Coriander (Coriandrum sativum L.) in Combination with Organic Amendments and Arbuscular Mycorrhizal Inoculation: An Efficient Option for the Phytomanagement of Trace Elements-Polluted Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Description and Sampling
2.2. Amendment Addition and Pot Experimentation
2.3. Sampling and Measurements
2.3.1. Plant Analysis
2.3.2. pH Measurement
2.3.3. TE Analysis
2.3.4. Phospholipid Fatty Acid (PLFA) Analysis
2.3.5. Community-Level Physiological Profiles
2.3.6. Soil Enzyme Activities
2.4. Statistical Analysis
3. Results
3.1. Effects of Organic Amendments and AMF Inoculation on Soil Chemical Parameters and Plant Growth
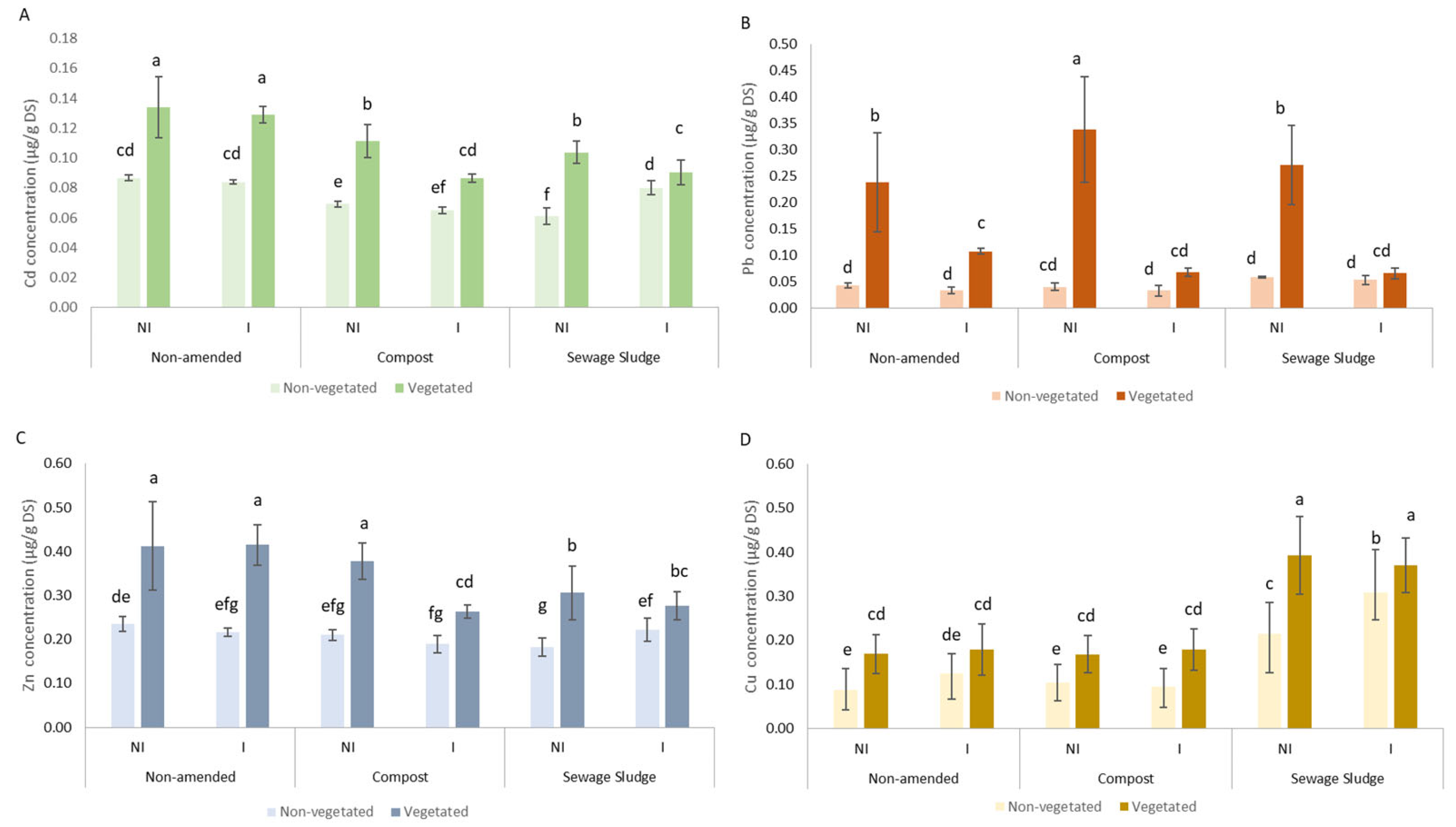
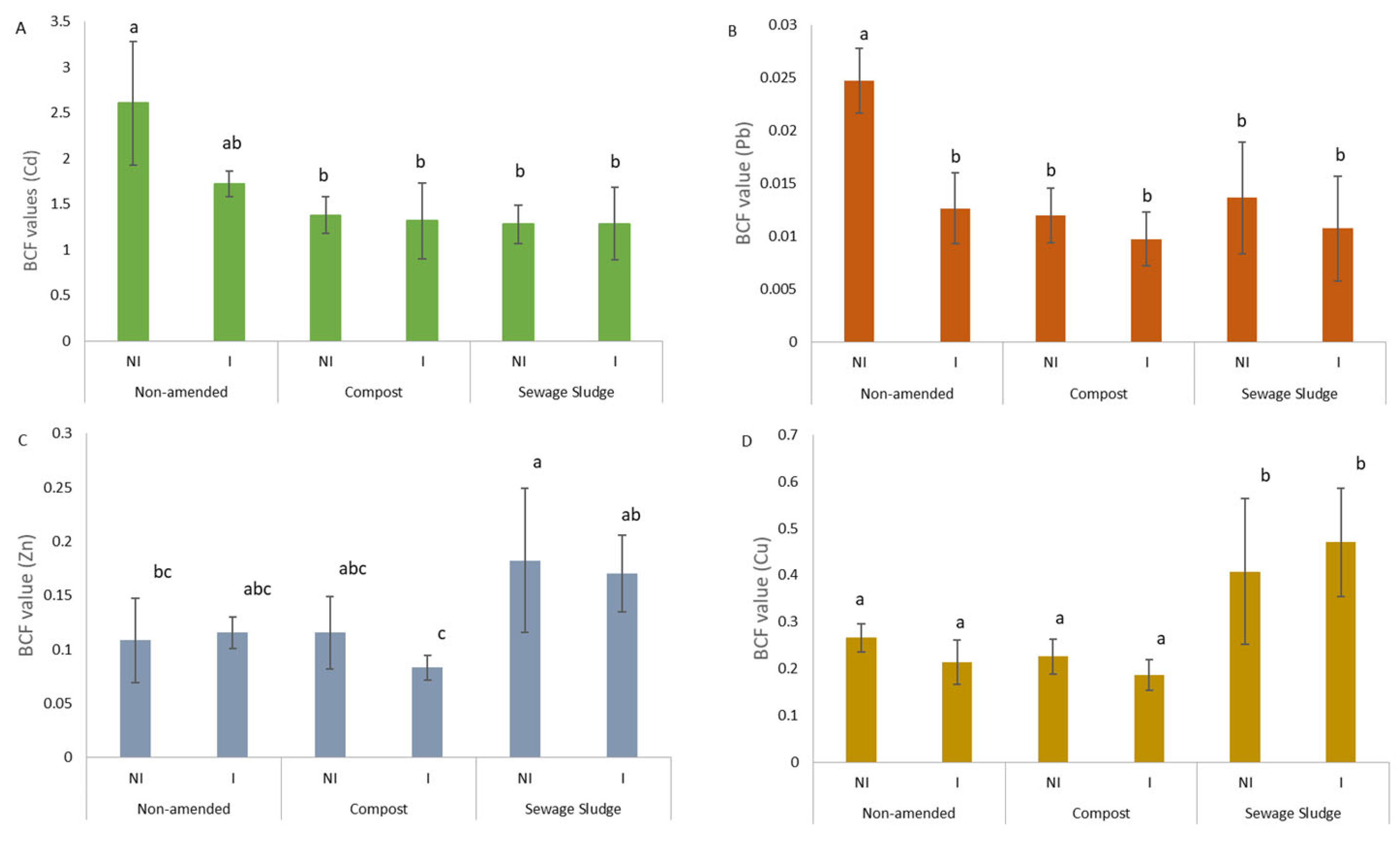
3.2. Effect of Organic Amendments and AMF Inoculation on TE Concentration in Soil and Plant Metal Uptake
3.3. Effect of Organic Amendments and AMF Inoculation on the Soil Microbial Biomass
3.4. Effect of Organic Amendments and AMF Inoculation on the Soil Microbial Community Functionality
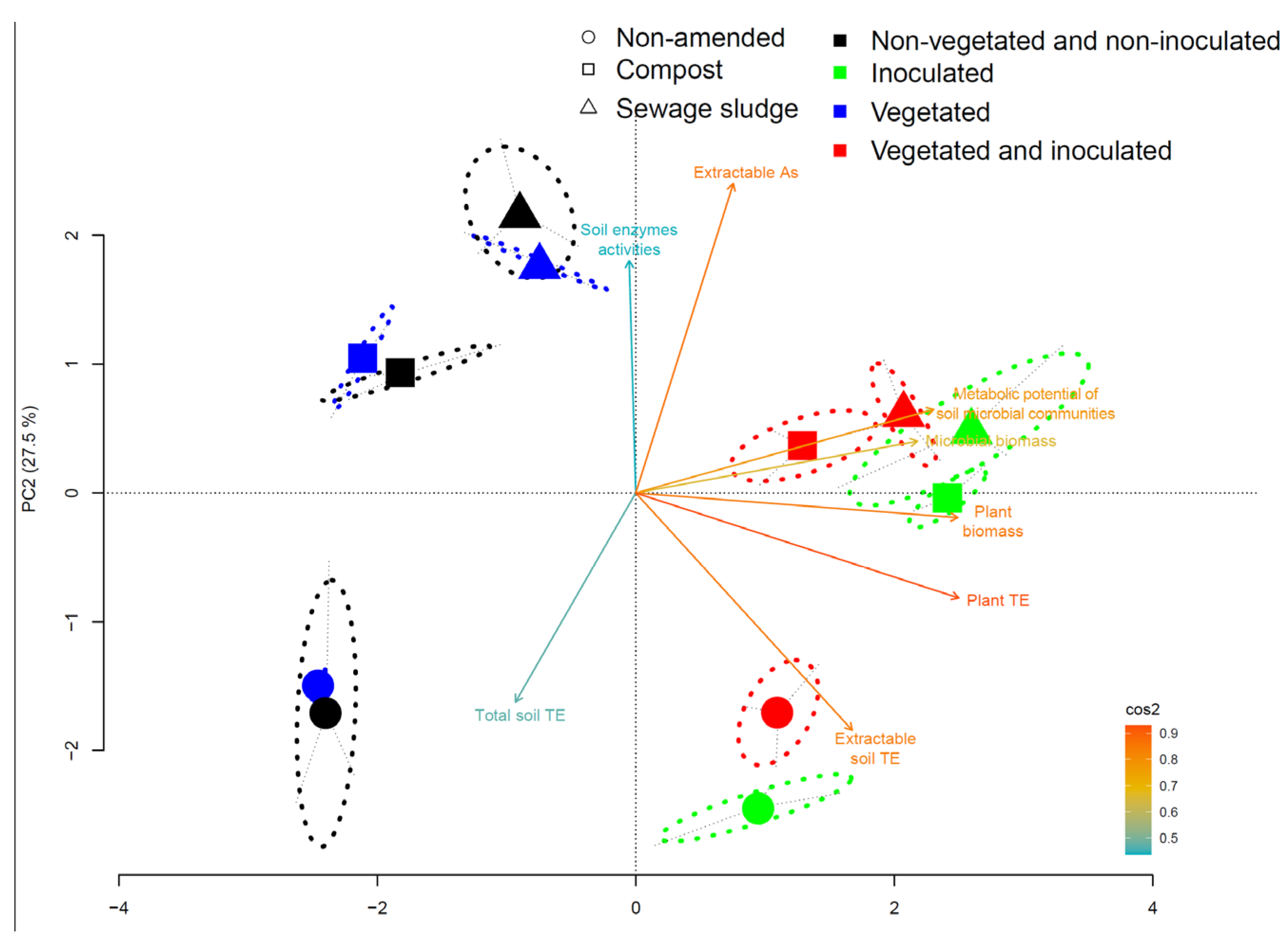
3.5. Relationships between Biological Parameters and Soil TE Contents
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garbisu, C.; Alkorta, I.; Kidd, P.; Epelde, L.; Mench, M. Keep and Promote Biodiversity at Polluted Sites under Phytomanagement. Environ. Sci. Pollut. Res. 2020, 27, 44820–44834. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Naushad, M.; Lima, E.C.; Zhang, S.; Shaheen, S.M.; Rinklebe, J. Global Soil Pollution by Toxic Elements: Current Status and Future Perspectives on the Risk Assessment and Remediation Strategies—A Review. J. Hazard. Mater. 2021, 417, 126039. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.; Ali, Q.; Zahir, Z.A.; Ashraf, S.; Asghar, H.N. Phytoremediation: Environmentally Sustainable Way for Reclamation of Heavy Metal Polluted Soils. Ecotoxicol. Environ. Saf. 2019, 174, 714–727. [Google Scholar] [CrossRef]
- Xue, K.; van Nostrand, J.D.; Vangronsveld, J.; Witters, N.; Janssen, J.O.; Kumpiene, J.; Siebielec, G.; Galazka, R.; Giagnoni, L.; Arenella, M.; et al. Management with Willow Short Rotation Coppice Increase the Functional Gene Diversity and Functional Activity of a Heavy Metal Polluted Soil. Chemosphere 2015, 138, 469–477. [Google Scholar] [CrossRef]
- Thakare, M.; Sarma, H.; Datar, S.; Roy, A.; Pawar, P.; Gupta, K.; Pandit, S.; Prasad, R. Understanding the Holistic Approach to Plant-Microbe Remediation Technologies for Removing Heavy Metals and Radionuclides from Soil. Curr. Res. Biotechnol. 2021, 3, 84–98. [Google Scholar] [CrossRef]
- Mench, M.; Lepp, N.; Bert, V.; Schwitzguébel, J.-P.; Gawronski, S.W.; Schröder, P.; Vangronsveld, J. Successes and Limitations of Phytotechnologies at Field Scale: Outcomes, Assessment and Outlook from COST Action 859. J. Soils Sediments 2010, 10, 1039–1070. [Google Scholar] [CrossRef]
- Oustriere, N.; Marchand, L.; Bouchardon, J.L.; Faure, O.; Moutte, J.; Mench, M. Aided Phytostabilization of a Trace Element-Contaminated Technosol Developed on Steel Mill Wastes. J. Hazard. Mater. 2016, 320, 458–468. [Google Scholar] [CrossRef]
