Comparative Analyses of the Exopalaemon carinicauda Gut Bacterial Community and Digestive and Immune Enzyme Activity during a 24-Hour Cycle
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement and Sample Collection
2.2. Enzyme Activity Determination
2.3. Extraction of Genomic DNA and Amplicon Generation
2.4. Library Preparation and Sequencing
2.5. Bioinformatics and Statistical Analysis
3. Results
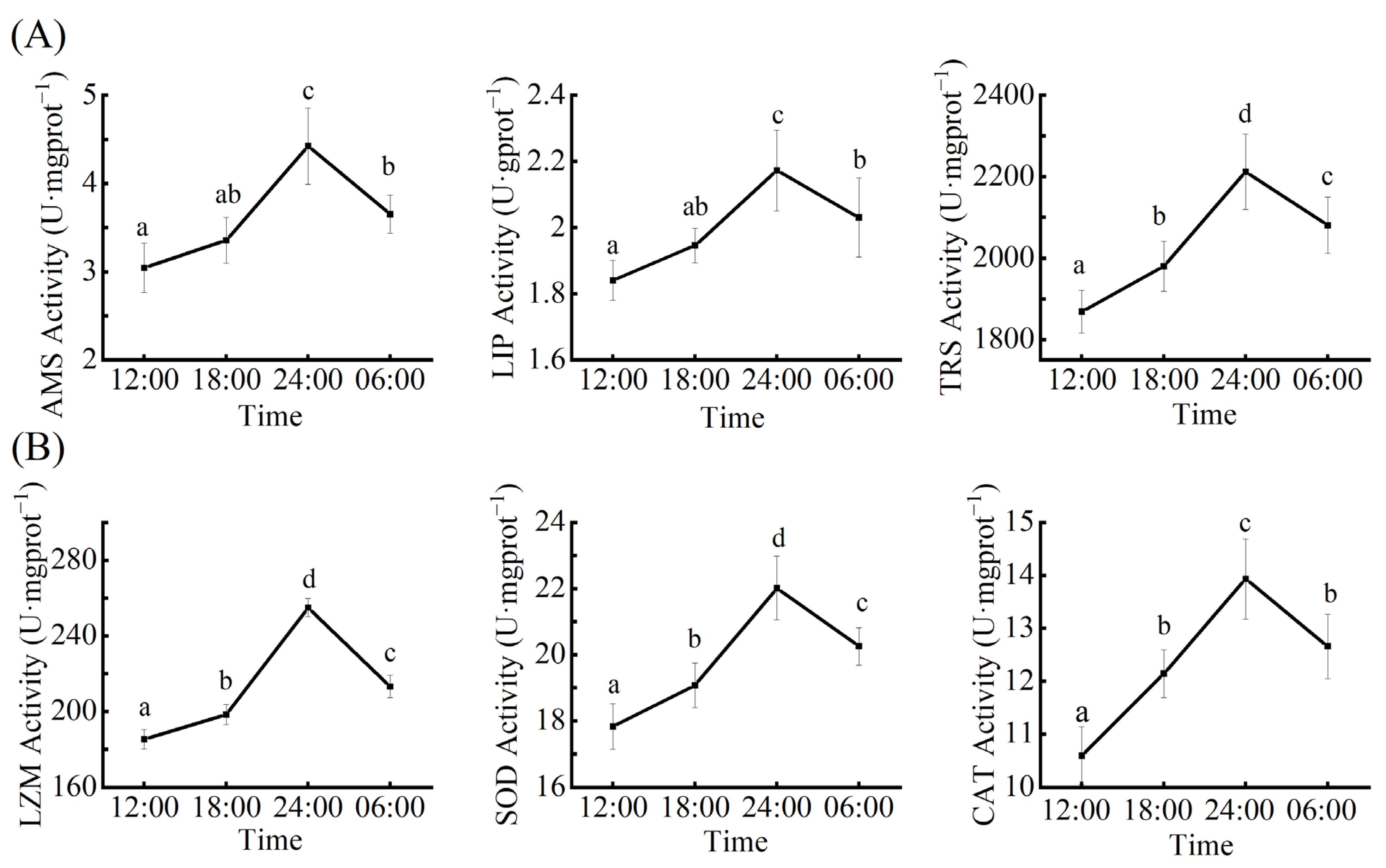
3.1. The Circadian Rhythm of Digestive Enzyme Activity
3.2. The Circadian Rhythm of Immune Enzyme Activity
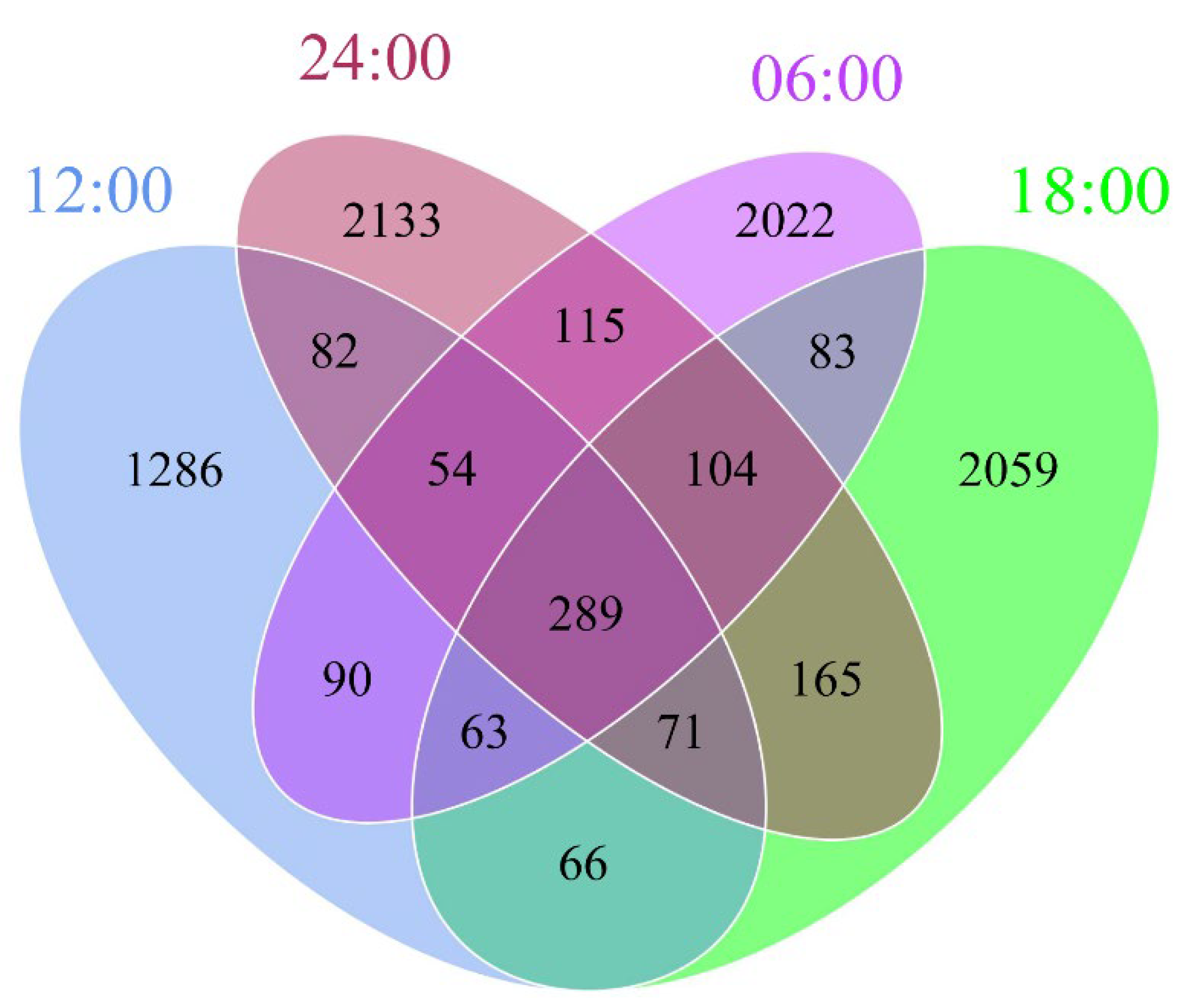
3.3. Amplicon Sequence Variants
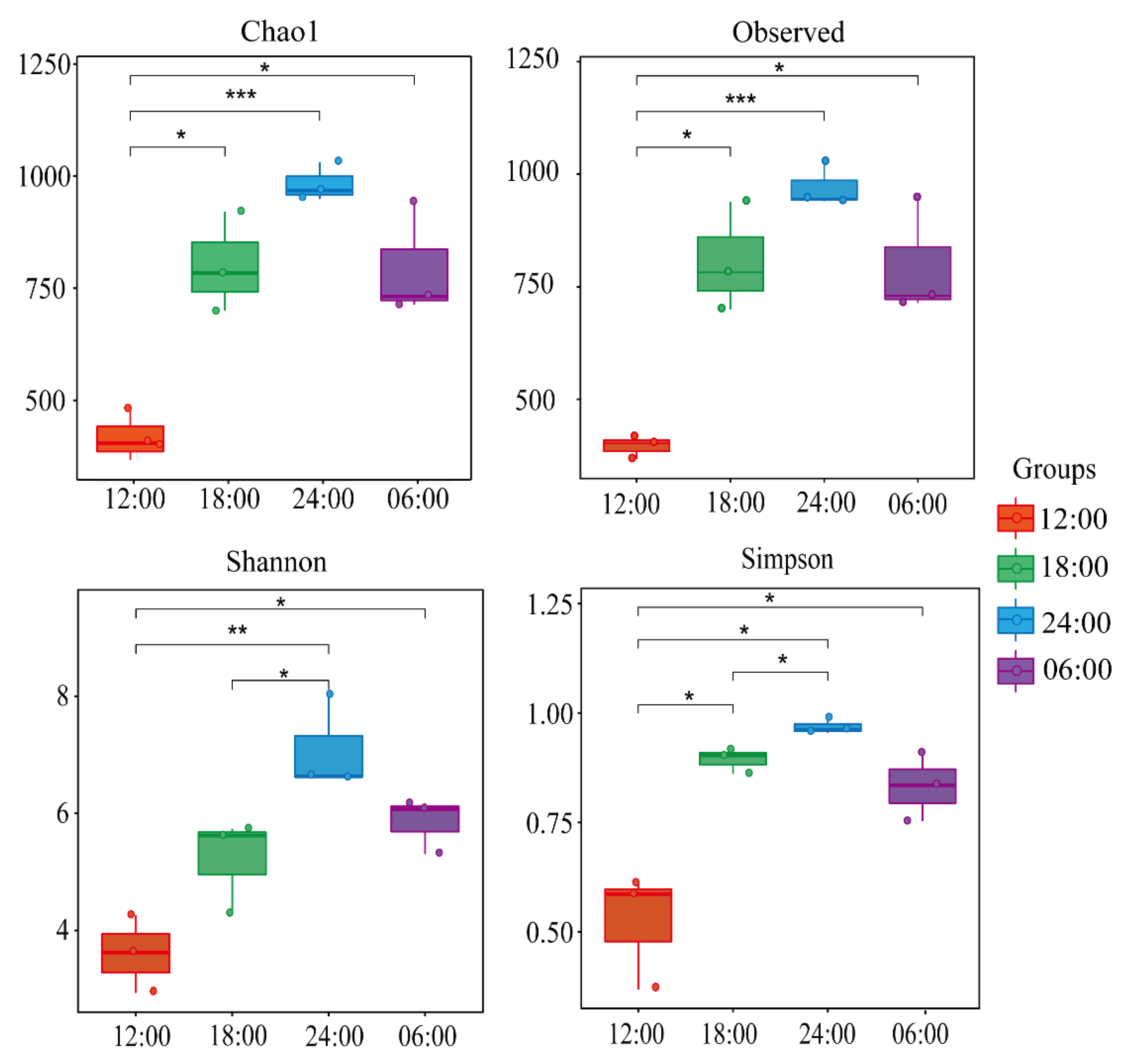
3.4. Alpha Diversity
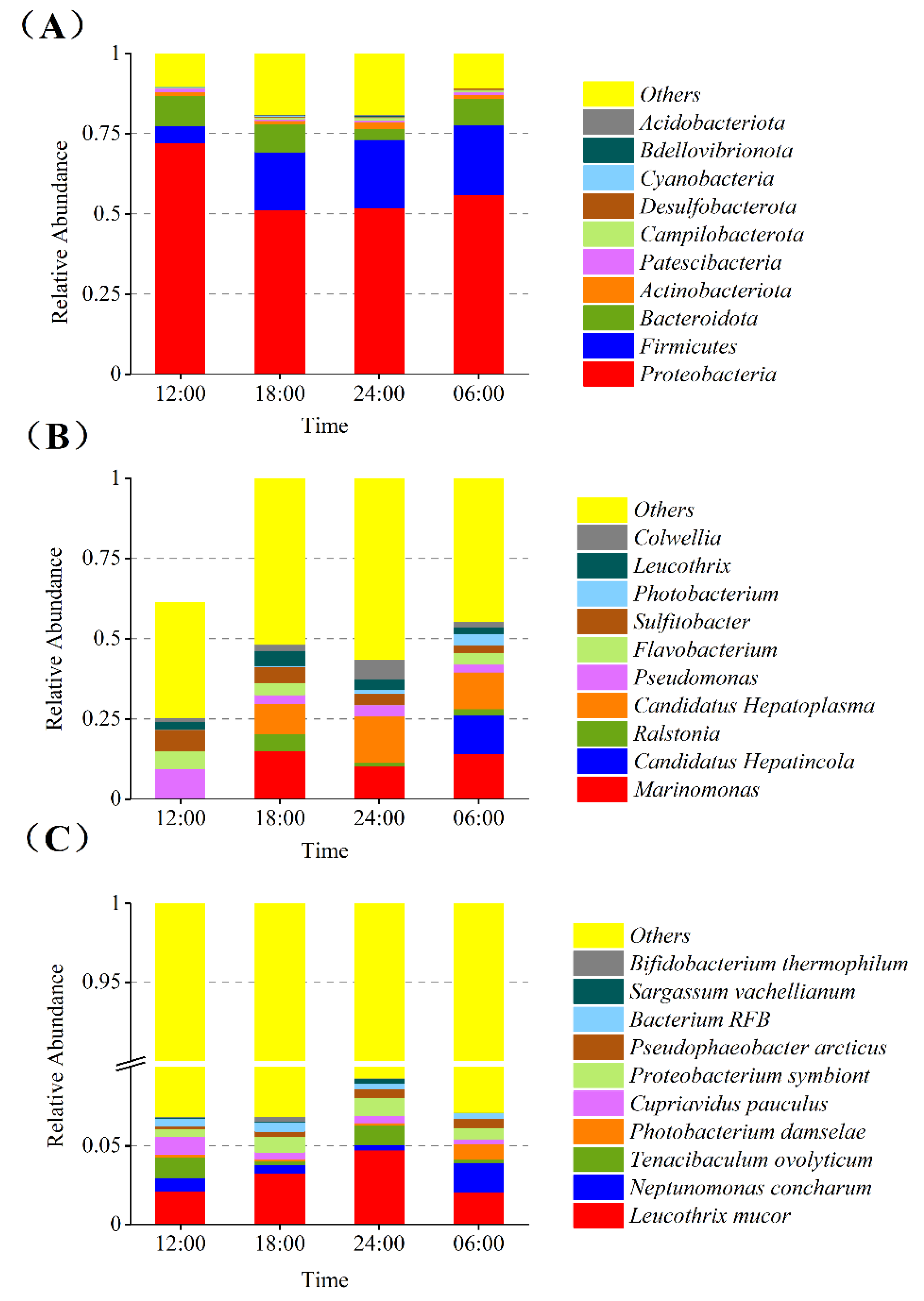
3.5. Relative Abundance of the Gut Bacterial Community
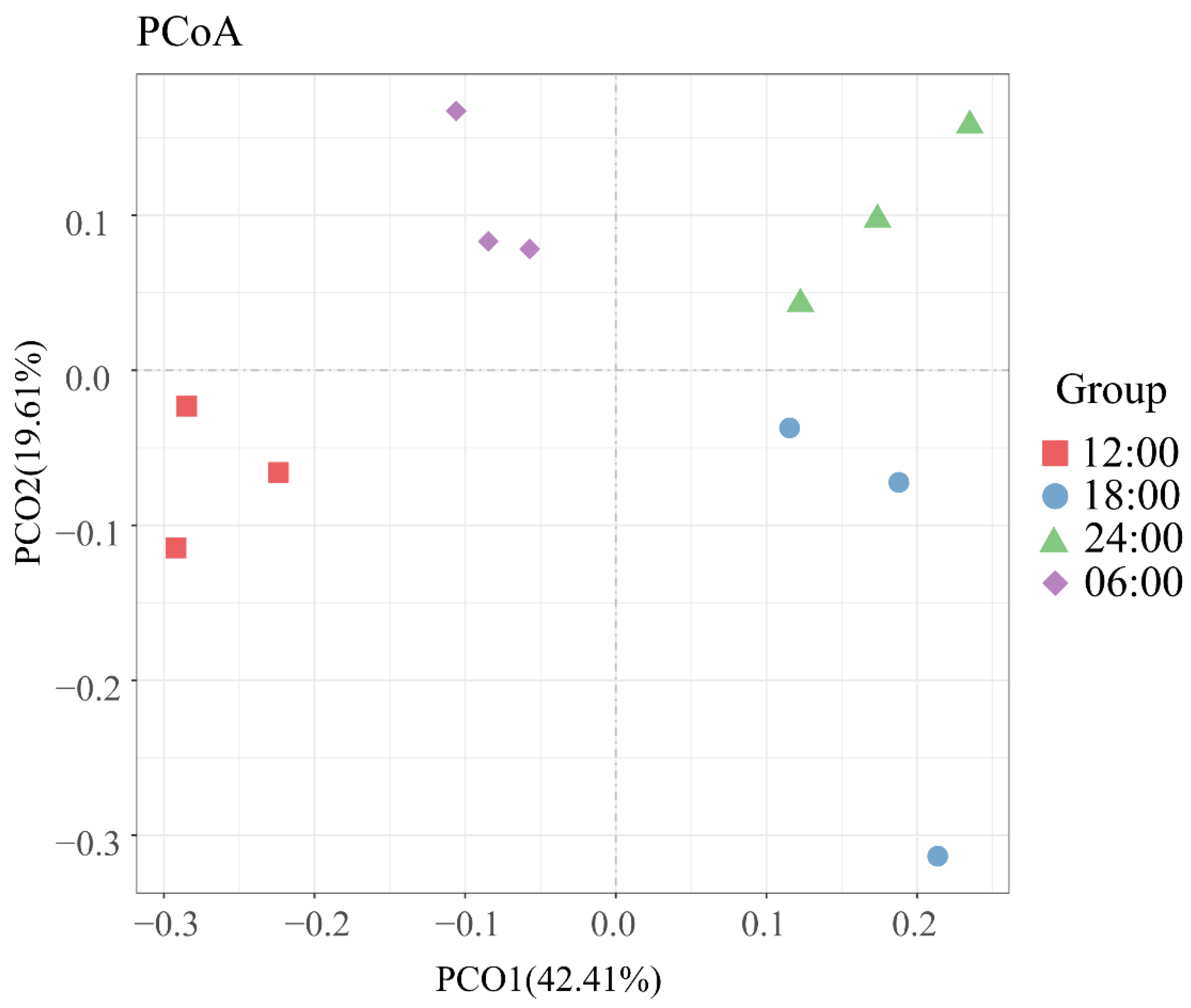
3.6. Beta Diversity
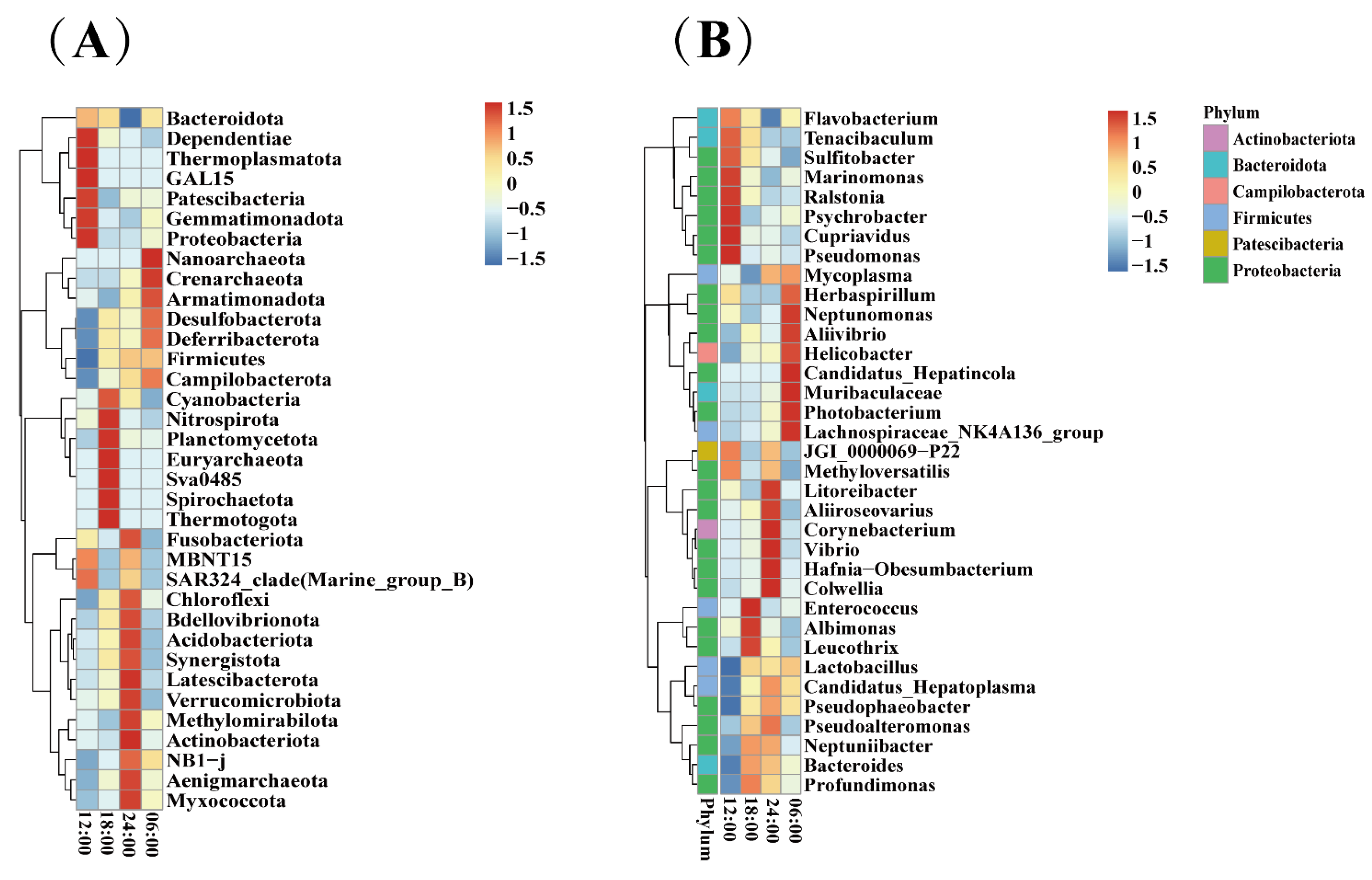
3.7. Species Abundance
3.8. Gene Function of the Gut Bacterial Community
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fustin, J.-M.; Doi, M.; Yamaguchi, Y.; Hida, H.; Nishimura, S.; Yoshida, M.; Isagawa, T.; Morioka Masaki, S.; Kakeya, H.; Manabe, I.; et al. RNA-Methylation-Dependent RNA Processing Controls the Speed of the Circadian Clock. Cell 2013, 155, 793–806. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Chen, P.; Hang, R.; Yang, S.Y.; Shen, J.L. Influence of salinity and day and night rhythm on feeding rate(FR) of Philippinarum ruditapes. Oceanogr. Tanwan Strait 2002, 21, 73–77. [Google Scholar]
- Baekelandt, S.; Mandiki, S.N.M.; Schmitz, M.; Kestemont, P. Influence of the light spectrum on the daily rhythms of stress and humoral innate immune markers in pikeperch Sander lucioperca. Aquaculture 2019, 499, 358–363. [Google Scholar] [CrossRef]
