Antimicrobial Activity of Honey against Oral Microorganisms: Current Reality, Methodological Challenges and Solutions
Abstract
1. Introduction
2. Oral Microbiome
3. What Explains the Antimicrobial Activity of Honey?
4. Analysis of the Antimicrobial Activity of Honey
5. Antimicrobial Activity of Honey against Oral Pathogens
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical Composition, Stability and Authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Cianciosi, D.; Forbes-Hernández, T.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.; Zhang, J.; Bravo Lamas, L.; Martínez Flórez, S.; Agudo Toyos, P.; et al. Phenolic Compounds in Honey and Their Associated Health Benefits: A Review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef] [PubMed]
- Alqarni, A.S.; Owayss, A.A.; Mahmoud, A.A. Physicochemical Characteristics, Total Phenols and Pigments of National and International Honeys in Saudi Arabia. Arab. J. Chem. 2016, 9, 114–120. [Google Scholar] [CrossRef]
- Konstantinidi, M.; Koutelidakis, A.E. Functional Foods and Bioactive Compounds: A Review of Its Possible Role on Weight Management and Obesity’s Metabolic Consequences. Medicines 2019, 6, 94. [Google Scholar] [CrossRef]
- Cooper, R. Honey for Wound Care in the 21st Century. J. Wound Care 2016, 25, 544–552. [Google Scholar] [CrossRef]
- Abd El-Malek, F.F.; Yousef, A.S.; El-Assar, S.A. Hydrogel Film Loaded with New Formula from Manuka Honey for Treatment of Chronic Wound Infections. J. Glob. Antimicrob. Resist. 2017, 11, 171–176. [Google Scholar] [CrossRef]
- Molan, P.; Rhodes, T. Honey: A Biologic Wound Dressing. Wounds 2015, 27, 141–151. [Google Scholar]
- Dreyfus, J.; Delhougne, G.; James, R.; Gayle, J.; Waycaster, C. Clostridial Collagenase Ointment and Medicinal Honey Utilization for Pressure Ulcers in US Hospitals. J. Med. Econ. 2018, 21, 390–397. [Google Scholar] [CrossRef]
- Colombo, A.P.V.; do Souto, R.M.; da Silva-Boghossian, C.M.; Miranda, R.; Lourenço, T.G.B. Microbiology of Oral Biofilm-Dependent Diseases: Have We Made Significant Progress to Understand and Treat These Diseases? Curr. Oral Health Rep. 2015, 2, 37–47. [Google Scholar] [CrossRef]
- Roberts, A.P.; Mullany, P. Oral Biofilms: A Reservoir of Transferable, Bacterial, Antimicrobial Resistance. Expert Rev. Anti-Infect. Ther. 2010, 8, 1441–1450. [Google Scholar] [CrossRef]
- Vieira Colombo, A.P.; Magalhães, C.B.; Hartenbach, F.A.R.R.; Martins do Souto, R.; Maciel da Silva-Boghossian, C. Periodontal-Disease-Associated Biofilm: A Reservoir for Pathogens of Medical Importance. Microb. Pathog. 2016, 94, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Ozen, A.E.; Pons, A.; Tur, J.A. Worldwide Consumption of Functional Foods: A Systematic Review. Nutr. Rev. 2012, 70, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Berger, D.; Rakhamimova, A.; Pollack, A.; Loewy, Z. Oral Biofilms: Development, Control, and Analysis. High-Throughput 2018, 7, 24. [Google Scholar] [CrossRef]
- Štšepetova, J.; Truu, J.; Runnel, R.; Nõmmela, R.; Saag, M.; Olak, J.; Nõlvak, H.; Preem, J.K.; Oopkaup, K.; Krjutškov, K.; et al. Impact of Polyols on Oral Microbiome of Estonian Schoolchildren. BMC Oral Health 2019, 19, 1–10. [Google Scholar] [CrossRef]
- Jepsen, S.; Blanco, J.; Buchalla, W.; Carvalho, J.C.; Dietrich, T.; Dörfer, C.; Eaton, K.A.; Figuero, E.; Frencken, J.E.; Graziani, F.; et al. Prevention and Control of Dental Caries and Periodontal Diseases at Individual and Population Level: Consensus Report of Group 3 of Joint EFP/ORCA Workshop on the Boundaries between Caries and Periodontal Diseases. J. Clin. Periodontol. 2017, 44, S85–S93. [Google Scholar] [CrossRef]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The Oral Microbiota: Dynamic Communities and Host Interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef]
- Marsh, P.D. Dental Plaque as a Biofilm and a Microbial Community—Implications for Health and Disease. BMC Oral Health 2006, 6 (Suppl. S1), S14. [Google Scholar] [CrossRef]