- Kumpiene, J.; Lagerkvist, A.; Maurice, C. Stabilization of As, Cr, Cu, Pb and Zn in Soil Using Amendments—A Review. Waste Manag. 2008, 28, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, J.; Ma, Y.; Bauddh, K. Arbuscular Mycorrhizal Fungi: An Ecological Accelerator of Phytoremediation of Metal Contaminated Soils. Arch. Agron. Soil Sci. 2020, 68, 283–296. [Google Scholar] [CrossRef]
- Janeeshma, E.; Puthur, J.T. Direct and Indirect Influence of Arbuscular Mycorrhizae on Enhancing Metal Tolerance of Plants. Arch. Microbiol. 2020, 202, 1–16. [Google Scholar] [CrossRef]
- Wang, F. Occurrence of Arbuscular Mycorrhizal Fungi in Mining-Impacted Sites and Their Contribution to Ecological Restoration: Mechanisms and Applications. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1901–1957. [Google Scholar] [CrossRef]
- Fernández-Gómez, M.J.; Quirantes, M.; Vivas, A.; Nogales, R. Vermicomposts and/or Arbuscular Mycorrhizal Fungal Inoculation in Relation to Metal Availability and Biochemical Quality of a Soil Contaminated with Heavy Metals. Water Air. Soil Pollut. 2012, 223, 2707–2718. [Google Scholar] [CrossRef]
- Medina, A.; Azcón, R. Effectiveness Of The Application Of Arbuscular Mycorrhiza Fungi And Organic Amendments To Improve Soil Quality and Plant Performance Under Stress Conditions. J. Soil Sci. Plant Nutr. 2010, 10, 354–372. [Google Scholar] [CrossRef]
- Wang, F.Y.; Shi, Z.Y.; Xu, X.F.; Wang, X.G.; Li, Y.J. Contribution of AM Inoculation and Cattle Manure to Lead and Cadmium Phytoremediation by Tobacco Plants. Environ. Sci. Process. Impacts 2013, 15, 794–801. [Google Scholar] [CrossRef]
- Gryndler, M.; Hršelová, H.; Cajthaml, T.; Havránková, M.; Řezáčová, V.; Gryndlerová, H.; Larsen, J. Influence of Soil Organic Matter Decomposition on Arbuscular Mycorrhizal Fungi in Terms of Asymbiotic Hyphal Growth and Root Colonization. Mycorrhiza 2009, 19, 255–266. [Google Scholar] [CrossRef]
- Hammer, E.C.; Nasr, H.; Wallander, H. Effects of Different Organic Materials and Mineral Nutrients on Arbuscular Mycorrhizal Fungal Growth in a Mediterranean Saline Dryland. Soil Biol. Biochem. 2011, 43, 2332–2337. [Google Scholar] [CrossRef]
- Yang, W.; Gu, S.; Xin, Y.; Bello, A.; Sun, W.; Xu, X. Compost Addition Enhanced Hyphal Growth and Sporulation of Arbuscular Mycorrhizal Fungi without Affecting Their Community Composition in the Soil. Front. Microbiol. 2018, 9, 169. [Google Scholar] [CrossRef]
- Kohler, J.; Caravaca, F.; Azcón, R.; Díaz, G.; Roldán, A. Suitability of the Microbial Community Composition and Function in a Semiarid Mine Soil for Assessing Phytomanagement Practices Based on Mycorrhizal Inoculation and Amendment Addition. J. Environ. Manag. 2016, 169, 236–246. [Google Scholar] [CrossRef]
- Pérez, R.; Tapia, Y.; Antilén, M.; Casanova, M.; Vidal, C.; Santander, C.; Aponte, H.; Cornejo, P. Interactive Effect of Compost Application and Inoculation with the Fungus Claroideoglomus claroideum in Oenothera picensis Plants Growing in Mine Tailings. Ecotoxicol. Environ. Saf. 2021, 208, 111495. [Google Scholar] [CrossRef]
- Meng, J.; Cui, Z.; Zhang, H.; Zhang, J.; Tang, X.; Wong, M.H.; Shan, S. Combined Effects of Arbuscular Mycorrhizae Fungus and Composted Pig Manure on the Growth of Ryegrass and Uptake of Cd and Zn in the Soil from an E-Waste Recycling Site. Environ. Sci. Pollut. Res. 2021, 28, 12677–12685. [Google Scholar] [CrossRef]
