Abstract
At present, there is an increasing interest in beverages of non-dairy origin, as alternatives to those based on milk, but having similar health-promoting properties. Fermentation with specific bacteria or consortia may enhance the functionality of these products. In our study, selected lactic acid bacteria, that have been previously shown to possess functional properties (antimicrobial activity, probiotic potential), were used for the fermentation of wheat bran combined with root vegetables. Strains were investigated for their safety, while the obtained beverages were characterized in terms of microbial content, physical, chemical, nutritional, and functional properties. None of the strains harbors virulence genes, but all of them possess genes for survival at low pH, starch metabolism, and vitamin biosynthesis. Three strains (Lactiplantibacillus plantarum BR9, L. plantarum P35, and Lactobacillus acidophilus IBB801) and two substrates (5% wheat bran with 10% red beetroot/carrots) were selected based on a preliminary assessment of the beverage’s sensory acceptability. These strains showed good growth and stability over time in the stored beverages. No enterobacteria were detected at the end of fermentations, while the final pH was, in most cases, below 3.5. Free phenolics, flavonoids, and DPPH scavenging effect increased during fermentation in all drinks, reaching 24h values that were much higher than in the unfermented substrates. Most of the obtained drinks were able to prevent the growth of certain pathogens, including Listeria monocytogenes ATCC 19111, Salmonella enterica ATCC 14028, Staphylococcus aureus ATCC 25923, and Escherichia coli ATCC 25922. The obtained beverages would combine the nutritiveness of the raw ingredients with the beneficial effect of fermentation (increasing shelf life, health-promoting effect, pleasant flavor, etc.). They would also fill a gap in the non-dairy probiotics sector, which is constantly increasing due to the increasing number of vegan people or people that cannot consume dairy products.
1. Introduction
Fermentation, in particular, lactic acid bacteria (LAB) fermentation is known as one of the oldest preservation methods, applied to various substrates, including milk, meat, fish, vegetables, and cereals. Besides preservation, LAB also contributes to the sensory, nutritional, and functional attributes of the final products [1]. Consumption of fermented foods and beverages implies health-promoting benefits [2,3], which include enhanced digestibility, antipathogenic [4,5,6], antihypertensive [5], antioxidant [7], and immune-stimulating properties [8]. Homemade products, obtained by spontaneous fermentation, may differ in aroma, flavor, texture, and quality due to other environmental factors affecting the process [9]. Therefore, there is an increased concern nowadays to enhance the functionality of these products by standardizing the process and using specific bacteria or consortia with proven benefits [1].
The concept of functional foods was born in Japan in the 1980s. They were developed specifically to promote health or reduce the risk of disease and are considered as those foods, which are intended to be consumed as part of the normal diet (not pills or capsules) and exert their effect when used in amounts that can normally be expected to be consumed in the diet [10,11]. Principal substances that impart functionality to a food/beverage include vitamins, minerals, fiber, omega-3 fatty acids, flavonoids, and probiotic bacterial strains [12]. Attention concerning this category of foods has grown once people became aware of the relationship between diet and health [13,14]. On the other hand, consumers are more and more interested in organic foods and they tend to avoid foods with artificial ingredients [15].
Compared with solid foods, beverages offer the advantages of greater possibility of incorporating bioactive compounds and easy delivery and storage of the final products [16]. Commercially, a wide variety of functional beverages exist, including dairy-based beverages, vegetable and fruit-based beverages, sports drinks, energy drinks, tea and tea-based beverages, and whey and soy proteins-based beverages [11]. Dairy beverages (yogurt drinks, fresh or fermented milk) are considered an excellent way of delivering probiotics. However, there is an increasing interest in beverages of non-dairy origin, as alternatives to those based on milk, but having similar health-promoting properties. These products are requested both by vegans and by lactose-intolerant people or those with a high cholesterol level [17]. In this context, various functional non-dairy beverages that contain probiotics have been launched, including fruit, cereals, soybeans, and vegetable-based beverages [18,19].
Fermented foods and drinks of non-dairy origin represent an important part of the daily food in Romania. Among these, fermented vegetables or cereals are in the top positions. Wheat bran, for instance, is widely used in Romania for the production of borș, an acidic liquid used in Romanian cuisine for the preparation of a traditional soup known as borş or ciorbă, but also consumed as a refreshing drink [20]. Wheat bran is the major by-product of wheat processing, but it has high utilization values because of its nutritional contents, such as enzymes, proteins, carbohydrates, vitamins, and minerals [21], with various health benefits, including: enhanced immunity, improved digestion, and metabolism, or antioxidant activity that leads to protection of normal cells from oxidative damage [22]. Moreover, it has been shown that diets rich in cereals play a crucial role in the prevention of chronic diseases such as cardiovascular disease and certain types of cancer [23].
On the other hand, fruit and vegetable juices are also known for their high nutritiveness and health benefits. Red beetroot, for instance, is known for its antihypertensive, hypoglycemic, anti-inflammatory, hepatoprotective, and antioxidant activity [24,25,26,27]. Recently, it has been shown that the antioxidant activity of many plant-based foods was enhanced by microbial fermentation [28]. Moreover, many vegetables, including red beetroot and carrots are rich in pigments with coloring potential but are seasonal and unstable. Fermentation with selected LAB strains may be used to increase their durability and enhance the pigment contents [29,30].
In this context, the aim of our study was to use selected functional LAB strains for the fermentation of substrates containing both wheat bran and root vegetables (red beetroot or carrots) and to characterize the obtained beverages in terms of microbial content, physical, chemical, nutritional, and functional properties.
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
Bacterial strains used as starters in this study have been previously isolated from various (fermented) foods/drinks, including both dairy and non-dairy products (Table 1). These strains were selected based on their functional properties, such as antibacterial/antifungal activity, production of bacteriocins/surfactants/exopolysaccharides (EPS), or probiotic potential, as mentioned in Table 1. All strains were maintained at −80 °C in MRS broth [31], in the presence of 25% (v/v) of glycerol for cryoprotection, as part of the Culture Collection of the Department of Microbiology, Institute of Biology Bucharest, Romania. Before use, strains were propagated twice, for 24 h, in MRS broth, at 28 °C (the two Leuconostoc strains) or 37 °C (all lactobacilli).