- Lazado, C.C.; Gesto, M.; Madsen, L.; Jokumsen, A. Interplay between daily rhythmic serum-mediated bacterial killing activity and immune defence factors in rainbow trout (Oncorhynchus mykiss). Fish Shellfish. Immunol. 2018, 72, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Lazado, C.C.; Skov, P.V.; Pedersen, P.B. Innate immune defenses exhibit circadian rhythmicity and differential temporal sensitivity to a bacterial endotoxin in Nile tilapia (Oreochromis niloticus). Fish Shellfish. Immunol. 2016, 55, 613–622. [Google Scholar] [CrossRef]
- Moran, D.; Softley, R.; Warrant, E.J. Eyeless Mexican cavefish save energy by eliminating the circadian rhythm in metabolism. PLoS ONE 2014, 9, e107877. [Google Scholar] [CrossRef]
- Zhao, E.; Tait, C.; Minacapelli, C.D.; Catalano, C.; Rustgi, V.K. Circadian Rhythms, the Gut Microbiome, and Metabolic Disorders. Gastro. Hep. Adv. 2022, 1, 93–105. [Google Scholar] [CrossRef]
- Gutierrez Lopez, D.E.; Lashinger, L.M.; Weinstock, G.M.; Bray, M.S. Circadian rhythms and the gut microbiome synchronize the host’s metabolic response to diet. Cell Metab. 2021, 33, 873–887. [Google Scholar] [CrossRef]
- Pickel, L.; Sung, H.-K. Feeding Rhythms and the Circadian Regulation of Metabolism. Front. Nutr. 2020, 7, 39. [Google Scholar] [CrossRef]
- Vera, L.M.; Madrid, J.A.; Sánchez-Vázquez, F.J. Locomotor, feeding and melatonin daily rhythms in sharpsnout seabream (Diplodus puntazzo). Physiol. Behav. 2006, 88, 167–172. [Google Scholar] [CrossRef]
- Santos, A.D.A.; López-Olmeda, J.F.; Sánchez-Vázquez, F.J.; Fortes-Silva, R. Synchronization to light and mealtime of the circadian rhythms of self-feeding behavior and locomotor activity of white shrimps (Litopenaeus vannamei). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2016, 199, 54–61. [Google Scholar] [CrossRef]