- García-Sánchez, M.; Cajthaml, T.; Filipová, A.; Tlustoš, P.; Száková, J.; García-Romera, I. Implications of Mycoremediated Dry Olive Residue Application and Arbuscular Mycorrhizal Fungi Inoculation on the Microbial Community Composition and Functionality in a Metal-Polluted Soil. J. Environ. Manag. 2019, 247, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Liao, B.; Yang, Z.; Chai, L.; Li, J. Revegetation of Extremely Acid Mine Soils Based on Aided Phytostabilization: A Case Study from Southern China. Sci. Total Environ. 2016, 562, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Raveau, R.; Lounès-Hadj Sahraoui, A.; Hijri, M.; Fontaine, J. Clary Sage Cultivation and Mycorrhizal Inoculation Influence the Rhizosphere Fungal Community of an Aged Trace-Element Polluted Soil. Microorganisms 2021, 9, 1333. [Google Scholar] [CrossRef] [PubMed]
- Ait Elallem, K.; Sobeh, M.; Boularbah, A.; Yasri, A. Chemically Degraded Soil Rehabilitation Process Using Medicinal and Aromatic Plants: Review. Environ. Sci. Pollut. Res. 2021, 28, 73–93. [Google Scholar] [CrossRef]
- Pandey, J.; Verma, R.K.; Singh, S. Suitability of Aromatic Plants for Phytoremediation of Heavy Metal Contaminated Areas: A Review. Int. J. Phytoremediation 2019, 21, 405–418. [Google Scholar] [CrossRef]
- Raveau, R.; Fontaine, J.; Bert, V.; Perlein, A.; Tisserant, B.; Ferrant, P.; Lounès-Hadj Sahraoui, A. In Situ Cultivation of Aromatic Plant Species for the Phytomanagement of an Aged-Trace Element Polluted Soil: Plant Biomass Improvement Options and Techno-Economic Assessment of the Essential Oil Production Channel. Sci. Total Environ. 2021, 789, 147944. [Google Scholar] [CrossRef]
- Raveau, R.; Fontaine, J.; Lounès-Hadj Sahraoui, A. Essential Oils as Potential Alternative Biocontrol Products against Plant Pathogens and Weeds: A Review. Foods 2020, 9, 365. [Google Scholar] [CrossRef]
- Perlein, A.; Zdanevitch, I.; Gaucher, R.; Robinson, B.; Papin, A.; Lounès-Hadj Sahraoui, A.; Bert, V. Phytomanagement of a Metal(Loid)-Contaminated Agricultural Site Using Aromatic and Medicinal Plants to Produce Essential Oils: Analysis of the Metal(Loid) Fate in the Value Chain. Environ. Sci. Pollut. Res. 2021, 28, 62155–62173. [Google Scholar] [CrossRef]
- Hamid, Y.; Tang, L.; Hussain, B.; Usman, M.; Lin, Q.; Rashid, M.S.; He, Z.; Yang, X. Organic Soil Additives for the Remediation of Cadmium Contaminated Soils and Their Impact on the Soil-Plant System: A Review. Sci. Total Environ. 2020, 707, 136121. [Google Scholar] [CrossRef]
- Labidi, S.; Fontaine, J.; Laruelle, F.; Tisserant, B.; Dalpé, Y.; Grandmougin-Ferjani, A.; Douay, F.; Lounès-Hadj Sahraoui, A. Fly Ash-Aided Phytostabilisation of Highly Trace Element Polluted Topsoils Improves the Telluric Fungal Biomass: A Long-Term Field Experiment. Appl. Soil Ecol. 2015, 85, 69–75. [Google Scholar] [CrossRef]
- Sterckeman, T.; Douay, F.; Fourrier, H.; Proix, N. Référentiel Pédo-Géochimique Du Nord-Pas de Calais: Méthode et Principaux Résultats. Etude Gest. Sols 2007, 14, 153–168. [Google Scholar]
