143.1 | MT031918.1 |
| M. hypsicladia | CBS 116202T | NG_042547.1 | NR_111563.1 |
| M. gemmifera | CBS 134.45T | NG_042543.1 | NR_111559.1 |
| M. gemmifera | XY01505 | MT521851.1 | MT521798.1 |
| M. polygonia | CBS 685.71T | NG_042546.1 | NR_111562.1 |
| M. polygonia | CBS 248.81 | JX976145.1 | JX975891.1 |
| M. globalpina | XY05427 | MT521812.1 | MT521758.1 |
| M. globalpina | CBS 360.70T | NG_064079.1 | NR_160121.1 |
| M. microzygospora | CBS 880.97T | NG_042553.1 | NR_111569.1 |
| M. indohii | CBS 720.71 | NG_042545.1 | NR_111561.1 |
| M. kuhlmanii | CBS 157.71T | NG_042544.1 | NR_111560.1 |
| M. alpina | CBS 219.35 | JX976018.1 | MH867163.1 |
| M. alpina | CBS 384.71C | MH860175.1 | JX976154.1 |
| M. turficola | CBS 432.76T | NG_042566.1 | NR_111583.1 |
| M. elongatula | CBS 488.70T | NG_042565.1 | NR_111582.1 |
| M. strangulata | CBS 455.67T | NG_057902.1 | JX975997.1 |
| Umbelopsis nana | CBS 730.70T | NG_058036.1 | MH859921.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sang, Y.; Jin, L.; Zhu, R.; Yu, X.-Y.; Hu, S.; Wang, B.-T.; Ruan, H.-H.; Jin, F.-J.; Lee, H.-G. Phosphorus-Solubilizing Capacity of Mortierella Species Isolated from Rhizosphere Soil of a Poplar Plantation. Microorganisms 2022, 10, 2361. https://doi.org/10.3390/microorganisms10122361
Sang Y, Jin L, Zhu R, Yu X-Y, Hu S, Wang B-T, Ruan H-H, Jin F-J, Lee H-G. Phosphorus-Solubilizing Capacity of Mortierella Species Isolated from Rhizosphere Soil of a Poplar Plantation. Microorganisms. 2022; 10(12):2361. https://doi.org/10.3390/microorganisms10122361
Chicago/Turabian StyleSang, Yue, Long Jin, Rui Zhu, Xing-Ye Yu, Shuang Hu, Bao-Teng Wang, Hong-Hua Ruan, Feng-Jie Jin, and Hyung-Gwan Lee. 2022. "Phosphorus-Solubilizing Capacity of Mortierella Species Isolated from Rhizosphere Soil of a Poplar Plantation" Microorganisms 10, no. 12: 2361. https://doi.org/10.3390/microorganisms10122361
APA StyleSang, Y., Jin, L., Zhu, R., Yu, X.-Y., Hu, S., Wang, B.-T., Ruan, H.-H., Jin, F.-J., & Lee, H.-G. (2022). Phosphorus-Solubilizing Capacity of Mortierella Species Isolated from Rhizosphere Soil of a Poplar Plantation. Microorganisms, 10(12), 2361. https://doi.org/10.3390/microorganisms10122361