- Marsh, P.D. Dental Plaque as a Microbial Biofilm. Caries Res. 2004, 38, 204–211. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L. Microbial Complexes in Subgingival Plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef]
- Haffajee, A.D.; Socransky, S.S.; Patel, M.R.; Song, X. Microbial Complexes in Supragingival Plaque. Oral Microbiol. Immunol. 2008, 23, 196–205. [Google Scholar] [CrossRef]
- Miranda, S.L.F.; Damasceno, J.T.; Faveri, M.; Figueiredo, L.; da Silva, H.D.; de Alencar, S.M.A.; Rosalen, P.L.; Feres, M.; Bueno-Silva, B. Brazilian Red Propolis Reduces Orange-Complex Periodontopathogens Growing in Multispecies Biofilms. Biofouling 2019, 35, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Hu, W.; Tian, Z.; Yuan, D.; Yi, G.; Zhou, Y.; Cheng, Q.; Zhu, J.; Li, M. Developing Natural Products as Potential Anti-Biofilm Agents. Chin. Med. 2019, 14, 11. [Google Scholar] [CrossRef] [PubMed]
- Israili, Z.H. Antimicrobial Properties of Honey. Am. J. Ther. 2014, 21, 304–323. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.L.; Molan, P.C.; Reid, G.M. A Survey of the Antibacterial Activity of Some New Zealand Honeys. J. Pharm. Pharmacol. 1991, 43, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Albaridi, N.A. Antibacterial Potency of Honey. Int. J. Microbiol. 2019, 2019, 1–10. [Google Scholar] [CrossRef]
- Matzen, R.D.; Zinck Leth-Espensen, J.; Jansson, T.; Nielsen, D.S.; Lund, M.N.; Matzen, S. The Antibacterial Effect In Vitro of Honey Derived from Various Danish Flora. Dermatol. Res. Pract. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Brudzynski, K.; Lannigan, R. Mechanism of Honey Bacteriostatic Action against MRSA and VRE Involves Hydroxyl Radicals Generated from Honey’s Hydrogen Peroxide. Front. Microbiol. 2012, 3, 1–8. [Google Scholar] [CrossRef]
- Bucekova, M.; Buriova, M.; Pekarik, L.; Majtan, V.; Majtan, J. Phytochemicals-Mediated Production of Hydrogen Peroxide Is Crucial for High Antibacterial Activity of Honeydew Honey. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Agbaje, E.O.; Ogunsanya, T.; Aiwerioba, O.I.R. Conventional use of honey as antibacterial agent. Ann. Afr. Med. 2006, 5, 78–81. [Google Scholar]
- Rabie, E.; Serem, J.C.; Oberholzer, H.M.; Gaspar, A.R.M.; Bester, M.J. How Methylglyoxal Kills Bacteria: An Ultrastructural Study. Ultrastruct. Pathol. 2016, 40, 107–111. [Google Scholar] [CrossRef]
- Szweda, P. Antimicrobial Activity of Honey. In Honey Analysis, 1st ed.; Toledo, V.A.A., Ed.; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef]
- Escuredo, O.; Dobre, I.; Fernández-González, M.; Seijo, M.C. Contribution of Botanical Origin and Sugar Composition of Honeys on the Crystallization Phenomenon. Food Chem. 2014, 149, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Molan, P.C.; Allen, K.L. The Effect of Gamma-Irradiation on the Antibacterial Activity of Honey. J. Pharm. Pharmacol. 1996, 48, 1206–1209. [Google Scholar] [CrossRef] [PubMed]
- Mandal, M.D.; Mandal, S. Honey: Its Medicinal Property and Antibacterial Activity. Asian Pac. J. Trop. Biomed. 2011, 1, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.J.; Boult, C.H.; Deadman, B.J.; Farr, J.M.; Grainger, M.N.C.; Manley-Harris, M.; Snow, M.J. Isolation by HPLC and Characterisation of the Bioactive Fraction of New Zealand Manuka (Leptospermum Scoparium) Honey. Carbohydr. Res. 2008, 343, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Mavric, E.; Wittmann, S.; Barth, G.; Henle, T. Identification and Quantification of Methylglyoxal as the Dominant Antibacterial Constituent of Manuka (Leptospermum Scoparium) Honeys from New Zealand. Mol. Nutr. Food Res. 2008, 52, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Carter, D.A.; Blair, S.E.; Cokcetin, N.N.; Bouzo, D.; Brooks, P.; Schothauer, R.; Harry, E.J. Therapeutic Manuka Honey: No Longer so Alternative. Front. Microbiol. 2016, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Packer, J.M.; Irish, J.; Herbert, B.R.; Hill, C.; Padula, M.; Blair, S.E.; Carter, D.A.; Harry, E.J. Specific Non-Peroxide Antibacterial Effect of Manuka Honey on the Staphylococcus aureus Proteome. Int. J. Antimicrob. Agents 2012, 40, 43–50. [Google Scholar] [CrossRef]