- El Fels, L.; Zamama, M.; El Asli, A.; Hafidi, M. Assessment of Biotransformation of Organic Matter during Co-Composting of Sewage Sludge-Lignocelullosic Waste by Chemical, FTIR Analyses, and Phytotoxicity Tests. Int. Biodeterior. Biodegrad. 2014, 87, 128–137. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved Procedures for Clearing Roots and Staining Parasitic and Vesicular-Arbuscular Mycorrhizal Fungi for Rapid Assessment of Infection. Trans. Br. Mycol. Soc. 1970, 55, 158-IN18. [Google Scholar] [CrossRef]
- Koske, R.E.; Gemma, J.N. A Modified Procedure for Staining Roots to Detect VA Mycorrhizas. Mycol. Res. 1989, 92, 486–488. [Google Scholar] [CrossRef]
- McGonigle, T.P.; Miller, M.H.; Evans, D.G.; Fairchild, G.L.; Swan, J.A. A New Method Which Gives an Objective Measure of Colonization of Roots by Vesicular—Arbuscular Mycorrhizal Fungi. New Phytol. 1990, 115, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Frostegård, Å.; Tunlid, A.; Bååth, E. Microbial Biomass Measured as Total Lipid Phosphate in Soils of Different Organic Content. J. Microbiol. Methods 1991, 14, 151–163. [Google Scholar] [CrossRef]
- Larsen, J.; Olsson, P.A.; Jakobsen, I. The Use of Fatty Acid Signatures to Study Mycelial Interactions between the Arbuscular Mycorrhizal Fungus Glomus intraradices and the Saprotrophic Fungus Fusarium culmorum in Root-Free Soil. Mycol. Res. 1998, 102, 1491–1496. [Google Scholar] [CrossRef]
- Olsson, P.A.; Wilhelmsson, P. The Growth of External AM Fungal Mycelium in Sand Dunes and in Experimental Systems. Plant Soil 2000, 226, 161. [Google Scholar] [CrossRef]
- Kieft, T.L.; Wilch, E.; O’connor, K.; Ringelberg, D.B.; White, D.C. Survival and Phospholipid Fatty Acid Profiles of Surface and Subsurface Bacteria in Natural Sediment Microcosms. Appl. Environ. Microbiol. 1997, 63, 1531–1542. [Google Scholar] [CrossRef]
- Alahmad, A.; Decocq, G.; Spicher, F.; Kheirbeik, L.; Kobaissi, A.; Tetu, T.; Dubois, F.; Duclercq, J. Cover Crops in Arable Lands Increase Functional Complementarity and Redundancy of Bacterial Communities. J. Appl. Ecol. 2019, 56, 651–664. [Google Scholar] [CrossRef]
- Weber, K.P.; Gehder, M.; Legge, R.L. Assessment of Changes in the Microbial Community of Constructed Wetland Mesocosms in Response to Acid Mine Drainage Exposure. Water Res. 2008, 42, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Bell, C.W.; Fricks, B.E.; Rocca, J.D.; Steinweg, J.M.; McMahon, S.K.; Wallenstein, M.D. High-Throughput Fluorometric Measurement of Potential Soil Extracellular Enzyme Activities. JoVE J. Vis. Exp. 2013, 81, e50961. [Google Scholar] [CrossRef] [PubMed]
- Nivelle, E.; Verzeaux, J.; Habbib, H.; Kuzyakov, Y.; Decocq, G.; Roger, D.; Lacoux, J.; Duclercq, J.; Spicher, F.; Nava-Saucedo, J.-E.; et al. Functional Response of Soil Microbial Communities to Tillage, Cover Crops and Nitrogen Fertilization. Appl. Soil Ecol. 2016, 108, 147–155. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, B.; Simpson, G.; Solymos, P.; Stevens, H.; Wagner, H. Vegan: Community Ecology Package, R Package Version 22-1; R Core Team: Vienna, Austria, 2015; Available online: http://CRAN.R-project.org/package=vegan (accessed on 14 January 2022).
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses, R Package Version 1.0.5.999; R Core Team: Vienna, Austria, 2017; Available online: http://CRAN.R-project.org/package=Factoextra (accessed on 14 January 2022).