- Gao, X.; Pang, G.; Luo, X.; You, W.; Ke, C. Effects of light cycle on circadian feeding activity and digestive physiology in Haliotis discus hannai. Aquaculture 2021, 539, 736642. [Google Scholar] [CrossRef]
- Solovyev, M.; Gisbert, E. Feeding regimes affected the circadian rhythms of pancreatic digestive enzymes and somatic growth in flathead grey mullet (Mugil cephalus) fry. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2022, 264, 111116. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, W.B.; Li, H.Y.; Xu, W.N.; He, J.X.; Li, X.F.; Jiang, G.Z. Effects of dietary supplementation of fructooligosaccharide on growth performance, body composition, intestinal enzymes activities and histology of blunt snout bream (Megalobrama amblycephala) fingerlings. Aquac. Nutr. 2013, 19, 886–894. [Google Scholar] [CrossRef]
- Chan, Q.; Wang, F.; Han, Y.; Ren, T.; Shi, L.; Ren, X.; Zeng, F.; Li, M.; Chen, W. An investigation on dietary chromium picolinate supplementation in the juvenile sea cucumber Apostichopus japonicus: Growth, digestive enzyme activity, growth-related genes expression, immune and antioxidant capacity. Aquac. Rep. 2022, 24, 101099. [Google Scholar] [CrossRef]
- Magouz, F.I.; Mahmoud, S.A.; El-Morsy, R.A.A.; Paray, B.A.; Soliman, A.A.; Zaineldin, A.I.; Dawood, M.A.O. Dietary menthol essential oil enhanced the growth performance, digestive enzyme activity, immune-related genes, and resistance against acute ammonia exposure in Nile tilapia (Oreochromis niloticus). Aquaculture 2021, 530, 735944. [Google Scholar] [CrossRef]
- Song, Y.; Song, X.; Wu, M.; Pang, Y.; Shi, A.; Shi, X.; Niu, C.; Cheng, Y.; Yang, X. The protective effects of melatonin on survival, immune response, digestive enzymes activities and intestinal microbiota diversity in Chinese mitten crab (Eriocheir sinensis) exposed to glyphosate. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 238, 108845. [Google Scholar] [CrossRef]