- Bucekova, M.; Jardekova, L.; Juricova, V.; Bugarova, V.; Di Marco, G.; Gismondi, A.; Leonardi, D.; Farkasovska, J.; Godocikova, J.; Laho, M.; et al. Antibacterial Activity of Different Blossom Honeys: New Findings. Molecules 2019, 24, 1573. [Google Scholar] [CrossRef]
- Sakihama, Y.; Cohen, M.F.; Grace, S.C.; Yamasaki, H. Plant Phenolic Antioxidant and Prooxidant Activities: Phenolics-Induced Oxidative Damage Mediated by Metals in Plants. Toxicology 2002, 177, 67–80. [Google Scholar] [CrossRef]
- Brudzynski, K.; Abubaker, K.; St-Martin, L.; Castle, A. Re-Examining the Role of Hydrogen Peroxide in Bacteriostatic and Bactericidal Activities of Honey. Front. Microbiol. 2011, 2, 1–9. [Google Scholar] [CrossRef]
- Brudzynski, K. Effect of Hydrogen Peroxide on Antibacterial Activities of Canadian Honeys. Can. J. Microbiol. 2006, 52, 1228–1237. [Google Scholar] [CrossRef] [PubMed]
- Kuś, P.M.; Szweda, P.; Jerković, I.; Tuberoso, C.I.G. Activity of Polish Unifloral Honeys against Pathogenic Bacteria and Its Correlation with Colour, Phenolic Content, Antioxidant Capacity and Other Parameters. Lett. Appl. Microbiol. 2016, 62, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Kwakman, P.H.S.; Zaat, S.A.J. Antibacterial Components of Honey. IUBMB Life 2012, 64, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Ramos, F.A.; Takaishi, Y.; Shirotori, M.; Kawaguchi, Y.; Tsuchiya, K.; Shibata, H.; Higuti, T.; Tadokoro, T.; Takeuchi, M. Antibacterial and Antioxidant Activities of Quercetin Oxidation Products from Yellow Onion (Allium Cepa) Skin. J. Agric. Food Chem. 2006, 54, 3551–3557. [Google Scholar] [CrossRef] [PubMed]
- Brudzynski, K.; Miotto, D.; Kim, L.; Sjaarda, C.; Maldonado-Alvarez, L.; Fukś, H. Active Macromolecules of Honey Form Colloidal Particles Essential for Honey Antibacterial Activity and Hydrogen Peroxide Production. Sci. Rep. 2017, 7, 7637. [Google Scholar] [CrossRef]
- Lu, J.; Carter, D.A.; Turnbull, L.; Rosendale, D.; Hedderley, D.; Stephens, J.; Gannabathula, S.; Steinhorn, G.; Schlothauer, R.C.; Whitchurch, C.B.; et al. The Effect of New Zealand Kanuka, Manuka and Clover Honeys on Bacterial Growth Dynamics and Cellular Morphology Varies According to the Species. PLoS ONE 2013, 8, e55898. [Google Scholar] [CrossRef]
- Henriques, A.F.; Jenkins, R.E.; Burton, N.F.; Cooper, R.A. The Intracellular Effects of Manuka Honey on Staphylococcus aureus. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 45–50. [Google Scholar] [CrossRef]
- Almasaudi, S. The Antibacterial Activities of Honey. Saudi J. Biol. Sci. 2021, 28, 2188–2196. [Google Scholar] [CrossRef]
- Anthimidou, E.; Mossialos, D. Antibacterial Activity of Greek and Cypriot Honeys Against Staphylococcus aureus and Pseudomonas Aaeruginosa in Comparison to Manuka Honey. J. Med. Food 2013, 16, 42–47. [Google Scholar] [CrossRef]
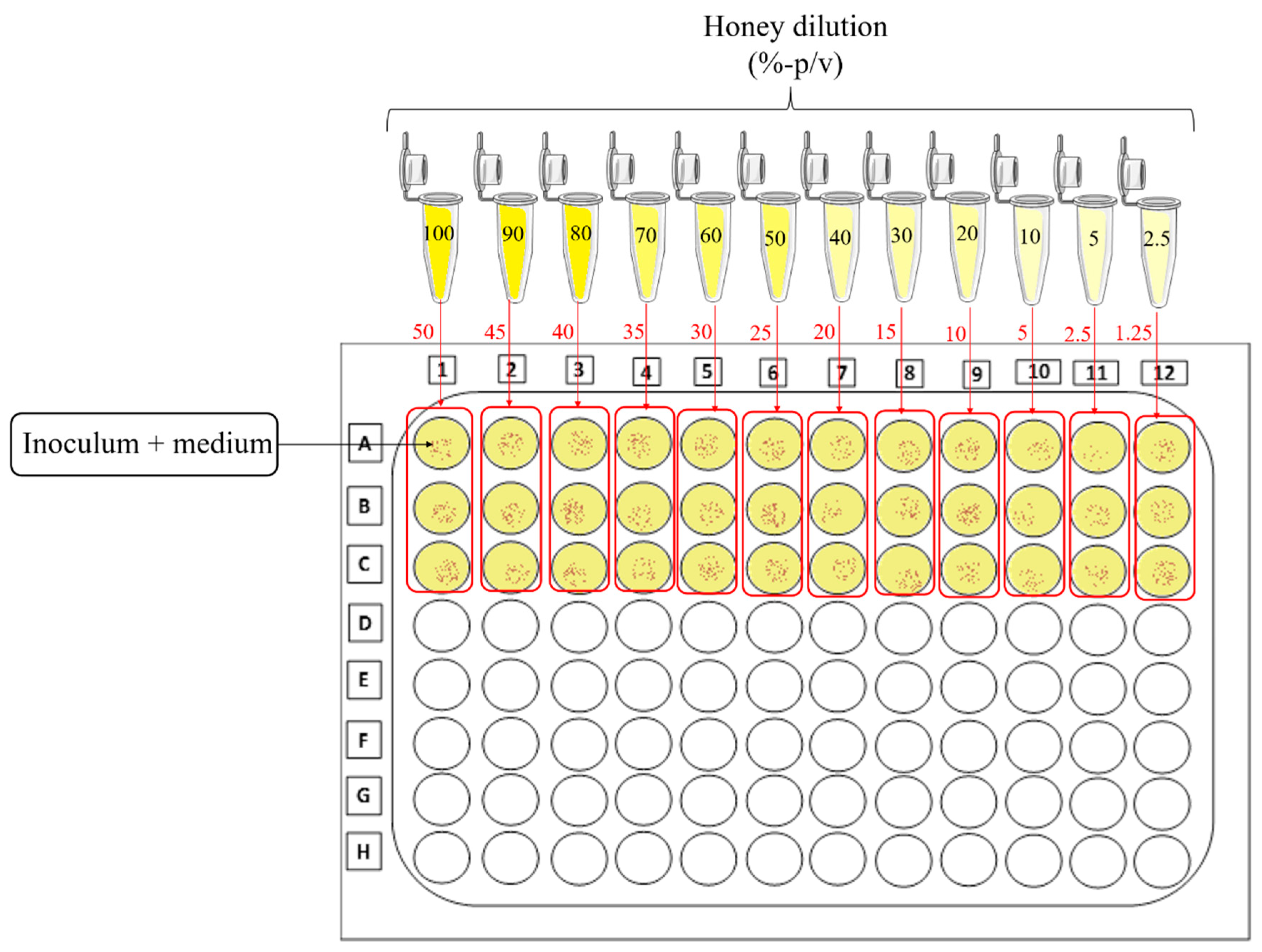
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro Evaluating Antimicrobial Activity: A Review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Sheehan, D.J.; Rex, J.H. Determination of Fungicidal Activities against Yeasts and Molds: Lessons Learned from Bactericidal Testing and the Need for Standardization. Clin. Microbiol. Rev. 2004, 17, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Almasaudi, S.B.; Al-Nahari, A.A.M.; Abd El-Ghany, E.S.M.; Barbour, E.; Al Muhayawi, S.M.; Al-Jaouni, S.; Azhar, E.; Qari, M.; Qari, Y.A.; Harakeh, S. Antimicrobial Effect of Different Types of Honey on Staphylococcus aureus. Saudi J. Biol. Sci. 2017, 24, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- Mama, M.; Teshome, T.; Detamo, J. Antibacterial Activity of Honey against Methicillin-Resistant Staphylococcus aureus: A Laboratory-Based Experimental Study. Int. J. Microbiol. 2019, 2019, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi-Motamayel, F.; Hendi, S.S.; Alikhani, M.Y.; Khamverdi, Z. Antibacterial Activity of Honey on Cariogenic Bacteria. J. Dent. Tehran 2013, 10, 10–15. [Google Scholar] [PubMed]