- Ozkutlu, F. Determination of Cadmium and Trace Elements in Some Spices Cultivated in Turkey. Asian J. Chem. 2008, 20, 1081–1088. [Google Scholar]
- Zheljazkov, V.D.; Craker, L.E.; Xing, B.; Nielsen, N.E.; Wilcox, A. Aromatic Plant Production on Metal Contaminated Soils. Sci. Total Environ. 2008, 395, 51–62. [Google Scholar] [CrossRef]
- Fattahi, B.; Arzani, K.; Souri, M.K.; Barzegar, M. Morphophysiological and Phytochemical Responses to Cadmium and Lead Stress in Coriander (Coriandrum sativum L.). Ind. Crops Prod. 2021, 171, 113979. [Google Scholar] [CrossRef]
- Hernández, T.; Moreno, J.I.; Costa, F. Influence of Sewage Sludge Application on Crop Yields and Heavy Metal Availability. Soil Sci. Plant Nutr. 1991, 37, 201–210. [Google Scholar] [CrossRef]
- Clemente, R.; Walker, D.J.; Pardo, T.; Martínez-Fernández, D.; Bernal, M.P. The Use of a Halophytic Plant Species and Organic Amendments for the Remediation of a Trace Elements-Contaminated Soil under Semi-Arid Conditions. J. Hazard. Mater. 2012, 223–224, 63–71. [Google Scholar] [CrossRef]
- Garau, G.; Silvetti, M.; Vasileiadis, S.; Donner, E.; Diquattro, S.; Deiana, S.; Lombi, E.; Castaldi, P. Use of Municipal Solid Wastes for Chemical and Microbiological Recovery of Soils Contaminated with Metal(loid)s. Soil Biol. Biochem. 2017, 111, 25–35. [Google Scholar] [CrossRef]
- Siebielec, G.; Kidd, P.; Pecio, M.; Galazka, R.; Mench, M.; Basta, N.; Chaney, R.L.; Álvarez-López, V.; Rodríguez-Garrido, B.; Vangronsveld, J.; et al. Testing Single and Combinations of Amendments for Stabilization of Metals in Contrasting Extremely Contaminated Soils. E3S Web Conf. 2013, 1, 01003. [Google Scholar] [CrossRef]
- Garaiyurrebaso, O.; Garbisu, C.; Blanco, F.; Lanzén, A.; Martín, I.; Epelde, L.; Becerril, J.M.; Jechalke, S.; Smalla, K.; Grohmann, E.; et al. Long-Term Effects of Aided Phytostabilisation on Microbial Communities of Metal-Contaminated Mine Soil. FEMS Microbiol. Ecol. 2017, 93, fiw252. [Google Scholar] [CrossRef] [PubMed]
- Kacprzak, M.; Grobelak, A.; Grosser, A.; Napora, A. The Potential of Biosolid Application for the Phytostabilisation of Metals. Desalination Water Treat. 2014, 52, 3955–3964. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Shaheen, S.M.; Chen, S.S.; Tsang, D.C.W.; Hashimoto, Y.; Hou, D.; Bolan, N.S.; Rinklebe, J.; Ok, Y.S. Soil Amendments for Immobilization of Potentially Toxic Elements in Contaminated Soils: A Critical Review. Environ. Int. 2020, 134, 105046. [Google Scholar] [CrossRef] [PubMed]
- Pérez-de-Mora, A.; Madejón, P.; Burgos, P.; Cabrera, F.; Lepp, N.W.; Madejón, E. Phytostabilization of Semiarid Soils Residually Contaminated with Trace Elements Using By-Products: Sustainability and Risks. Environ. Pollut. 2011, 159, 3018–3027. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Medina, A.; Vassilev, N.; Barea, J.M.; Azcón, R. Application of Aspergillus niger-Treated Agrowaste Residue and Glomus Mosseae for Improving Growth and Nutrition of Trifolium repens in a Cd-Contaminated Soil. J. Biotechnol. 2005, 116, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, O.; Kehri, H.K.; Zoomi, I. Arbuscular Mycorrhiza and Aspergillus terreus Inoculation along with Compost Amendment Enhance the Phytoremediation of Cr-Rich Technosol by Solanum lycopersicum under Field Conditions. Ecotoxicol. Environ. Saf. 2020, 201, 110869. [Google Scholar] [CrossRef]
- Madejón, E.; Doronila, A.I.; Madejón, P.; Baker, A.J.M.; Woodrow, I.E. Biosolids, Mycorrhizal Fungi and Eucalypts for Phytostabilization of Arsenical Sulphidic Mine Tailings. Agrofor. Syst. 2012, 84, 389–399. [Google Scholar] [CrossRef][Green Version]
- Marques, A.P.G.C.; Oliveira, R.S.; Rangel, A.O.S.S.; Castro, P.M.L. Application of Manure and Compost to Contaminated Soils and Its Effect on Zinc Accumulation by Solanum Nigrum Inoculated with Arbuscular Mycorrhizal Fungi. Environ. Pollut. 2008, 151, 608–620. [Google Scholar] [CrossRef]
- Medina, A.; Vassileva, M.; Barea, J.-M.; Azcón, R. The Growth-Enhancement of Clover by Aspergillus-Treated Sugar Beet Waste and Glomus Mosseae Inoculation in Zn Contaminated Soil. Appl. Soil Ecol. 2006, 33, 87–98. [Google Scholar] [CrossRef]
- Huang, M.; Zhu, Y.; Li, Z.; Huang, B.; Luo, N.; Liu, C.; Zeng, G. Compost as a Soil Amendment to Remediate Heavy Metal-Contaminated Agricultural Soil: Mechanisms, Efficacy, Problems, and Strategies. Water Air. Soil Pollut. 2016, 227, 359. [Google Scholar] [CrossRef]
- Curaqueo, G.; Schoebitz, M.; Borie, F.; Caravaca, F.; Roldán, A. Inoculation with Arbuscular Mycorrhizal Fungi and Addition of Composted Olive-Mill Waste Enhance Plant Establishment and Soil Properties in the Regeneration of a Heavy Metal-Polluted Environment. Environ. Sci. Pollut. Res. 2014, 21, 7403–7412. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-T.; Wang, Y.; Yeh, K.-C. Role of Root Exudates in Metal Acquisition and Tolerance. Curr. Opin. Plant Biol. 2017, 39, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.H.; Wang, X.; Ali, S.; Zafar, S.; Nawaz, M.; Adnan, M.; Fahad, S.; Shah, A.; Alyemeni, M.N.; Hefft, D.I.; et al. Interactive Effects of Gibberellic Acid and NPK on Morpho-Physio-Biochemical Traits and Organic Acid Exudation Pattern in Coriander (Coriandrum sativum L.) Grown in Soil Artificially Spiked with Boron. Plant Physiol. Biochem. 2021, 167, 884–900. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhao, H.; Zou, C.; Li, Y.; Chen, Y.; Wang, Z.; Jiang, Y.; Liu, A.; Zhao, P.; Wang, M.; et al. Combined Inoculation with Multiple Arbuscular Mycorrhizal Fungi Improves Growth, Nutrient Uptake and Photosynthesis in Cucumber Seedlings. Front. Microbiol. 2017, 8, 2516. [Google Scholar] [CrossRef]