Table 1.
Bacterial strains used in this study as inoculum for fermentation.
2.2. Safety Evaluation of the Bacterial Strains Used as Inoculum
Detection of Virulence Genes
LAB strains were screened for the presence of virulence genes ace (adhesion collagen protein), agg (aggregation), and asa (aggregation) by PCR, following the method/primers of Pieniz et al. [37], as mentioned in Table 2. Genomic DNA was extracted from overnight LAB cultures, using a Pure Link Genomic DNA kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s guidelines. The PCR was performed by adding 50 ng/μL of DNA extract to a final volume of 25 µL reaction mixture containing 1× GoTaq Flexi buffer, 2.5 mM MgCl2, dNTP Mix (containing 0.2 mM each dNTP), 0.5 µM forward and reverse primer (Table 2), and 1.25 U GoTaq G2 Hot Start Polymerase (Invitrogen, Waltham, MA, USA). The PCR conditions for virulence genes, ace, asa, and agg, were as follows: initial denaturation at 92 °C for 2 min followed by 35 cycles of denaturation at 92 °C for 30 s, primers annealing at optimum temperature for each set for 1 min (Table 2), 72 °C extension for 1 min and final extension at 72 °C for 5 min. The PCR products were analyzed on 1.5% agarose gels supplemented with SYBR Safe DNA Gel Stain (Invitrogen).

Table 2.
Primers used for detection of virulence/functional genes.
2.3. Genetic Screening of LAB Functional Properties
The bacterial strains used as inoculum were screened by PCR multiplex reactions for the presence in their genome of some functional genes involved in stress resistance, production of vitamins, and starch metabolism, respectively. The function of each investigated gene and the primers used for the screening are listed in Table 2. KAPA2G Fast Multiplex PCR kit (KAPA Biosystems, Wilmington, MA, USA) was used and each mixture (25 µL) contained 2× multiplex mix, 10 µM of each primer, and 100 ng DNA template. The PCR conditions were as follows: initial denaturation at 95 °C for 3 min followed by 30 cycles of denaturation at 95 °C for 15 s, primers annealing at a temperature depending on the primer (Table 2) for 30 s, elongation at 72 °C for 90 s, and a final elongation at 72 °C for 10 min. PCR products were separated on a 2% agarose gel, supplemented with ethidium bromide, and UV-analyzed for the presence of a unique amplicon.
2.4. Fermentation of Wheat Bran and Root Vegetables with Selected LAB Strains
Various combinations of wheat bran (WB) with either red beetroot (BR), carrots (C), or both, were used as fermentation substrates (Table 3). Both WB and vegetables were purchased from local markets. Fresh BR and C were blended, added to the WB, and suspended in hot water. After cooling down to about 40 °C, each combination was inoculated (2%) with one of the LAB strains listed in Table 1. For this, bacterial cells were recovered from overnight MRS cultures by centrifugation (10,000× g, 4 °C, 10 min), washed twice with saline water, and suspended in saline water at a concentration of about log 8–9 CFU/mL before being inoculated. Mixtures were incubated for 24 h at the optimal growth temperature of each bacterial strain.

Table 3.
Combination of substrates used for fermentation by selected LAB strains.
Based on a preliminary sensory analysis (data not shown) of the fermented products, two substrate combinations (numbers 3 and 4 from Table 3), inoculated with L. plantarum BR9, L. plantarum P35, and L. acidophilus IBB801 (2% inoculum prepared as above), or various mixtures of them (1% inoculum of each strain), were selected for further studies. During fermentation, 50 mL samples were taken from each batch at 0, 3, 6, 9, 12, and 24 h for analyses.
2.5. Microbiological Analyses
LAB viability was evaluated by counting the CFU numbers on MRS agar plates (1.5% agar), in each sample collected during fermentation. The viability of the starter strains was further determined 1 week after the end of fermentation, during storage at 4 °C.
Undiluted samples were also plated on violet-red-bile-glucose (VRBG) agar medium (Merck KGaA, Darmstadt, Germany) supplemented with 0.1 g/L of cycloheximide (incubated at 37 °C), and yeast extract-peptone-glucose (YPG) agar medium (Merck) supplemented with 0.1 g/L of chloramphenicol (incubated at 28 °C), for the enumeration of enterobacteria and yeasts, respectively.
2.6. Physical, Chemical, and Nutritional Characteristics
pH measurements. Acidification during the fermentation was analyzed by measuring the pH with an InoLab 720 pH meter (WTW, Weilheim, Germany).
Lactic acid production. A Jasco HPLC System (Jasco Europe, Cremella, Italy), equipped with a PRPx300 (Hamilton, Switzerland) column, maintained at 60 °C, and coupled with a photodiode array (PDA) detector, was used for lactic acid quantification. Elution was performed with 2.5 mM H2SO4, at a flow rate of 0.5 mL/min.
Free phenolics. The free phenolic content was measured using the Folin–Ciocalteu method [39] and the results were expressed as µg of gallic acid equivalents per ml of sample (µg GAE/mL).
Flavonoids content. The content of total flavonoids was assessed based on the method described by Dewanto et al. [40]. Briefly, 1 mL of each sample was mixed with 4 mL of deionized water and 0.3 mL of 5% NaNO2. After 5 min at room temperature, 0.3 mL of 10% AlCl3 was added to the mixture and kept for another 6 min. Finally, 2 mL of 4% NaOH and 2.4 mL of deionized water were added and the absorption was read after 15 min at 510 nm against water. Rutin was used as standard, and results were expressed as µg of rutin equivalents per ml of sample (µg RE/mL).
DPPH free radical scavenging activity. The free radical scavenging activity was determined using DPPH (2,2-diphenyl-1-picrylhydrazyl radical), based on the method of Moon and Terao [41]. Briefly, 0.1 mL of sample was added to 2.25 mL methanol and vortexed. DPPH (1.27 mM) was then added (0.15 mL), vortexed again, and incubated for 30 min in the dark, at room temperature. The absorbance was finally measured at 517 nm, using a control without sample. The scavenging effect (Se) was calculated using the formula [42]:
where Abs means absorbance of the sample/control.