- Johnston, M.; McBride, M.; Dahiya, D.; Owusu-Apenten, R.; Singh Nigam, P. Antibacterial Activity of Manuka Honey and Its Components: An Overview. AIMS Microbiol. 2018, 4, 655–664. [Google Scholar] [CrossRef]
- Anand, S.; Deighton, M.; Livanos, G.; Morrison, P.D.; Pang, E.C.K.; Mantri, N. Antimicrobial Activity of Agastache Honey and Characterization of Its Bioactive Compounds in Comparison with Important Commercial Honeys. Front. Microbiol. 2019, 10, 263. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.; Gasparrini, M.; Forbes-Hernández, T.; Mazzoni, L.; Giampieri, F. The Composition and Biological Activity of Honey: A Focus on Manuka Honey. Foods 2014, 3, 420–432. [Google Scholar] [CrossRef]
- Sanz, M.; Beighton, D.; Curtis, M.A.; Cury, J.A.; Dige, I.; Dommisch, H.; Ellwood, R.; Giacaman, R.A.; Herrera, D.; Herzberg, M.C.; et al. Role of Microbial Biofilms in the Maintenance of Oral Health and in the Development of Dental Caries and Periodontal Diseases. Consensus Report of Group 1 of the Joint EFP/ORCA Workshop on the Boundaries between Caries and Periodontal Disease. J. Clin. Periodontol. 2017, 44, S5–S11. [Google Scholar] [CrossRef]
- Colombo, A.P.V.; Tanner, A.C.R. The Role of Bacterial Biofilms in Dental Caries and Periodontal and Peri-Implant Diseases: A Historical Perspective. J. Dent. Res. 2019, 98, 373–385. [Google Scholar] [CrossRef]
- Kırmusaoğlu, S. The Methods for Detection of Biofilm and Screening Antibiofilm Activity of Agents. In Antimicrobials, Antibiotic Resistance, Antibiofilm Strategies and Activity Methods; Kırmusaoğlu, S., Ed.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Wilson, C.; Lukowicz, R.; Merchant, S.; Valquier-Flynn, H.; Caballero, J.; Sandoval, J.; Okuom, M.; Huber, C.; Brooks, T.D.; Wilson, E.; et al. Quantitative and Qualitative Assessment Methods for Biofilm Growth: A Mini-Review. Res. Rev. J. Eng. Technol. 2017, 6, 4–42. [Google Scholar]
- Ceri, H.; Olson, M.E.; Stremick, C.; Read, R.R.; Morck, D.; Buret, A. The Calgary Biofilm Device: New Technology for Rapid Determination of Antibiotic Susceptibilities of Bacterial Biofilms. J. Clin. Microbiol. 1999, 37, 1771–1776. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Mavelli, G.V.; Nacharaju, P.; Li, K.; Cleare, L.G.; Nosanchuk, J.D.; Friedman, J.M.; Abuzeid, W.M. Novel Nitric Oxide–generating Platform Using Manuka Honey as an Anti-biofilm Strategy in Chronic Rhinosinusitis. Int. Forum Allergy Rhinol. 2020, 10, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Cremers, N.; Belas, A.; Santos Costa, S.; Couto, I.; de Rooster, H.; Pomba, C. In Vitro Antimicrobial Efficacy of Two Medical Grade Honey Formulations against Common High-risk Meticillin-resistant Staphylococci and Pseudomonas Spp. Pathogens. Vet. Dermatol. 2020, 31, 90. [Google Scholar] [CrossRef] [PubMed]
- Ghramh, H.A.; Khan, K.A.; Alshehri, A.M.A. Antibacterial Potential of Some Saudi Honeys from Asir Region against Selected Pathogenic Bacteria. Saudi J. Biol. Sci. 2019, 26, 1278–1284. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.; Ribeiro, H.G.; Silva, A.C.; Silva, M.D.; Sousa, J.C.; Rodrigues, C.F.; Melo, L.D.R.; Henriques, A.F.; Sillankorva, S. Synergistic Antimicrobial Interaction between Honey and Phage against Escherichia coli Biofilms. Front. Microbiol. 2017, 8, 2407. [Google Scholar] [CrossRef] [PubMed]
- Hannan, A.; Bajwa, A.E.; Riaz, S.; Arshad, U.; Saleem, S.; Bajwa, U.I. In vitro Salmonella typhi Biofilm Formation on Gallstones and Its Disruption by Manuka Honey. Pak. J. Pharm. Sci. 2018, 31, 129–135. [Google Scholar]