- Li, B.; Chen, B.; Qi, Z.; Jiang, Z.; Zhang, J.; Fang, J. Relationship between differential retention of Escherichia coli and Enterococcus faecalis and variations in enzyme activity in the scallop Patinopecten yessoensis. Mar. Pollut. Bull. 2010, 60, 1600–1605. [Google Scholar] [CrossRef]
- Javahery, S.; Noori, A.; Hoseinifar, S.H. Growth performance, immune response, and digestive enzyme activity in Pacific white shrimp, Penaeus vannamei Boone, 1931, fed dietary microbial lysozyme. Fish Shellfish. Immunol. 2019, 92, 528–535. [Google Scholar] [CrossRef]
- Angela, C.; Wang, W.; Lyu, H.; Zhou, Y.; Huang, X. The effect of dietary supplementation of Astragalus membranaceus and Bupleurum chinense on the growth performance, immune-related enzyme activities and genes expression in white shrimp, Litopenaeus vannamei. Fish Shellfish. Immunol. 2020, 107, 379–384. [Google Scholar] [CrossRef]
- Bai, Z.; Ren, T.; Han, Y.; Hu, Y.; Schohel, M.R.; Jiang, Z. Effect of dietary Bio-fermented selenium on growth performance, nonspecific immune enzyme, proximate composition and bioaccumulation of zebrafish (Danio rerio). Aquac. Rep. 2019, 13, 100180. [Google Scholar] [CrossRef]
- Sonnenburg, J.L.; Bäckhed, F. Diet–microbiota interactions as moderators of human metabolism. Nature 2016, 535, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Parma, L.; Yúfera, M.; Navarro-Guillén, C.; Moyano, F.J.; Soverini, M.; D’Amico, F.; Candela, M.; Fontanillas, R.; Gatta, P.P.; Bonaldo, A. Effects of calcium carbonate inclusion in low fishmeal diets on growth, gastrointestinal pH, digestive enzyme activity and gut bacterial community of European sea bass (Dicentrarchus labrax L.) juveniles. Aquaculture 2019, 510, 283–292. [Google Scholar] [CrossRef]