- Veresoglou, S.D.; Menexes, G.; Rillig, M.C. Do Arbuscular Mycorrhizal Fungi Affect the Allometric Partition of Host Plant Biomass to Shoots and Roots? A Meta-Analysis of Studies from 1990 to 2010. Mycorrhiza 2012, 22, 227–235. [Google Scholar] [CrossRef]
- Fatemi, H.; Esmaielpour, B.; Sefidkon, F.; Soltani, A.-A.; Nematollahzadeh, A. How Mycorrhiza Symbiosis Help Coriander (Coriandrum sativum L.) Plants Grow Better under Contaminated Soil? J. Plant Nutr. 2020, 43, 2040–2053. [Google Scholar] [CrossRef]
- Firmin, S.; Labidi, S.; Fontaine, J.; Laruelle, F.; Tisserant, B.; Nsanganwimana, F.; Pourrut, B.; Dalpé, Y.; Grandmougin, A.; Douay, F.; et al. Arbuscular Mycorrhizal Fungal Inoculation Protects Miscanthus × giganteus against Trace Element Toxicity in a Highly Metal-Contaminated Site. Sci. Total Environ. 2015, 527–528, 91–99. [Google Scholar] [CrossRef]
- Kohler, J.; Caravaca, F.; Azcón, R.; Díaz, G.; Roldán, A. The Combination of Compost Addition and Arbuscular Mycorrhizal Inoculation Produced Positive and Synergistic Effects on the Phytomanagement of a Semiarid Mine Tailing. Sci. Total Environ. 2015, 514, 42–48. [Google Scholar] [CrossRef]
- Liu, L.; Li, J.; Yue, F.; Yan, X.; Wang, F.; Bloszies, S.; Wang, Y. Effects of Arbuscular Mycorrhizal Inoculation and Biochar Amendment on Maize Growth, Cadmium Uptake and Soil Cadmium Speciation in Cd-Contaminated Soil. Chemosphere 2018, 194, 495–503. [Google Scholar] [CrossRef]
- Alguacil, M.M.; Torrecillas, E.; Caravaca, F.; Fernández, D.A.; Azcón, R.; Roldán, A. The Application of an Organic Amendment Modifies the Arbuscular Mycorrhizal Fungal Communities Colonizing Native Seedlings Grown in a Heavy-Metal-Polluted Soil. Soil Biol. Biochem. 2011, 43, 1498–1508. [Google Scholar] [CrossRef]
- Qin, H.; Lu, K.; Strong, P.J.; Xu, Q.; Wu, Q.; Xu, Z.; Xu, J.; Wang, H. Long-Term Fertilizer Application Effects on the Soil, Root Arbuscular Mycorrhizal Fungi and Community Composition in Rotation Agriculture. Appl. Soil Ecol. 2015, 89, 35–43. [Google Scholar] [CrossRef]
- Zhu, C.; Ling, N.; Guo, J.; Wang, M.; Guo, S.; Shen, Q. Impacts of Fertilization Regimes on Arbuscular Mycorrhizal Fungal (AMF) Community Composition Were Correlated with Organic Matter Composition in Maize Rhizosphere Soil. Front. Microbiol. 2016, 7, 1840. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.X.; Wang, Y.P.; Lin, Q.; Luo, Y.M. Effect of Copper-Tolerant Rhizosphere Bacteria on Mobility of Copper in Soil and Copper Accumulation by Elsholtzia Splendens. Environ. Int. 2005, 31, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Dell’Amico, E.; Cavalca, L.; Andreoni, V. Improvement of Brassica Napus Growth under Cadmium Stress by Cadmium-Resistant Rhizobacteria. Soil Biol. Biochem. 2008, 40, 74–84. [Google Scholar] [CrossRef]
- Khalid, S.; Shahid, M.; Niazi, N.K.; Murtaza, B.; Bibi, I.; Dumat, C. A Comparison of Technologies for Remediation of Heavy Metal Contaminated Soils. J. Geochem. Explor. 2017, 182, 247–268. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, X.; He, X.; Lü, Q.; Qian, X.; Xiao, Q.; Lin, R. Effects of Pseudomonas TCd-1 on Rice (Oryza sativa) Cadmium Uptake, Rhizosphere Soils Enzyme Activities and Cadmium Bioavailability under Cadmium Contamination. Ecotoxicol. Environ. Saf. 2021, 218, 112249. [Google Scholar] [CrossRef]
- Siebielec, S.; Siebielec, G.; Marzec-Grządziel, A.; Pecio, M.; Stuczyński, T. Testing Combined Effect of Amendments and Inoculation with Bacteria for Improving Phytostabilisation of Smelter Waste Extremely Contaminated with Trace Elements. Agronomy 2021, 11, 2064. [Google Scholar] [CrossRef]
- Faucon, M.-P.; Houben, D.; Lambers, H. Plant Functional Traits: Soil and Ecosystem Services. Trends Plant Sci. 2017, 22, 385–394. [Google Scholar] [CrossRef]
- Covino, S.; Fabianová, T.; Křesinová, Z.; Čvančarová, M.; Burianová, E.; Filipová, A.; Vořísková, J.; Baldrian, P.; Cajthaml, T. Polycyclic Aromatic Hydrocarbons Degradation and Microbial Community Shifts during Co-Composting of Creosote-Treated Wood. J. Hazard. Mater. 2016, 301, 17–26. [Google Scholar] [CrossRef]
- Lorenz, N.; Hintemann, T.; Kramarewa, T.; Katayama, A.; Yasuta, T.; Marschner, P.; Kandeler, E. Response of Microbial Activity and Microbial Community Composition in Soils to Long-Term Arsenic and Cadmium Exposure. Soil Biol. Biochem. 2006, 38, 1430–1437. [Google Scholar] [CrossRef]
- Bailey, V.L.; Smith, J.L.; Bolton, H. Fungal-to-Bacterial Ratios in Soils Investigated for Enhanced C Sequestration. Soil Biol. Biochem. 2002, 34, 997–1007. [Google Scholar] [CrossRef]
- Bardgett, R.; Hobbs, P.; Frostegard, A. Changes in Soil Fungal:Bacterial Biomass Ratios Following Reductions in the Intensity of Management of an Upland Grassland. Biol. Fertil. Soils 1996, 22, 261–264. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Manzoni, S.; Moorhead, D.L.; Richter, A. Carbon Use Efficiency of Microbial Communities: Stoichiometry, Methodology and Modelling. Ecol. Lett. 2013, 16, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Misra, P.; Maji, D.; Awasthi, A.; Pandey, S.S.; Yadav, A.; Pandey, A.; Saikia, D.; Babu, C.S.V.; Kalra, A. Vulnerability of Soil Microbiome to Monocropping of Medicinal and Aromatic Plants and Its Restoration through Intercropping and Organic Amendments. Front. Microbiol. 2019, 10, 2604. [Google Scholar] [CrossRef]