- Morroni, G.; Alvarez-Suarez, J.M.; Brenciani, A.; Simoni, S.; Fioriti, S.; Pugnaloni, A.; Giampieri, F.; Mazzoni, L.; Gasparrini, M.; Marini, E.; et al. Comparison of the Antimicrobial Activities of Four Honeys from Three Countries (New Zealand, Cuba, and Kenya). Front. Microbiol. 2018, 9, 1378. [Google Scholar] [CrossRef]
- Habluetzel, A.; Schmid, C.; Carvalho, T.S.; Lussi, A.; Eick, S. Impact of Honey on Dental Erosion and Adhesion of Early Bacterial Colonizers. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Mathai, K.; Anand, S.; Aravind, A.; Dinatius, P.; Krishnan, A.V.; Mathai, M. Antimicrobial Effect of Ginger, Garlic, Honey, and Lemon Extracts on Streptococcus mutans. J. Contemp. Dent. Pract. 2017, 18, 1004–1008. [Google Scholar] [CrossRef]
- Safii, S.H.; Tompkins, G.R.; Duncan, W.J. Periodontal Application of Manuka Honey: Antimicrobial and Demineralising Effects In Vitro. Int. J. Dent. 2017, 2017, 4535. [Google Scholar] [CrossRef]
- Schmidlin, P.R.; English, H.; Duncan, W.; Belibasakis, G.N.; Thurnheer, T. Antibacterial Potential of Manuka Honey against Three Oral Bacteria in Vitro. Swiss Dent. J. 2014, 124, 922–924. [Google Scholar] [PubMed]
- Badet, C.; Quero, F. The in vitro Effect of Manuka Honeys on Growth and Adherence of Oral Bacteria. Anaerobe 2011, 17, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Basson, N.J.; du Toit, I.J.; Grobler, S.R. Antibacterial Action of Honey on Oral Streptococci. J. Dent. Assoc. S. Afr. 1994, 49, 339–341. [Google Scholar] [PubMed]
- Basson, N.J.; Grobler, S.R. Antimicrobial Activity of Two South African Honeys Produced from Indigenous Leucospermum Cordifolium and Erica Species on Selected Micro-Organisms. BMC Complement. Altern. Med. 2008, 8, 41. [Google Scholar] [CrossRef]
- Eslami, H.; Ariamanesh, N.; Ariamanesh, A.; Kafil, H.S. Synergistic Effect of Honey and Azarian Propolis on Oral Microorganisms: An in vitro Study. J. Adv. Oral Res. 2016, 7, 31–36. [Google Scholar]
- Nassar, H.M.; Li, M.; Gregory, R.L. Effect of Honey on Streptococcus mutans Growth and Biofilm Formation. Appl. Environ. Microbiol. 2012, 78, 536–540. [Google Scholar] [CrossRef]
- Eick, S.; Schäfer, G.; Kwieciński, J.; Atrott, J.; Henle, T.; Pfister, W. Honey—A Potential Agent against Porphyromonas Gingivalis: An in vitro Study. BMC Oral Health 2014, 14, 1–9. [Google Scholar] [CrossRef]
- Aparna, S.; Srirangarajan, S.; Malgi, V.; Setlur, K.P.; Shashidhar, R.; Setty, S.; Thakur, S. A Comparative Evaluation of the Antibacterial Efficacy of Honey in vitro and Antiplaque Efficacy in a 4-Day Plaque Regrowth Model in vivo: Preliminary Results. J. Periodontol. 2012, 83, 1116–1121. [Google Scholar] [CrossRef]
- English, H.K.P.; Pack, A.R.C.; Molan, P.C. The Effects of Manuka Honey on Plaque and Gingivitis: A Pilot Study. J. Int. Acad. Periodontol. 2004, 6, 63–67. [Google Scholar]
- Abdelmegid, F.; Al-Agamy, M.; Alwohaibi, A.; Ka’abi, H.; Salama, F. Effect of Honey and Green Tea Solutions on Streptococcus mutans. J. Clin. Pediatr. Dent. 2015, 39, 435–441. [Google Scholar] [CrossRef]
- Rupesh, S.; Winnier, J.; Nayak, U.; Rao, A.; Reddy, N.; Peter, J. Evaluation of the Effects of Manuka Honey on Salivary Levels of mutans Streptococci in Children: A Pilot Study. J. Indian Soc. Pedod. Prev. Dent. 2014, 32, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Nandlal, B.; Sreenivasan, P.K.; Shashikumar, P.; Devishree, G.; Bettahalli Shivamallu, A. A randomized clinical study to examine the oral hygiene efficacy of a novel herbal toothpaste with zinc over a 6-month period. Int. J. Dent. Hyg. 2021, 19, 440–449. [Google Scholar] [CrossRef] [PubMed]
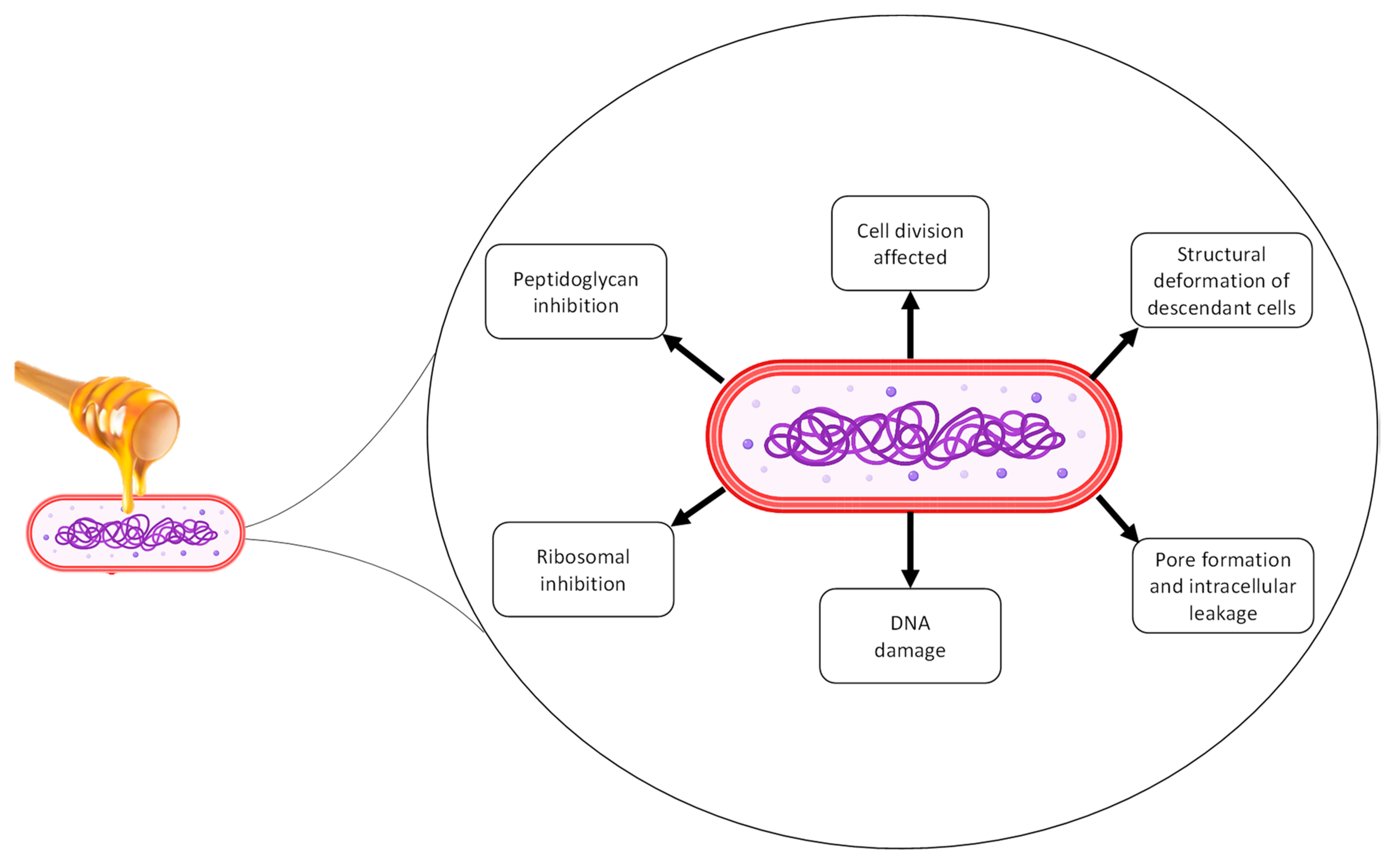
- Al-Sayaghi, A.M.; Al-Kabsi, A.M.; Abduh, M.S.; Saghir, S.A.M.; Alshawsh, M.A. Antibacterial Mechanism of Action of Two Types of Honey against Escherichia coli through Interfering with Bacterial Membrane Permeability, Inhibiting Proteins, and Inducing Bacterial DNA Damage. Antibiotics 2022, 11, 1182. [Google Scholar] [CrossRef] [PubMed]