- Zhou, L.; Qu, Y.; Qin, J.G.; Chen, L.; Han, F.; Li, E. Deep insight into bacterial community characterization and relationship in the pond water, sediment and the gut of shrimp (Penaeus japonicus). Aquaculture 2021, 539, 736658. [Google Scholar] [CrossRef]
- Shen, H.; Zhang, X.; Qian, D.; Chen, J.; Xiong, J. Pathobiology of Enterocytozoon hepatopenaei (EHP) in shrimp: Diagnosis and interpretation from the gut bacterial community. Aquaculture 2022, 554, 738169. [Google Scholar] [CrossRef]
- Dai, W.; Dong, Y.; Ye, J.; Xue, Q.; Lin, Z. Gut microbiome composition likely affects the growth of razor clam Sinonovacula constricta. Aquaculture 2022, 550, 737847. [Google Scholar] [CrossRef]
- Voigt, R.M.; Forsyth, C.B.; Green, S.J.; Engen, P.A.; Keshavarzian, A. Chapter Nine—Circadian Rhythm and the Gut Microbiome. Int. Rev. Neurobiol. 2016, 131, 193–205. [Google Scholar]
- Matenchuk, B.A.; Mandhane, P.J.; Kozyrskyj, A.L. Sleep, circadian rhythm, and gut microbiota. Sleep Med. Rev. 2020, 53, 101340. [Google Scholar] [CrossRef]
- Cicala, F.; Lago-Lestón, A.; Gomez-Gil, B.; Gollas-Galván, T.; Chong-Robles, J.; Cortés-Jacinto, E.; Martínez-Porchas, M. Gut microbiota shifts in the giant tiger shrimp, Penaeus monodon, during the postlarvae, juvenile, and adult stages. Aquac. Int. 2020, 28, 1421–1433. [Google Scholar] [CrossRef]
- Ock Kim, Y.; Mahboob, S.; Viayaraghavan, P.; Biji, D.; Abdullah Al-Ghanim, K.; Al-Misned, F.; Ahmed, Z.; Kwon, J.-T.; Won Na, S.; Kim, H.-J. Growth promoting activity of Penaeus indicus by secondary metabolite producing probiotic bacterium Bacillus subtilis isolated from the shrimp gut. J. King Saud Univ. Sci. 2020, 32, 1641–1646. [Google Scholar] [CrossRef]