- Raveau, R.; Fontaine, J.; Hijri, M.; Lounès-Hadj Sahraoui, A. The Aromatic Plant Clary Sage Shaped Bacterial Communities in the Roots and in the Trace Element-Contaminated Soil More Than Mycorrhizal Inoculation—A Two-Year Monitoring Field Trial. Front. Microbiol. 2020, 11, 2779. [Google Scholar] [CrossRef]
- Choudhary, S.; Mishra, B.K.; Singh, R.; Sharma, R. Bacterial Diversity and Bio-Chemical Properties in the Rhizosphere Soils of Cumin and Coriander. Trop. Ecol. 2021, 62, 368–376. [Google Scholar] [CrossRef]
- Bebber, D.P.; Richards, V.R. A Meta-Analysis of the Effect of Organic and Mineral Fertilizers on Soil Microbial Diversity. Appl. Soil Ecol. 2022, 175, 104450. [Google Scholar] [CrossRef]
- Rodríguez-Berbel, N.; Soria, R.; Ortega, R.; Bastida, F.; Miralles, I. Quarry Restoration Treatments from Recycled Waste Modify the Physicochemical Soil Properties, Composition and Activity of Bacterial Communities and Priming Effect in Semi-Arid Areas. Sci. Total Environ. 2021, 774, 145693. [Google Scholar] [CrossRef]
- Carrasco, L.; Gattinger, A.; Fliessbach, A.; Roldán, A.; Schloter, M.; Caravaca, F. Estimation by PLFA of Microbial Community Structure Associated with the Rhizosphere of Lygeum Spartum and Piptatherum Miliaceum Growing in Semiarid Mine Tailings. Microb. Ecol. 2010, 60, 265–271. [Google Scholar] [CrossRef]
- Fernández, D.; Roldán, A.; Azcón, R.; Caravaca, F.; Bååth, E. Effects of Water Stress, Organic Amendment and Mycorrhizal Inoculation on Soil Microbial Community Structure and Activity During the Establishment of Two Heavy Metal-Tolerant Native Plant Species. Microb. Ecol. 2011, 63, 794–803. [Google Scholar] [CrossRef]
- Fanin, N.; Kardol, P.; Farrell, M.; Nilsson, M.-C.; Gundale, M.J.; Wardle, D.A. The Ratio of Gram-Positive to Gram-Negative Bacterial PLFA Markers as an Indicator of Carbon Availability in Organic Soils. Soil Biol. Biochem. 2019, 128, 111–114. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, J.; Ren, L.; Zhou, Y.; Gao, J.; Luo, L.; Yang, Y.; Peng, Q.; Huang, H.; Chen, A. Diagnosis of Soil Contamination Using Microbiological Indices: A Review on Heavy Metal Pollution. J. Environ. Manag. 2019, 242, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Ndiaye, E.L.; Sandeno, J.M.; McGrath, D.; Dick, R.P. Integrative Biological Indicators for Detecting Change in Soil Quality. Am. J. Altern. Agric. 2000, 15, 26–36. [Google Scholar] [CrossRef]
- Sun, Y.H.; Yang, Z.H.; Zhao, J.J.; Li, Q. Functional Diversity of Microbial Communities in Sludge-Amended Soils. Phys. Procedia 2012, 33, 726–731. [Google Scholar] [CrossRef][Green Version]
- Zhao, F.; Zhang, Y.; Li, Z.; Shi, J.; Zhang, G.; Zhang, H.; Yang, L. Vermicompost Improves Microbial Functions of Soil with Continuous Tomato Cropping in a Greenhouse. J. Soils Sediments 2020, 20, 380–391. [Google Scholar] [CrossRef]
| Parameters | Amended Soil Before Cultivation | Amendments | ||||
|---|---|---|---|---|---|---|
| Non-Amended | Compost | Sewage Sludge | Compost | Sewage Sludge | Mycorrhizal Inoculum | |
| pH-H2O | 7.9 ± 0.00 a | 7.9 ± 0.10 a | 7.7 ± 0.05 a | 7.11 | 8.92 | 8.3 |
| Clay (%) | 22.8 ± 0.71 a | 22.45 ± 0.07 a | 23.45 ± 0.35 ab | ND | ND | ND |
| Silt (%) | 56.95 ± 0.21 a | 56.45 ± 0.49 a | 56.5 ± 0.14 a | ND | ND | ND |
| Sand (%) | 20.2 ± 0.42 a | 21.1 ± 0.28 a | 20.1 ± 0.28 a | ND | ND | ND |
| Total organic carbon (g/Kg) | 11.75 ± 1.20 a | 16.1 ± 1.27 b | 13.3 ± 0.85 ab | 206.8 | 257.5 | 13.8 |
| Organic matter (g/Kg) | 19.4 ± 0.99 a | 27.75 ± 2.19 b | 22.85 ± 1.48 ab | 355.7 | 442.8 | 23.8 |
| C/N | 10 ± 1.41 a | 10 ± 0.01 a | 7.5 ± 0.71 a | 13 | 6 | 25 |
| CEC Metson (me/Kg) | 238.5 ± 4.95 a | 248.5 ± 17.68 a | 234.5 ± 4.95 a | 328 | 167 | 524 |
| N (g/Kg) | 1.2 ± 0.14 a | 1.55 ± 0.07 a | 1.6 ± 0.14 a | 15.1 | 40.9 | 0.5 |
| Available P Olsen (g/Kg) | 0.05 ± 0.01 a | 0.09 ± 0.01 c | 0.202 ± 0.02 b | 0.91 | 2.85 | 0.03 |
| Available K (g/Kg)—K2O | 0.76 ± 0.01 a | 0.72 ± 0.04 a | 0.74 ± 0.00 a | 1.55 | 0.78 | 1.66 |
| Available Mg (g/Kg)—MgO | 6.78 ± 0.13 a | 7.86 ± 0.31 c | 9.62 ± 0.65 b | 1.66 | 1.75 | 1.05 |
| Available Ca (g/Kg)—CaO | 0.38 ± 0.04 a | 0.43 ± 0.05 a | 0.43 ± 0.01 a | 12.84 | 25.5 | 10.72 |
| Cd (mg/Kg) | 5.29 ± 0.18 | ND | ND | 0.0 | 0.81 | ND |
| Cr (mg/Kg) | 32.18 ± 8.07 | ND | ND | 30.8 | 25.8 | ND |
| Cu (mg/Kg) | 21.29 ± 0.86 | ND | ND | 51.5 | 122 | ND |
| Ni (mg/Kg) | 24.46 ± 0.79 | ND | ND | 15.9 | 16.8 | ND |
| Pb (mg/Kg) | 326,84 ± 10.12 | ND | ND | 77.5 | 38.1 | ND |
| Zn (mg/Kg) | 409.80 ± 12.54 | ND | ND | 163 | 418 | ND |
| Germination index (%) | 63 | 58 | ND | |||
| Biomass (mg/plant) | Mycorrhizal Rate (%) | ||
|---|---|---|---|
| Non-amended | NI | 0.44 ± 0.24 a | 53.6 ± 8.1 a |
| I | 0.47 ± 0.15 a | 56.7 ± 3.5 a | |
| Compost | NI | 0.97 ± 0.26 c | 54.54 ± 7.4 a |
| I | 0.65 ± 0.13 b | 49.8 ± 8.0 a | |
| Sewage sludge | NI | 0.88 ± 0.40 bc | 59.9 ± 1.3 a |
| I | 0.66 ± 0.41 ab | 55.2 ± 9.3 a |
| Gram-Positive Bacteria PLFA | Gram-Negative Bacteria PLFA | Fungal PLFA C18:2ω 6,9 | AMF PLFA C16:1ω 5 | Total Microbial ∑ PLFA | Stress Indicator | |||
|---|---|---|---|---|---|---|---|---|
| Non-amended | Non-vegetated | NI | 1.44 ± 0.22 c | 2.45 ± 0.39 c | 0.80 ± 0.06 bc | 0.81 ± 0.36 bc | 5.85 ± 0.78 cd | 0.69 ± 0.02 a |