All determination were done in triplicate and the results are given as mean value ± standard deviation (SD).
2.7. Functional Properties
Antibacterial activity. The antagonistic activity of the samples collected during fermentation was assayed by the agar spot method [43] against L. delbrueckii subsp. bulgaricus LMG6901T as indicator strain. Samples were centrifuged to remove cells, and the supernatant was serially diluted up to 1/8. From each dilution, 10 µL were spotted onto a fresh indicator lawn (0.7% agar MRS with 100 µL of the sensitive strain). The activity was defined as the reciprocal of the highest dilution for which a clear inhibition zone could be observed, and was expressed in activity units (AU) per milliliter of sample.
Prevention of pathogens growth. Aliquots of the fermented drinks were filter sterilized and inoculated with one of the following pathogens: Listeria monocytogenes ATCC 19111, Salmonella enterica ATCC 14028, Staphylococcus aureus ATCC 25923, Escherichia coli ATCC 25922, and Bacillus cereus CBAB, in two different concentrations (2%, and 10%, respectively). OD600nm was measured hourly, for 24h, in a plate reader (Spectra Max, Molecular Devices, San Jose, CA, USA). Filter-sterilized non-fermented substrates inoculated with the pathogens served as controls.
2.8. Statistical Analysis
Experimental data were recorded as mean ± standard deviation of triplicates, generated by GraphPad Outlier calculator. Outliers were calculated using a significance level (alpha) set at 0.05. GraphPad Prism (GraphPad Software LLC, San Diego, CA, USA) was used for the statistical analysis of the results (One-way ANOVA with a post hoc test: Dunnett’s multiple comparisons test), also with a significance level (alpha) set at 0.05.
3. Results
3.1. Safety Evaluation of the Bacterial Strains Used as Inoculum
PCR reactions with three primer sets used for the detection of virulence genes did not result in specific amplification for any of the genomic DNA extracted from the seven tested strains (Figure S1).
3.2. Genetic Screening of LAB Functional Properties
As seen in Table 4, most LAB strains selected for this study harbor genes involved in survival at low pH (LBA1272), in starch metabolism (agl, α-amy, and for some strains malL), and in vitamin production (folP and ribA). None of the strains harbors dltD or folK genes.

Table 4.
Functional genes present in the genome of tested bacteria.
3.3. Fermentation of Wheat Bran and Root Vegetables with Selected LAB Strains
3.3.1. Microbiological Analysis
LAB counts were variable at the start of the fermentation, ranging from about 6 to about 8 log CFU/mL, the highest being for L. plantarum P35 (Table 5). The counts increased fast during the first 6h for all cultures, and reached, after 24h, about 8 log CFU/mL in the case of L. acidophilus IBB801, and about 9 log CFU/mL for the other strains, regardless the substrate used for fermentation. A slight difference could be detected in the case of L. plantarum P35, both as single culture, and in combination with L. acidophilus IBB801, for which the growth was faster when carrots were used instead of red beetroot.

Table 5.
Fermentation parameters, antibacterial activity and nutritional properties of the beverages obtained with selected strains.
The presence of yeasts was not detected in any sample (results not shown), but low numbers of enterobacteria (up to about 3 log CFU/mL) were counted in the single cultures of L. plantarum BR9 and L. acidophilus IBB801, at certain times, in the middle of the fermentation. No enterobacteria were present at the end of the fermentation (24 h) when the liquid was removed and stored at 4 °C.
After one week of storage, the CFU counts decreased with a maximum of 1.4 log CFU/mL in the liquids obtained from the carrots fermented with the single cultures of L. plantarum BR9, but there were drinks in which CFU counts were not affected (Table 5).
3.3.2. Physical, Chemical, and Nutritional Characteristics
PH Value
During the fermentations, the pH drop was fast, starting within the first 3 h, as the bacterial growth occurred. At 24 h, the pH reached values of about 3.0 in most cases (Table 5). The highest pH values measured at 24 h (3.4–3.6) were for the single culture of L. acidophilus IBB801, in both substrates.
Lactic Acid Production
Lactic acid could not be detected in the first 3 h of fermentation in any of the collected liquids. At 6 h, small amounts of lactic acid were present in almost all samples. The concentration increased afterward, directly related to bacterial growth (Table 5). L. plantarum P35 and L. acidophilus IBB801, both in single cultures and in their co-culture, produced more lactic acid in the substrate based on carrots, where the growth was also faster. However, L acidophilus produced the lowest amount of lactic acid (about 8 mg/mL at 24 h), compared to the other strains (between about 11 and 15 mg/mL at 24 h).
Free Phenolics
Total free phenolics were higher when red beetroot was used as a substrate, and increased during fermentation from 112–128 µg GAE/mL, immediately after inoculation, to about 158–188 µg GAE/mL, at 24 h (about 50% increase) (Table 5). When carrots were used in combination with wheat bran, total free phenolics were lower during the whole fermentation process, but the increase in the end samples was over 75%, and even over 100% in the case of L. acidophilus IBB801 compared to the initial values. Moreover, all end values were higher than the ones of the unfermented substrates, regardless of the vegetable type (Figure 1).
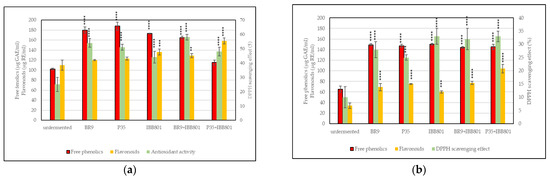
Figure 1.
Free phenolics, flavonoids, and DPPH scavenging effect of the fermented beverages: (a) WB + BR; (b) WB + C. Differences between fermented and unfermented samples were statistically analyzed (One-way ANOVA with post hoc test: Dunnett’s multiple comparisons test) and p value summary was marked with asterisks on the graph; no asterisk means no significant difference, while **, ***, and **** mean p < 0.01, p < 0.001, and p < 0.0001, respectively.