- Yu, C.; Li, L.; Jin, J.; Zhang, B.; Wei, H.; Zhao, Y.; Li, X.; Li, Y. Comparative analysis of gut bacterial community composition during a single day cycle in Chinese mitten crab (Eriocheir sinensis). Aquac. Rep. 2021, 21, 100907. [Google Scholar] [CrossRef]
- Xu, W.; Xie, J.; Shi, H.; Li, C. Hematodinium infections in cultured ridgetail white prawns, Exopalaemon carinicauda, in eastern China. Aquaculture 2010, 300, 25–31. [Google Scholar] [CrossRef]
- Wang, J.; Ge, Q.; Li, J.; Li, J. Effects of inbreeding on growth and survival rates, and immune responses of ridgetail white prawn Exopalaemon carinicauda against the infection of Vibrio parahaemolyticus. Aquaculture 2020, 519, 734755. [Google Scholar] [CrossRef]
- Fortes-Silva, R.; Oliveira, I.E.; Vieira, V.P.; Winkaler, E.U.; Guerra-Santos, B.; Cerqueira, R.B. Daily rhythms of locomotor activity and the influence of a light and dark cycle on gut microbiota species in tambaqui (Colossoma macropomum). Biol. Rhythm. Res. 2016, 47, 183–190. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, H.-H.; Zhou, T.; Lin, Z.; Dong, Y. The discovery of circadian rhythm of feeding time on digestive enzymes activity and their gene expression in Sinonovacula constricta within a light/dark cycle. Front. Mar. Sci. 2021, 8, 744212. [Google Scholar] [CrossRef]
- Parkar, S.G.; Kalsbeek, A.; Cheeseman, J.F. Potential role for the gut microbiota in modulating host circadian rhythms and metabolic health. Microorganisms 2019, 7, 41. [Google Scholar] [CrossRef]
- Liang, X.; FitzGerald, G.A. Timing the Microbes: The Circadian Rhythm of the Gut Microbiome. J. Biol. Rhythm. 2017, 32, 505–515. [Google Scholar] [CrossRef]
- Nagendra, H. Opposite trends in response for the Shannon and Simpson indices of landscape diversity. Appl. Geogr. 2002, 22, 175–186. [Google Scholar] [CrossRef]