- Otreba, M.; Marek, L.; Tyczynska, N.; Stojko, J.; Rzepecka-Stojko, A. Bee Venom, Honey, and Royal Jelly in the Treatment of Bacterial Infections of the Oral Cavity: A Review. Life 2021, 11, 1311. [Google Scholar] [CrossRef] [PubMed]
- Deglovic, J.; Majtanova, N.; Majtan, J. Antibacterial and Antibiofilm Effect of Honey in the Prevention of Dental Caries: A Recent Perspective. Foods 2022, 11, 2670. [Google Scholar] [CrossRef]
- Prince, A.; Roy, S.; McDonald, D. Exploration of the Antimicrobial Synergy between Selected Natural Substances on Streptococcus mutans to Identify Candidates for the Control of Dental Caries. Microbiol. Spectr. 2022, 10, e0235721. [Google Scholar] [CrossRef]

| Component/Physical Characteristic | Considerations about the Antimicrobial Activity of Honey | References |
|---|---|---|
| Low pH | Due to the high concentration of organic acids (mainly gluconic acid), most honey samples have a pH ranging between 3.4 and 6.0 which, in combination with high osmotic pressure, can eliminate and/or prevent microbial colonization. | [25] |
| Osmotic effect | High osmolarity has been shown to play a role in the antimicrobial properties of honey. Despite this, high concentrations of glucose alone showed no inhibitory effect on bacterial growth. Thus, sugar-induced osmolarity in honey is only considered an adjuvant that provides an unfavorable environment for pathogens. | [26] |
| Hydrogen peroxide (H2O2) | Hydrogen peroxide (H2O2) levels are directly proportional to the antibacterial activity of honey. Therefore, H2O2 is considered a predictive biomarker of its antibacterial activity. Glucose oxidase is an enzyme present in honey that is activated when the honey is diluted. This enzyme acts on endogenous glucose to produce hydrogen peroxide. H2O2 is involved with oxidative damage, causing inhibition of bacterial growth and DNA degradation. However, these effects are synergistically modulated by other components present in honey, since higher concentrations of pure H2O2 are needed to obtain similar effects as compared to the low H2O2 levels present in honey samples. | [25,27,28] |
| Phenolic compounds | The therapeutic effect of honey is attributed to the presence of several antioxidants, including phenolic compounds, such as flavonoids and phenolic acids. Some phenolic compounds, such as pinocembrin and serum acid, have been strongly associated with the antimicrobial activity of honey. Nevertheless, information on the mechanism of action and effective doses of these compounds is yet to be determined. | [2,29] |
| Methylglyoxal (MGO) | MGO is an organic compound derived from dihydroxyacetone. The presence of MGO in honey contributes to its antimicrobial activity, even in honeys with low levels of peroxide (e.g., Manuka honey). MGO causes loss of membrane integrity and changes the structure of bacterial fimbriae and flagella, which impairs microbial adhesion and motility. | [30] |
| Bee peptides (Defensin-1) | Defensin-1 is a peptide secreted by the hypopharyngeal glands of bees. This peptide is active against Gram-positive bacteria, including Bacillus subtilis and Staphylococcus aureus. Although bees produce other peptides, only defensin-1 has been detected in honey and was found to have antimicrobial activity. | [31] |
| MIC (%, w/v) | Antimicrobial Activity |
|---|---|
| 1.0% to 12.5% | Strong |
| 12.5% to 50.0% | Moderate |
| >50.0% | Weak |
| Type of Honey (Plant Origin) | Strain | Method | Results | Reference |
|---|---|---|---|---|
| Honey from central Switzerland, honey from the German plain, and Manuka honey (Leptospermum scoparium). | Streptococcus gordonii, Streptococcus sanguinis, Streptococcus mutans, Streptococcus sobrinus, Lactobacillus acidophilus, Actinomyces naeslundii. | Antimicrobial activity (MIC determination) and anti-adherent activity by counting colony-forming units per mL (CFU/mL). | The three honey samples inhibited the specific growth of oral bacterial strains. When using a multispecies biofilm model, none of the samples were significantly effective. | [70] |