| I | 1.43 ± 0.24 c | 1.80 ± 0.14 c | 0.84 ± 0.24 bc | 0.56 ± 0.03 c | 5.78 ± 0.43 d | 0.38 ± 0.27 abc | ||
| Vegetated | NI | 1.83 ± 0.36 bc | 2.87 ± 0.67 bc | 0.82 ± 0.13 bc | 0.97 ± 0.18 abc | 7.28 ± 10.3 cd | 0.52 ± 0.10 bcd | |
| I | 2.29 ± 0.52 bc | 3.40 ± 0.34 ab | 1.36 ± 0.26 abc | 0.96 ± 0.18 ab | 8.17 ± 1.65 ab | 0.28 ± 0.02 d | ||
| Compost | Non-vegetated | NI | 1.50 ± 0.37 c | 2.83 ± 1.41 bc | 0.95 ± 0.19 abc | 0.98 ± 0.20 abc | 7.31 ± 1.46 bcd | 0.58 ± 0.11 ab |
| I | 1.98 ± 0.44 bc | 2.13 ± 0.24 c | 0.65 ± 0.05 c | 1.12 ± 0.35 ab | 7.51 ± 0.88 cd | 0.27 ± 0.08 abc | ||
| Vegetated | NI | 1.99 ± 0.09 abc | 3.49 ± 0.32 ab | 1.24 ± 0.45 abc | 1.40 ± 0.44 a | 8.13 ± 2.11 ab | 0.50 ± 0.10 d | |
| I | 2.21 ± 0.55 abc | 3.50 ± 0.93 ab | 1.32 ± 0.22 abc | 1.08 ± 0.25 ab | 8.67 ± 2.11 ab | 0.26 ± 0.04 d | ||
| Sewage sludge | Non-vegetated | NI | 2.01 ± 0.32 bc | 2.74 ± 0.06 bc | 0.86 ± 0.19 bc | 0.73 ± 0.11 bc | 6.79 ± 0.01 bcd | 0.39 ± 0.02 bcd |
| I | 1.84 ± 0.96 ab | 2.87 ± 1.14 bc | 0.89 ± 0.47 ab | 0.53 ± 0.22 bc | 7.46 ± 1.43 bc | 0.26 ± 0.02 ab | ||
| Vegetated | NI | 2.29 ± 0.63 bc | 4.46 ± 0.65 a | 1.61 ± 0.17 a | 1.45 ± 0.43 a | 10.28 ± 0.74 a | 0.54 ± 029 d | |
| I | 2.85 ± 1.14 a | 4.05 ± 0.48 a | 1.50 ± 0.28 ab | 1.18 ± 0.11 ab | 10.24 ± 1.78 a | 0.31 ± 0.03 cd | ||
| Vegetation (V) | ** | *** | *** | *** | *** | *** | ||
| Mycorrhizal inoculation (M) | NS | NS | NS | NS | NS | NS | ||
| Amendments (A) | ** | ** | NS | NS | ** | NS | ||
| M × V | NS | NS | NS | NS | NS | NS | ||
| M × A | NS | NS | NS | NS | NS | NS | ||
| V × A | NS | NS | NS | * | NS | NS | ||
| M × V × A | NS | NS | NS | NS | NS | NS | ||
| ACWD | Richness | Dehydrogenase | Arylsulfatase | Glucosidase | Cellubiosidase | Phosphomonoesterase | |||
|---|---|---|---|---|---|---|---|---|---|
| Non-amended | Non- vegetated | NI | 19.24 ± 9.77 a | 9.00 ± 2.73 a | 5.69 ± 1.84 d | 0.85 ± 0.03 b | 1.95 ± 0.17 c | 0.60 ± 0.09 b | 2.37 ± 0.22 ab |
| I | 28.56 ± 8.79 a | 10.11 ± 2.34 a | 51.38 ± 14.61 d | 0.68 ± 0.08 b | 1.57 ± 0.52 c | 0.52 ± 0.01 b | 1.68 ± 0.06 b | ||
| Vegetated | NI | 95.44 ± 20.44 b | 20.78 ± 1.35 b | 131.08 ± 91.90 bcd | 0.83 ± 0.04 b | 1.64 ± 0.08 c | 0.52 ± 0.00 ab | 3.71 ± 1.51 ab | |
| I | 91.90 ± 18.34 b | 19.56 ± 3.56 b | 216.09 ± 163.09 abcd | 0.88 ± 0.10 ab | 2.18 ± 0.31 bc | 0.61 ± 0.03 ab | 6.07 ± 0.06 a | ||
| Compost | Non- vegetated | NI | 24.49 ± 9.64 a | 9.22 ± 1.02 a | 22.69 ± 11.49 d | 0.85 ± 0.04 b | 1.95 ± 0.27 bc | 0.60 ± 0.01 ab | 2.37 ± 0.38 ab |
| I | 24.23 ± 18.08 a | 9.11 ± 6.55 a | 52.44 ± 61.93 d | 0.75 ± 0.06 b | 2.10 ± 0.44 bc | 0.55 ± 0.04 ab | 2.78 ± 1.01 ab | ||
| Vegetated | NI | 110.42 ± 14.83 b | 21.44 ± 1.90 b | 343.61 ± 13.27 abc | 0.86 ± 0.03 b | 2.28 ± 0.35 bc | 0.62 ± 0.02 b | 3.37 ± 0.29 ab | |
| I | 104.8 ± 17.19 b | 20.89 ± 3.67 b | 177.83 ± 95.44 bcd | 0.83 ± 0.11 b | 3.24 ± 1.34 ab | 0.62 ± 0.06 bc | 3.58 ± 0.73 ab | ||
| Sewage sludge | Non- vegetated | NI | 104.8 ± 43.65 b | 21.33 ± 2.73 b | 76.88 ± 35.93 cd | 0.86 ± 0.09 b | 2.41 ± 0.26 bc | 0.63 ± 0.05 ab | 4.27 ± 0.72 ab |
| I | 109.84 ± 9.30 b | 21.00 ± 0.58 b | 107.70 ± 108.64 bcd | 1.07 ± 0.08 a | 3.29 ± 0.29 ab | 0.70 ± 0.04 a | 5.98 ± 0.96 a | ||
| Vegetated | NI | 136.88 ± 37.83 b | 24.67 ± 3.79 b | 355.29 ± 60.85 ab | 0.77 ± 0.01 b | 3.71 ± 1.21 a | 0.62 ± 0.08 ab | 5.13 ± 1.54 ab | |
| I | 141.50 ± 5.66 b | 24.67 ± 1.45 b | 459.43 ± 204.7 a | 0.79 ± 0.05 b | 3.86 ± 1.54 a | 0.62 ± 0.06 | 4.38 ± 1.28 ab | ||
| Vegetation (V) | *** | *** | *** | NS | NS | NS | NS | ||
| Mycorrhizal inoculation (M) | NS | NS | NS | NS | NS | NS | NS | ||
| Amendments (A) | *** | *** | ** | NS | ** | ** | * | ||
| M × V | NS | NS | NS | NS | NS | NS | NS | ||
| M × A | NS | NS | NS | ** | NS | NS | NS | ||
| V × A | ** | * | NS | ** | NS | NS | NS | ||
| M × V × A | NS | NS | NS | ** | NS | ** | ** | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fontaine, J.; Duclercq, J.; Facon, N.; Dewaele, D.; Laruelle, F.; Tisserant, B.; Lounès-Hadj Sahraoui, A. Coriander (Coriandrum sativum L.) in Combination with Organic Amendments and Arbuscular Mycorrhizal Inoculation: An Efficient Option for the Phytomanagement of Trace Elements-Polluted Soils. Microorganisms 2022, 10, 2287. https://doi.org/10.3390/microorganisms10112287
Fontaine J, Duclercq J, Facon N, Dewaele D, Laruelle F, Tisserant B, Lounès-Hadj Sahraoui A. Coriander (Coriandrum sativum L.) in Combination with Organic Amendments and Arbuscular Mycorrhizal Inoculation: An Efficient Option for the Phytomanagement of Trace Elements-Polluted Soils. Microorganisms. 2022; 10(11):2287. https://doi.org/10.3390/microorganisms10112287
Chicago/Turabian StyleFontaine, Joël, Jérome Duclercq, Natacha Facon, Dorothée Dewaele, Frédéric Laruelle, Benoit Tisserant, and Anissa Lounès-Hadj Sahraoui. 2022. "Coriander (Coriandrum sativum L.) in Combination with Organic Amendments and Arbuscular Mycorrhizal Inoculation: An Efficient Option for the Phytomanagement of Trace Elements-Polluted Soils" Microorganisms 10, no. 11: 2287. https://doi.org/10.3390/microorganisms10112287
APA StyleFontaine, J., Duclercq, J., Facon, N., Dewaele, D., Laruelle, F., Tisserant, B., & Lounès-Hadj Sahraoui, A. (2022). Coriander (Coriandrum sativum L.) in Combination with Organic Amendments and Arbuscular Mycorrhizal Inoculation: An Efficient Option for the Phytomanagement of Trace Elements-Polluted Soils. Microorganisms, 10(11), 2287. https://doi.org/10.3390/microorganisms10112287