- Thaiss Christoph, A.; Zeevi, D.; Levy, M.; Zilberman-Schapira, G.; Suez, J.; Tengeler Anouk, C.; Abramson, L.; Katz Meirav, N.; Korem, T.; Zmora, N.; et al. Transkingdom Control of Microbiota Diurnal Oscillations Promotes Metabolic Homeostasis. Cell 2014, 159, 514–529. [Google Scholar] [CrossRef]
- Zarrinpar, A.; Chaix, A.; Yooseph, S.; Panda, S. Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell Metab. 2014, 20, 1006–1017. [Google Scholar] [CrossRef]
- Liang, X.; Bushman, F.D.; FitzGerald, G.A. Rhythmicity of the intestinal microbiota is regulated by gender and the host circadian clock. Proc. Natl. Acad. Sci. USA 2015, 112, 10479–10484. [Google Scholar] [CrossRef]
- Gao, S.; Pan, L.; Huang, F.; Song, M.; Tian, C.; Zhang, M. Metagenomic insights into the structure and function of intestinal microbiota of the farmed Pacific white shrimp (Litopenaeus vannamei). Aquaculture 2019, 499, 109–118. [Google Scholar] [CrossRef]
- Rungrassamee, W.; Klanchui, A.; Maibunkaew, S.; Chaiyapechara, S.; Jiravanichpaisal, P.; Karoonuthaisiri, N. Characterization of intestinal bacteria in wild and domesticated adult black tiger shrimp (Penaeus monodon). PLoS ONE 2014, 9, e91853. [Google Scholar] [CrossRef]
- Zeng, C.; Lin, M.; Li, Z.; Ma, Y.; Wang, S. The structural and functional characteristics of the gut microbiota of Marsupenaeus japonicus as revealed by 16S rRNA gene amplicon sequencing. Microbiol. China 2020, 47, 1857–1866. [Google Scholar]
- Allemann, M.N.; Allen, E.E. Chapter One—Characterization and Application of Marine Microbial Omega-3 Polyunsaturated Fatty Acid Synthesis. Methods Enzymol. 2018, 605, 3–32. [Google Scholar]
- Li, W.; Pu, N.; Liu, C.-X.; Yuan, Q.-P.; Li, Z.-J. Metabolic engineering of the marine bacteria Neptunomonas concharum for the production of acetoin and meso-2,3-butanediol from acetate. Biochem. Eng. J. 2019, 151, 107311. [Google Scholar] [CrossRef]