Flavonoids Content
The flavonoid content was higher in the fermented substrates than in the unfermented samples, but the amounts depended on the vegetable and on the strain used as inoculum (Figure 1). In the beetroot fermentations, the initial values ranged from about 83 µg RE/mL (for L. acidophilus IBB801) to about 116 µg RE/mL (L. plantarum P35) (Table 5). However, the increase of the flavonoid content over time was the highest in L. acidophilus IBB801 final samples (about 64%) and the lowest in L. plantarum P35 final samples (about 6%). In the carrot fermentations, even if lower concentrations were measured, the increase over time was much higher, the lowest being 45% for the co-culture BR9+IBB801, and the highest for P35, in single culture (114%), and in co-culture with IBB801 (148%).
Antioxidant Activity
The antioxidant activity determined in the red beetroot fermentations as a DPPH scavenging effect, increased, in general, in the first 3h from inoculation, and remained afterward approximately constant till the end (Table 5). Compared with the unfermented samples, the antioxidant activity is about double (p < 0.0001) in all drinks obtained with this substrate (Figure 1a). When carrots were used instead of red beetroot, the antioxidant activity did not register significant changes during fermentation, and the values were, in general, lower (maximum 33%) than in beetroot (maximum 58%). However, the final values, at 24 h (between 25–33%), were significantly (p < 0.0001) higher than for the unfermented samples (10%) (Figure 1b).
3.3.3. Functional Properties
Antibacterial Activity
Except for the two variants fermented with L. plantarum BR9, all drinks showed antibacterial activity when tested against L. delbrueckii subsp. bulgaricus LMG6901T as indicator strain (Table 5). In general, the activity was higher in WB + C drinks and reached a maximum of 200 AU/mL. When L. plantarum P35 was used in single culture or in co-culture, the activity was detected much earlier (after 3–6 h of fermentation) compared with the other strains (after 9–12 h).
Prevention of Pathogens Growth
Pathogens used in this experiment were able to grow in both substrates used for fermentation (WB+C, and WB+BR, respectively), reaching after 24 h, in general, OD600nm between 0.2 and 0.4, depending on the strain, the concentration of inoculum, and the substrate (Figure 2 and Figure 3). Lower OD600nm values were measured for L. monocytogenes, S. enterica, and S. aureus in WB + C medium, when inoculated in concentrations of 2%.
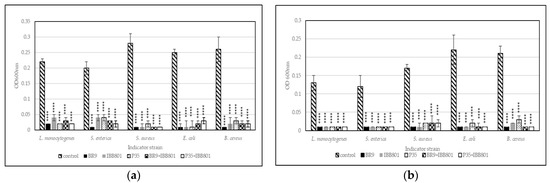
Figure 2.
Growth (recorded as maximum OD600nm) of pathogens inoculated (2%) in sterilized supernatants of the fermented beverages obtained with: (a) WB + BR; (b) WB + C. Differences between fermented and control (unfermented) samples were statistically analyzed (One-way ANOVA with post hoc test: Dunnett’s multiple comparisons test) for each pathogen and p value summary was marked with asterisks on the graph. **** means p < 0.0001.
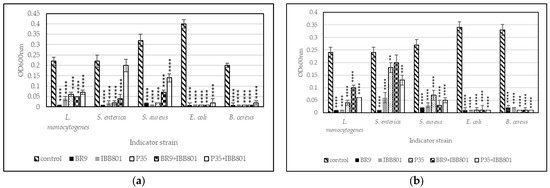
Figure 3.
Growth (recorded as maximum OD600nm) of pathogens inoculated (10%) in sterilized supernatants of the fermented beverages obtained with: (a) WB + BR; (b) WB + C. Differences between fermented and control (unfermented) samples were statistically analyzed (One-way ANOVA with post hoc test: Dunnett’s multiple comparisons test) and p value summary was marked with asterisks on the graph; no asterisk means no significant difference, while **, ***, and **** mean p < 0.01, p < 0.001, and p < 0.0001, respectively.
Figure 2 shows the comparative growth of the five pathogens in control media (WB+BR, and WB+C, respectively) and in sterilized supernatant of the fermented drinks when 2% of the pathogen was used as inoculum. All 10 variants of fermented drinks succeeded at preventing the growth of the pathogens at this concentration, the OD600nm of the culture after 24h being below 0.05.
When a higher concentration of inoculum was used (10%), E. coli and B. cereus growth was completely inhibited in all drinks (Figure 3). The other pathogens, but especially S. enterica, were able to grow in some drinks (such as those obtained with L. plantarum P35 and with the co-cultures), up to OD600nm of 0.1–0.2. The values were higher, in general, in the WB + C variants. However, the growth of the pathogens started much later (after 16–20 h) in these drinks as compared with the controls (Figure 4).
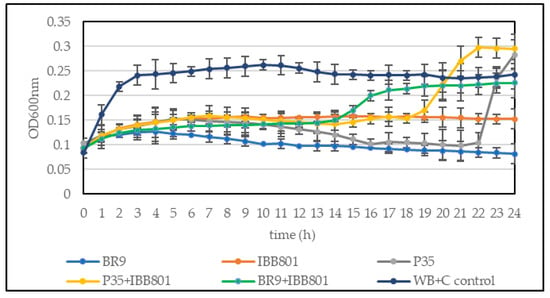
Figure 4.
Growth curves of S. enterica ATCC 14028 inoculated (10%) in sterilized supernatant of fermented beverages obtained with WB + C. Control: unfermented WB + C.
4. Discussion
Consumption of foods rich in dietary fiber has many health benefits [44]. However, many products, including wastes from cereal processing (i.e., wheat or rice bran), that contain fiber, along with protein, starch, and other beneficial ingredients, are not being fully utilized [45]. One reason is that such products cause lower overall acceptability, darker color, and poor consistency and texture [46]. Fermentation with selected LAB strains may be used to increase the bioavailability of nutrients and to improve sensory properties [47].