| Kerala commercial honey, India. | Streptococcus mutans | Antimicrobial activity determined by the agar diffusion method. | A discrete zone of inhibition of the growth of Streptococcus mutans was observed. | [71] |
| Manuka honey and white clover honey (Trifolium repens). | Staphylococcus aureus, Escherichia coli, Streptococcus mutans, Streptococcus sobrinus, Streptococcus sanguinis, Streptococcus gordonii, Fusobacterium nucleatum, Porphyromonas gingivalis, and Prevotella intermedia | Antimicrobial activity by broth microdilution for determination of MIC and MBC values. | Both honeys inhibited most of the tested stains, except Streptococcus mutans. Manuka honey displayed slightly greater inhibitory efficacy, with MICs ranging between 6.3% and 25%, whereas the MICs of clover honey ranged from 6.3% to 50%. Honeys with neutral pH had little antimicrobial activity. | [72] |
| Swiss multifloral honey, Manuka honey NPA 5+, Manuka honey NPA 15+ (Leptospermum scoparium), Manuka honey label “MGO 400+” (Leptospermum scoparium), equivalent to NPA 20+, Manuka honey NPA 25+, MediHoneyTM medicinal honey, and MediHoneyTM gel sheet. | Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, and Streptococcus mutans | Initial screening for antimicrobial activity by the agar diffusion method. The most active samples were selected for the determination of MIC and MBC values. | Manuka honey below an NPA value of 15 showed the least potential to inhibit bacterial growth, even less—although not significantly—than Swiss multifloral honey. Manuka honey above an NPA value of 15 showed a significantly greater antibacterial effect compared to the other honeys tested. All Manuka honey preparations were more effective in inhibiting the growth of Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans as compared to S. mutans. | [73] |
| Manuka honey 1 and 2 (Leptospermum scoparium). | Streptococcus mutans, Streptococcus sobrinus, Lactobacillus rhamnosus, Actinomyces viscosus, Porphyromonas gingivalis, and Fusobacterium nucleatum. | Antimicrobial activity (MIC determination) and anti-adherent activity by counting colony-forming units per mL (CFU/mL). | The antibacterial activity of Manuka 1 was the most important. The two honeys tested showed a weak ability to inhibit the adhesion of S. mutans cells onto a glass surface at sub-MIC concentrations. Manuka 1 completely inhibited multispecies biofilm formation at a concentration of 200 μg/mL. Manuka 2 inhibited biofilm formation weakly at a concentration of 200 μg/mL, but strongly at a concentration of 500 μg/mL. | [74] |
| Eucalyptus honey (Eucalyptus cladocalyx). | Steptococcus mutans, Steptococcus sobrinus, Steptococcus gordonii, Steptococcus salivarius, Steptococcus sanguinis, Steptococcus anginosus, Steptococcus oralis, and Escherichia coli. | Antimicrobial activity by broth microdilution for determination of MIC values. | Eucalyptus honey had MIC of 25% (v/v) on the tested strains, except for Streptococcus anginosus and Streptococcus oralis, whose MIC values were 17% (v/v) and 12% (v/v), respectively. Hypertonic sugar control had MIC of 25% (vol/vol) on all bacterial strains. | [75] |
| Manuka honey (Leptospermum scoparium), eucalyptus honey (Eucalyptus cladocalyx), pin cushion honey (Leucospermum cordifolium), and Erica honey (Erica species—Fynbos). | Streptococcus mutans, Streptococcus salivarius, Streptococcus sanguis, Streptococcus anginosus, Streptococcus gordonii, Streptococcus oralis, Streptococcus sobrinus, Candida albicans, Escherichia coli and Staphylococcus aureus. | Antimicrobial activity by broth microdilution for determination of MIC values. | Candida albicans yeast (MIC of 40%) was more resistant to the tested honeys than were the bacterial strains. Streptococcus anginosus (MIC of 17%) and S. oralis (MIC of 12.5%) were more sensitive to honey than the other strains. The honey samples showed MIC of 25% against other oral streptococci. | [76] |