- Von Ah, U.; Mozzetti, V.; Lacroix, C.; Kheadr, E.E.; Fliss, I.; Meile, L. Classification of a moderately oxygen-tolerant isolate from baby faeces as Bifidobacterium thermophilum. BMC Microbiol. 2007, 7, 79. [Google Scholar] [CrossRef]
- Jiao, L.; Dai, T.; Tao, X.; Lu, J.; Zhou, Q. Influence of Light/Dark Cycles on Body Color, Hepatopancreas Metabolism, and Intestinal Microbiota Homeostasis in Litopenaeus vannamei. Front. Mar. Sci. 2021, 8, 750384. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, S.; Liu, R.; Zhang, H.; Yu, F.; Shi, T.; Zhu, J.; Zhou, X.; Yan, B.; Gao, H.; Wang, P.; et al. Comparative Analyses of the Exopalaemon carinicauda Gut Bacterial Community and Digestive and Immune Enzyme Activity during a 24-Hour Cycle. Microorganisms 2022, 10, 2258. https://doi.org/10.3390/microorganisms10112258
Xie S, Liu R, Zhang H, Yu F, Shi T, Zhu J, Zhou X, Yan B, Gao H, Wang P, et al. Comparative Analyses of the Exopalaemon carinicauda Gut Bacterial Community and Digestive and Immune Enzyme Activity during a 24-Hour Cycle. Microorganisms. 2022; 10(11):2258. https://doi.org/10.3390/microorganisms10112258
Chicago/Turabian StyleXie, Shumin, Runyao Liu, Huiling Zhang, Fei Yu, Tingting Shi, Jiawei Zhu, Xinlei Zhou, Binlun Yan, Huan Gao, Panpan Wang, and et al. 2022. "Comparative Analyses of the Exopalaemon carinicauda Gut Bacterial Community and Digestive and Immune Enzyme Activity during a 24-Hour Cycle" Microorganisms 10, no. 11: 2258. https://doi.org/10.3390/microorganisms10112258
APA StyleXie, S., Liu, R., Zhang, H., Yu, F., Shi, T., Zhu, J., Zhou, X., Yan, B., Gao, H., Wang, P., & Xing, C. (2022). Comparative Analyses of the Exopalaemon carinicauda Gut Bacterial Community and Digestive and Immune Enzyme Activity during a 24-Hour Cycle. Microorganisms, 10(11), 2258. https://doi.org/10.3390/microorganisms10112258