In general, LAB can be safely included in products intended for human use due to their GRAS (generally recognized as safe) status. In particular, the strains used in our study do not harbor virulence genes and they are sensitive to many common antibiotics, showing their safety for consumption. These LAB strains have been selected based on some particular properties, including antimicrobial activity and probiotic potential, and have been used to obtain fermented beverages using wheat bran as substrate. Red beetroot and carrots were added to this substrate, in order to accelerate bacterial growth and to improve the sensory and nutritional properties of the final products. Indeed, all seven LAB strains were able to grow in all tested combinations of cereals/vegetables, resulting in products characterized, in general, by pleasant flavor and color. Regarding the overall acceptability, the volunteers gave very good scores (over 8) to many of these beverages. Based on their reports, the best two substrates have been selected for further fermentations: 5% WB + 10% vegetable (BR or C). Products obtained with L. plantarum BR9 and L. plantarum P35 received the best scores on these substrates, due to their strong acidic taste, very similar to the Romanian traditional borș. Such fermented products can be succesfully used for the preparation of sour soups. Besides their specific flavor, beverages obtained from WB and BR also have a pleasant color, very well appreciated by most of the volunteers. It has been shown that thermal treatment of BR juice may lead to a change in color to brown [48]. However, fermentation resulted in nice red color, close to that of natural juice, and this may be due to the low pH, of about 4.0, which offers the greatest stability of the red pigment in BR [48]. Fermented drinks obtained with L. acidophilus IBB801 were also preferred by many tasters due to the delicate acidic taste, which can be desired in beverages intended to be consumed as refreshing drinks. This strain was used, therefore, in the controlled fermentations, both as single strain and in co-culture with one of the two L. plantarum.
The good bacterial growth in all variants of cereal-based substrates may be explained by the fact that most of the tested LAB strains harbor in their genome genes involved in starch metabolism. Several LAB species, such as L. amylolyticus, L. amylotrophicus, and L. amylovorus, but also strains of L. plantarum, isolated from fermented cassava, maize, sorghum, rice, and beer malt, have been shown to degrade starch in the presence of easier fermentable carbohydrates [49].
In the followed-up fermentations, the three selected strains caused a significant decrease of the pH, down to 3.1–3.6, depending on the strain and on the substrate, with a concomitant increase in the viable cell numbers and in the lactic acid production, as it has been previously reported for LAB grown in various plant-based substrates [50]. On the other hand, due to the low pH values, no Enterobacteria were found in the end-products, proving their good fermentation quality. Similar tendencies were reported by Krungleviciute et al. [51] who found that fermentation of barley and wheat bran with LAB reduced the Enterobacteria and total aerobic bacteria count in the substrate. This result can be explained by the drop in the pH value below 3.8, which inhibits most food spoilage bacteria [52]. All the fermented beverages could be stored at 4 °C in well-sealed recipients for a couple of weeks without being spoiled or losing their organoleptic properties, as observed before with laboratory-made bors [20]. Moreover, it seems that yeasts were also inhibited by the low pH value since they were not found in any fermented product.
The nutritional characteristics and functional properties of the fermented beverages were also investigated. In general, beneficial health effects of diets rich in cereals have been ascribed to dietary fiber or to some of the components associated with the fiber, including phenolic acids [53]. Together with fibers, polyphenols are important for antioxidant and anti-inflammatory properties.
Fermentation had an enhancing effect on the total phenolics, flavonoids, and antioxidant activity, in variable degrees, depending on the bacterial strain/combination used as inoculum. The same effect was reported by other studies, using various cereal substrates [54], and it may be explained by activation at low pH of endogenous enzymes of the cereals, causing a structural breakdown of plant cell walls, and a release of bioactive compounds [55,56].
Antioxidants (such as vitamin C, phenolic compounds, or glutathione) are able to transform reactive oxygen forms of the oxidoreduction reaction into more stable and non-reactive forms, preventing oxidative cell damage and degenerative diseases [57]. On the other hand, antioxidant enzymes found in plants, including superoxide dismutases, catalases, or glutathione peroxidases, contribute to the detoxification process by converting the reactive oxygen species to hydrogen peroxide, further degraded by peroxidases or catalases to water [58]. It has been also reported that LAB strains might have antioxidant activity themselves, due to the production of antioxidant compounds, such as exo-polysaccharides, peptides, glutathione, benzoic acid, and benzaldehyde, but also antioxidant enzymes, such as superoxide dismutase [57]. This can be the case with our strains since the antioxidant activity determined immediately after inoculation was higher than in the uninoculated samples.
Moreover, total phenolics, but also flavonoids and antioxidant activities, were higher in the red beetroot fermentations than in carrot fermentations. In general, a direct correlation existed between the phenolic content and the scavenging of DPPH radical, as shown by other authors, as well [59]. Therefore, our study reveals the potential of wheat bran and root vegetables, such as red beetroot and carrots, as value-added ingredients for fermented functional foods/beverages. They may be exploited as a potential source of antioxidants in these fermented products and may replace synthetic antioxidants in food formulations [53].
On the other hand, fermented beverages, especially the ones based on red beetroot, can be good matrices for delivering probiotic strains, since they harbor, even after one week of storage at 4 °C, all tested strains, including the potential probiotic L. plantarum BR9, at viabilities much higher than log 6 CFU/mL, which is the minimum number for optimal therapeutic effects [60].
Last, but not least, most of the fermented products showed inhibitory activity against several bacterial strains used as indicators. They were able to prevent the growth of pathogens/spoilage bacteria when these were intentionally added (in concentrations up to 10%) to the cell-free supernatants. It is known that LAB are antagonistic toward various bacteria that may be present in raw materials and may cause unpleasant changes in the organoleptic properties [48], and therefore, they may find application in the food industry. This might be also the case for the strains used in this study, especially for L. plantarum BR9 and L. acidophilus IBB801, which inhibited the growth of most indicator bacteria, even when these were inoculated in a high concentration. Such strains may be used to reduce the amounts of preservatives added to foods, being a much healthier, non-toxic, and non-allergenic alternative to these chemical compounds.