| Azarian honey. | Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus mutans, MRSA, and Enterococcus faecalis | Antimicrobial activity by broth microdilution for the determination of MIC values. | The lowest MIC value of the tested honeys was found for Staphylococcus aureus. The MIC of honey for Escherichia coli was higher than that for the other strains. The mean MIC for Staphylococcus epidermidis was similar to that of Staphylococcus aureus. The MBC values for S. mutans, MRSA, Staphylococcus aureus, Staphylococcus epidermidis, and Enterococcus faecalis were 7.81%, 8.52%, 7.55%, 12.03%, and 7.81% (v/v), respectively. The combination of propolis and honey reduced the MIC for all bacterial strains. | [77] |
| Commercial honey from Saudi Arabia (Langnese Honig, Germany). | Streptococcus mutans | Antimicrobial activity (microdilution method) and inhibition of biofilm formation. | Natural honey reduced Streptococcus mutans growth more effectively than artificial honey (control) at the concentrations of 25% and 12.5%. At 50% and 25%, both honeys significantly reduced bacterial growth and biofilm formation as compared to the TSB control. Natural honey was also able to decrease the maximum growth rate of Streptococcus mutans compared to artificial honey. | [78] |
| Ramadan natural honey. | Streptococcus mutans | Antimicrobial activity by the agar diffusion method. | Significant antibacterial activity was detected against Streptococcus mutans at concentrations greater than 20% and against Lactobacillus at a concentration of 100%. | [55] |
| Manuka honey (Leptospermum scoparium) and commercial multifloral honey from Germany. | Porphyromonas gingivalis | Antimicrobial activity by the microdilution method (determination of the MIC) and antibiofilm activity. | Manuka honey and commercial honey inhibited 50% of Porphyromonas gingivalis growth at concentrations of 2% and 5%, respectively. Manuka honey contained 1.87 mg/kg of hydrogen peroxide, whereas the commercial honey had 3.74 mg/kg. The amount of methylglyoxal was 2 mg/kg in the domestic honey and 982 mg/kg in Manuka honey. At 10%, both types of honey inhibited Porphyromonas gingivalis biofilm formation and reduced the number of viable bacteria in 42-hour-old biofilms. | [79] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romário-Silva, D.; Alencar, S.M.; Bueno-Silva, B.; Sardi, J.d.C.O.; Franchin, M.; Carvalho, R.D.P.d.; Ferreira, T.E.d.S.A.; Rosalen, P.L. Antimicrobial Activity of Honey against Oral Microorganisms: Current Reality, Methodological Challenges and Solutions. Microorganisms 2022, 10, 2325. https://doi.org/10.3390/microorganisms10122325
Romário-Silva D, Alencar SM, Bueno-Silva B, Sardi JdCO, Franchin M, Carvalho RDPd, Ferreira TEdSA, Rosalen PL. Antimicrobial Activity of Honey against Oral Microorganisms: Current Reality, Methodological Challenges and Solutions. Microorganisms. 2022; 10(12):2325. https://doi.org/10.3390/microorganisms10122325
Chicago/Turabian StyleRomário-Silva, Diego, Severino Matias Alencar, Bruno Bueno-Silva, Janaína de Cássia Orlandi Sardi, Marcelo Franchin, Rafaela Durrer Parolina de Carvalho, Thayná Ellen de Sousa Alves Ferreira, and Pedro Luiz Rosalen. 2022. "Antimicrobial Activity of Honey against Oral Microorganisms: Current Reality, Methodological Challenges and Solutions" Microorganisms 10, no. 12: 2325. https://doi.org/10.3390/microorganisms10122325
APA StyleRomário-Silva, D., Alencar, S. M., Bueno-Silva, B., Sardi, J. d. C. O., Franchin, M., Carvalho, R. D. P. d., Ferreira, T. E. d. S. A., & Rosalen, P. L. (2022). Antimicrobial Activity of Honey against Oral Microorganisms: Current Reality, Methodological Challenges and Solutions. Microorganisms, 10(12), 2325. https://doi.org/10.3390/microorganisms10122325