5. Conclusions
The strains selected in this study may find application as starters for controlled cereal/vegetable-based fermentations. The obtained beverages would combine the nutritiveness of the raw ingredients with the beneficial effect of fermentation (increasing shelf life, health-promoting effect, pleasant flavor, etc.). They would also fill a gap in the non-dairy probiotics sector, which is constantly increasing due to the increasing number of vegan people or people that cannot consume dairy products.
Products obtained with L. plantarum BR9 and L. plantarum P35, with a strong acidic taste, are suitable, for instance, as ingredients for sour soups, while the less acidic products obtained with L. acidophilus IBB801 may be used as refreshing drinks. All three strains are safe to be used in such products, and their fermentation increases the total phenolic content and antioxidant activity, but also lowers the pH, preventing the growth of undesired bacteria or fungi.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms10122314/s1, Figure S1: PCR amplification of genomic DNA extracted from various LAB strains, using specific primers for agg (a), ace (b), and asa (c) virulence genes.
Author Contributions
Conceptualization, M.Z.; methodology, M.Z., I.-R.A. and S.-S.G.-T.; formal analysis and investigation, M.Z., I.-R.A., C.V., O.B.-S., C.-P.C. and S.-S.G.-T.; writing—original draft preparation, M.Z.; writing—review and editing, M.Z., I.-R.A., C.V., O.B.-S., C.-P.C. and S.-S.G.-T.; supervision, S.-S.G.-T.; project administration, M.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Romanian Ministry of Education and Research, CCCDI–UEFISCDI, project number PN-III-P2-2.1-PED-2019-1675, within PNCDI III, and by the Institute of Biology Bucharest of the Romanian Academy, project number RO1567-IBB05/2022.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article and in the supplementary files linked to it.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pérez-Armendáriz, B.; Cardoso-Ugarte, G.A. Traditional fermented beverages in Mexico: Biotechnological, nutritional, and functional approaches. Food Res. Int. 2020, 136, 109307. [Google Scholar] [CrossRef] [PubMed]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gänzle, M.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health benefits of fermented foods: Microbiota and beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Zannou, O.; Dehouegnon, J.A.; Yann, M.; Oyeniran, B.A.; Malthus, D.A.; Ifagbémi, B.C.; Yénoukounmè, E.K.; Paulin, A.; Ilkay, K. Traditional fermented foods and beverages: Indigenous practices of food processing in Benin Republic. Int. J. Gastron. Food Sci. 2022, 27, 100450. [Google Scholar] [CrossRef]
- Cordeiro, M.A.; Souza, E.L.S.; Arantes, R.M.E.; Balthazar, C.F.; Guimaraes, J.T.; Scudino, H.; Silva, H.; Rocha, R.; Freitas, M.; Esmerino, E.; et al. Fermented whey dairy beverage offers protection against Salmonella enterica ssp. enterica serovar typhimurium infection in mice. J. Dairy Sci. 2019, 102, 6756–6765. [Google Scholar] [CrossRef] [PubMed]
- Luz, C.; Izzo, L.; Graziani, G.; Gaspari, A.; Ritieni, A.; Manes, J.; Meca, G. Evaluation of biological and antimicrobial properties of freeze-dried whey fermented by different strains of Lactobacillus plantarum. Food Funct. 2018, 9, 3688–3697. [Google Scholar] [CrossRef] [PubMed]
- Sugiharto, S.; Lauridsen, C.; Jensen, B.B. Gastrointestinal ecosystem and immunological responses in E. coli challenged pigs after weaning fed liquid diets containing whey permeate fermented with different lactic acid bacteria. Anim. Feed Sci. Technol. 2015, 207, 278–282. [Google Scholar] [CrossRef]
- Zhao, Z.W.; Pan, D.D.; Wu, Z.; Sun, Y.Y.; Guo, Y.X.; Zeng, X.Q. Antialcoholic liver activity of whey fermented by Lactobacillus casei isolated from koumiss. J. Dairy Sci. 2014, 97, 4062–4071. [Google Scholar] [CrossRef] [PubMed]
- García, G.; Agosto, M.E.; Cavaglieri, L.; Dogi, C. Effect of fermented whey with a probiotic bacterium on gut immune system. J. Dairy Res. 2020, 87, 134–137. [Google Scholar] [CrossRef]
- Ramos, C.L.; Schwan, R.F. Technological and nutritional aspects of indigenous Latin America fermented foods. Curr. Opin. Food Sci. 2017, 13, 97–102. [Google Scholar] [CrossRef]
- Serafini, M.; Stanzione, A.; Foddai, S. Functional foods: Traditional use and European legislation. Int. J. Food Sci. Nutr. 2012, 63 (Suppl. 1), 7–9. [Google Scholar] [CrossRef]
- Nazir, M.; Arif, S.; Khan, R.S.; Nazir, W.; Khalid, N.; Maqsood, S. Opportunities and challenges for functional and medicinal beverages: Current and future trends. Trends Food Sci. Technol. 2019, 88, 513–526. [Google Scholar] [CrossRef]
- Keservani, R.K.; Kesharwani, R.K.; Vyas, N.; Jain, S.; Raghuvanshi, R.; Sharma, A.K. Nutraceutical and functional food as future food: A review. Der. Pharmacia. Lett. 2010, 2, 106–116. [Google Scholar]
- Granato, D.; Branco, G.F.; Nazzaro, F.; Cruz, A.G.; Faria, J.A. Functional foods and nondairy probiotic food development: Trends, concepts, and products. Compr. Rev. Food Sci. Food Saf. 2010, 9, 292–302. [Google Scholar] [CrossRef]
- Pázmándi, M.; Kovács, Z.; Maráz, A. Potential of Lactobacillus strains for the production of fermented functional beverages enriched in galacto-oligosaccharides. LWT Food Sci. Technol. 2021, 143, 111097. [Google Scholar] [CrossRef]
- Iqbal, J.; Yu, D.; Zubair, M.; Rasheed, M.I.; Khizar, H.M.U.; Imran, M. Health Consciousness, Food Safety Concern, and Consumer Purchase Intentions Toward Organic Food: The Role of Consumer Involvement and Ecological Motives. SAGE Open 2021, 11, 21582440211015727. [Google Scholar] [CrossRef]
- Kausar, H.; Saeed, S.; Mushtaq Ahmad, M.; Salam, A. Studies on the development and storage stability of cucumber-melon functional drink. J. Agric. Res. 2012, 50, 239–248. [Google Scholar] [CrossRef]
- Prado, F.C.; Parada, J.L.; Pandey, A.; Soccol, C.R. Trends in non-dairy probiotic beverages. Food Res. Int. 2008, 41, 111–123. [Google Scholar] [CrossRef]
- Hui, Y.H.; Evranuz, E.Ö. Handbook of Plant-Based Fermented Food and Beverage Technology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Saarela, M. Probiotics as ingredients in functional beverages. In Functional and Speciality Beverage Technology; Paquin, P., Ed.; Woodhead Publishing: Cambridge, UK, 2009; pp. 55–70. [Google Scholar] [CrossRef]
- Grosu-Tudor, S.S.; Stefan, I.R.; Stancu, M.M.; Cornea, C.P.; De Vuyst, L.; Zamfir, M. Microbial and nutritional characteristics of fermented wheat bran in traditional Romanian borş production. Rom. Biotechnol. Lett. 2019, 24, 440–447. [Google Scholar] [CrossRef]
- Shang, X.-L.; Liu, C.-Y.; Dong, H.-Y.; Peng, H.-H.; Zhu, Z.-Y. Extraction, purification, structural characterization, and antioxidant activity of polysaccharides from Wheat Bran. J. Mol. Struct 2021, 1233, 130096. [Google Scholar] [CrossRef]
- Yan, X.; Ye, R.; Chen, Y. Blasting extrusion processing: The increase of soluble dietary fiber content and extraction of soluble-fiber polysaccharides from wheat bran. Food Chem. 2015, 180, 106–115. [Google Scholar] [CrossRef]
- Esposito, F.; Arlotti, G.; Bonifati, A.M.; Napolitano, A.; Vitale, D.; Fogliano, V. Antioxidant activity and dietary fibre in durum wheat bran by products. Food Res. Int. 2005, 38, 1167–1173. [Google Scholar] [CrossRef]
- Ciz, M.; Cizova, H.; Denev, P.; Kratchanova, M.; Slavov, A.; Lojek, A. Different methods for control and comparison of the antioxidant properties of vegetables. Food Control 2010, 21, 518–523. [Google Scholar] [CrossRef]
- Ninfali, P.; Angelino, D. Nutritional and functional potential of Beta vulgaris cicla and rubra. Fitoterapia 2013, 89, 188–199. [Google Scholar] [CrossRef]
- Singh, A.; Garg, V.K.; Sharma, P.K.; Gupta, S. Wound healing activity of ethanolic extract of Beta vulgaris. Pharmacologyonline 2011, 1, 1031–1038. [Google Scholar] [CrossRef]
- Jain, S.; Garg, V.K.; Sharma, P.K. Anti-inflammatory activity of aqueous extract of Beta vulgaris L. J. Basic Clin. Pharm. 2011, 2, 83–86. [Google Scholar] [PubMed]
- Xiao, Y.; Wang, L.; Rui, X.; Li, W.; Chen, X.; Jiang, M.; Dong, M. Enhancement of the antioxidant capacity of soy whey by fermentation with Lactobacillus plantarum B1-6. J. Funct. Foods 2015, 12, 33–44. [Google Scholar] [CrossRef]
- Janiszewska-Turak, E.; Tracz, K.; Bielińska, P.; Rybak, K.; Pobiega, K.; Gniewosz, M.; Woźniak, Ł.; Gramza-Michałowska, A. The Impact of the Fermentation Method on the Pigment Content in Pickled Beetroot and Red Bell Pepper Juices and Freeze-Dried Powders. Appl. Sci. 2022, 12, 5766. [Google Scholar] [CrossRef]
- Janiszewska-Turak, E.; Walczak, M.; Rybak, K.; Pobiega, K.; Gniewosz, M.; Woźniak, Ł.; Witrowa-Rajchert, D. Influence of Fermentation Beetroot Juice Process on the Physico-Chemical Properties of Spray Dried Powder. Molecules 2022, 27, 1008. [Google Scholar] [CrossRef]
- De Man, J.C.; Rogosa, M.; Sharpe, M.E. A medium for the cultivation of lactobacilli. J. Appl. Bacteriol. 1960, 23, 130–135. [Google Scholar] [CrossRef]
- Zamfir, M.; Callewaert, R.; Cornea, C.P.; Savu, L.; Vatafu, I.; De Vuyst, L. Purification and characterization of a bacteriocin produced by Lactobacillus acidophilus IBB801. J. Appl. Microbiol. 1999, 87, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Angelescu, I.R.; Zamfir, M.; Stancu, M.M.; Grosu-Tudor, S.S. Identification and probiotic properties of lactobacilli isolated from two different fermented beverages. Ann. Microbiol. 2019, 69, 1557–1565. [Google Scholar] [CrossRef]
- Grosu-Tudor, S.S.; Zamfir, M.; Van der Meulen, R.; De Vuyst, L. Isolation of novel homopolysaccharide-producing lactic acid bacteria from Romanian raw milk and fermented dairy products. Eur. Food Res. Technol. 2013, 237, 609–615. [Google Scholar] [CrossRef]
- Grosu-Tudor, S.S.; Zamfir, M. Functional properties of lactic acid bacteria isolated from Romanian fermented vegetables. Food Biotechnol. 2013, 27, 235–248. [Google Scholar] [CrossRef]
- Cornea, C.P.; Israel Roming, F.; Sicuia, O.A.; Voaideș, C.; Zamfir, M.; Grosu-Tudor, S.S. Biosurfactant production by Lactobacillus spp. strains isolated from Romanian traditional fermented food products. Rom. Biotechnol. Lett. 2016, 21, 11312–11320. [Google Scholar]
- Pieniz, S.; de Moura, T.M.; Cassenego, A.P.V.; Andreazza, R.; Frazzon, A.P.G.; de Oliveira Camargo, F.A.; Brandelli, A. Evaluation of resistance genes and virulence factors in a food isolated Enterococcus durans with potential probiotic effect. Food Control 2015, 2, 49–54. [Google Scholar] [CrossRef]
- Turpin, W.; Humblot, C.; Guyot, J.P. Genetic Screening of Functional Properties of Lactic Acid Bacteria in a Fermented Pearl Millet Slurry and in the Metagenome of Fermented Starchy Foods. Appl. Environ. Microbiol. 2011, 77, 8722–8734. [Google Scholar] [CrossRef]
- Slinkard, K.; Singleton, V.L. Total phenol analysis: Automation and comparison with manual methods. Am. J. Enol. Vitic. 1997, 28, 49–55. [Google Scholar]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef]
- Moon, J.H.; Terao, J. Antioxidant effect of caffeic acid and dihydrocaffeic acid in lard and human low-density lipoprotein. J. Agric. Food Chem. 1998, 46, 5062–5065. [Google Scholar] [CrossRef]
- Kumari, S.; Deori, M.; Elancheran, R.; Kotoky, J.; Devi, R. In vitro and In vivo Antioxidant, Anti-hyperlipidemic Properties and Chemical Characterization of Centella asiatica (L.) Extract. Front. Pharmacol. 2016, 7, 400. Available online: https://www.frontiersin.org/articles/10.3389/fphar.2016.00400 (accessed on 8 October 2022). [CrossRef]
- De Vuyst, L.; Callewaert, R.; Pot, B. Characterization of the antagonistic activity of Lactobacillus amylovorus DCE 471 and large scale isolation of its bacteriocin amylovorin L471. Syst. Appl. Microbiol. 1996, 19, 9–20. [Google Scholar] [CrossRef]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Health Benefits of Dietary Fibre. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Lei, M.; Samina, N.; Chen, L.L.; Liu, C.L.; Yin, T.T.; Yan, X.T.; Wu, C.; He, H.; Yi, C.P. Impact of Lactobacillus plantarum 423 fermentation on the antioxidant activity and flavor properties of rice bran and wheat bran. Food Chem. 2020, 330, 127156. [Google Scholar] [CrossRef]
- Hemdane, S.; Jacobs, P.J.; Dornez, E.; Verspreet, J.; Delcour, J.A.; Courtin, C.M. Wheat (Triticum aestivum L.) bran in bread making: A critical review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 28–42. [Google Scholar] [CrossRef]
- Verni, M.; Rizzello, C.G.; Coda, R. Fermentation biotechnology applied to cereal industry by-products: Nutritional and functional insights. Front. Nutr. 2019, 6, 42. [Google Scholar] [CrossRef]
- Klewicka, E.; Motyl, I.; Libudzisz, Z. Fermentation of beet juice by bacteria of genus Lactobacillus sp. Eur. Food Res. Technol. 2004, 218, 178–183. [Google Scholar] [CrossRef]
- Hattingh, M.; Alexander, A.; Meijering, I.; Van Reenen, C.A.; Dicks, L.M.T. Amylolytic strains of Lactobacillus plantarum isolated from barley. Afr. J. Biotechnol. 2015, 14, 310–318. [Google Scholar] [CrossRef]
- Gupta, S.; Abu-Ghannam, N.; Scannell, A.G. Growth and kinetics of Lactobacillus plantarum in the fermentation of edible Irish brown seaweeds. Food Bioprod. Process. 2011, 89, 346–355. [Google Scholar] [CrossRef]
- Krungleviciute, V.; Zelvyte, R.; Monkeviciene, I.; Kantautaite, J.; Stankevicius, R.; Ruzauskas, M.; Bartkiene, E. Applicability of Pediococcus strains for fermentation of cereal bran and its influence on the milk yield of dairy cattle. Zemdirb. -Agric. 2017, 104, 63–70. [Google Scholar] [CrossRef]
- Avila, C.L.S.; Carvalho, B.F. Silage fermentation-Updates focusing on the performance of microorganisms. J. Appl. Microbiol. 2019, 128, 966–984. [Google Scholar] [CrossRef]
- Keddari, S.; Aldib, I.; Riazi, A. In vivo stimulatory effects of wheat bran on intestinal microbial ecosystem of mice. South Asian J. Exp. Biol. 2014, 4, 24–32. [Google Scholar] [CrossRef]
- Shumoy, H.; Gabaza, M.; Vandevelde, J.; Raes, K. Soluble and bound phenolic contents and antioxidant capacity of tef injera as affected by traditional fermentation. J. Food Compos. Anal. 2017, 58, 52–59. [Google Scholar] [CrossRef]
- Mateo Anson, N.; Aura, A.M.; Selinheimo, E.; Mattila, I.; Poutanen, K.; van den Berg, R.; Havenaar, R.; Bast, A.; Haenen Guido, R.M.M. Bioprocessing of Wheat Bran in Whole Wheat Bread Increases the Bioavailability of Phenolic Acids in Men and Exerts Antiinflammatory Effects ex Vivo. J. Nutr. 2011, 141, 137–143. [Google Scholar] [CrossRef]
- Hur, S.J.; Lee, S.Y.; Kim, Y.C.; Choi, I.; Kim, G.B. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef]
- Zhang, X.; Duan, W.; Zou, J.; Zhou, H.; Liu, C.; Yang, H. Flavor and antioxidant activity improvement of carrot juice by fermentation with Lactobacillus plantarum WZ-01. Food Meas. 2019, 13, 3366–3375. [Google Scholar] [CrossRef]
- Matamoros, M.A.; Loscos, J.; Dietz, K.J.; Aparicio-Tejo, P.M.; Becana, M. Function of antioxidant enzymes and metabolites during maturation of pea fruits. J. Exp. Bot. 2010, 61, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Kosanic, M.; Rankovic, B.; Vukojevic, J. Antioxidant properties of some lichen species. J. Food Sci. Technol. 2011, 48, 584–590. [Google Scholar] [CrossRef]
- Angmo, K.; Kumari, A.; Savitri; Bhalla, T.C. Probiotic characterization of lactic acid bacteria isolated from fermented foods and beverage of Ladakh. LWT Food Sci. Technol. 2016, 66, 428–435. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).