Immunomodulatory Effects of Probiotics: A Novel Preventive Approach for the Control of Bovine Mastitis
Abstract
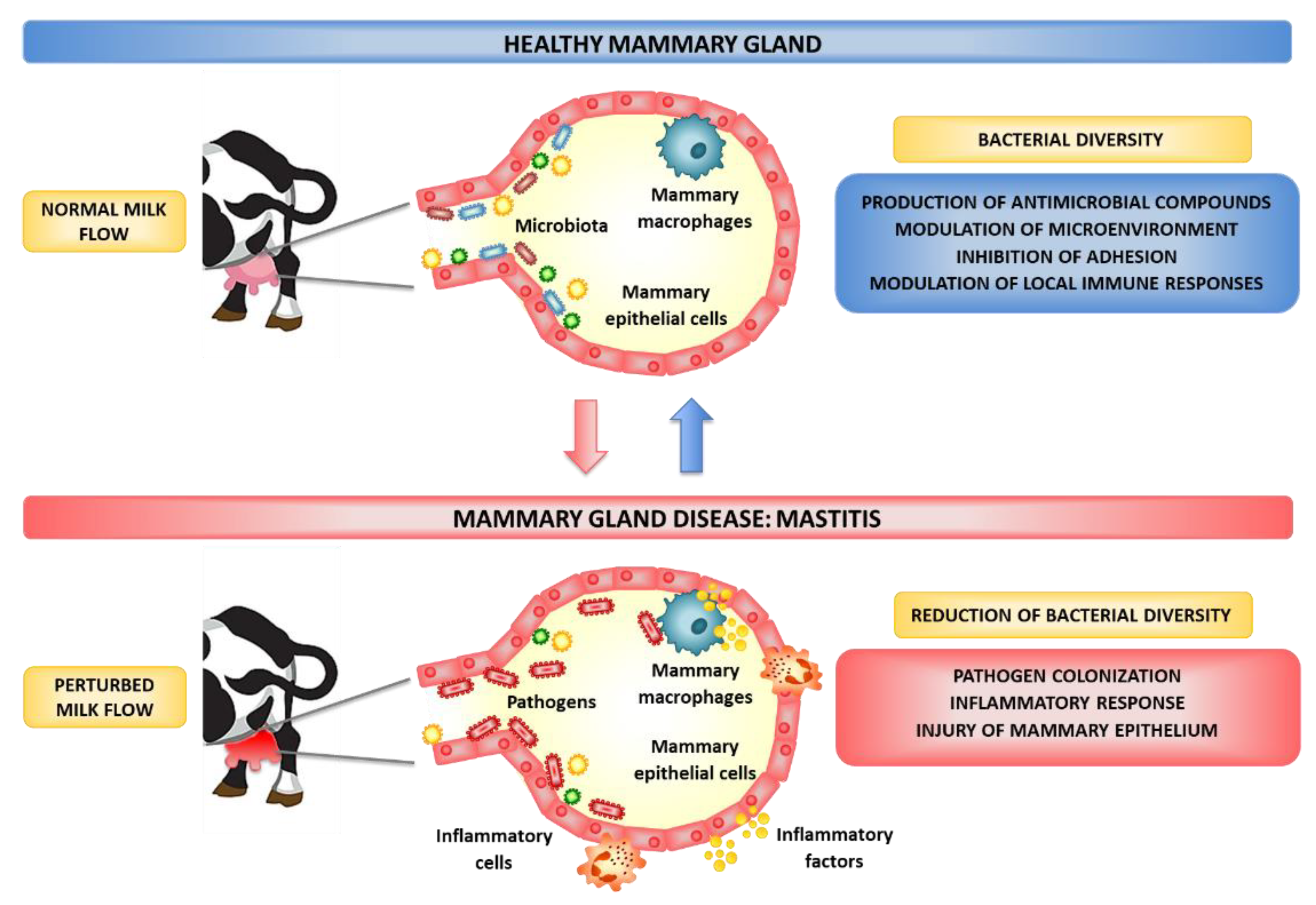
1. Introduction
2. Global Economic Impact of Bovine Mastitis
3. Prevention and Control Strategies of Bovine Mastitis
4. In Vivo Studies of Probiotics for the Prevention or Treatment of Bovine Mastitis
| Species | Administration Route | In-Vivo Model | Mechanism of Probiotics against Mastitis | References |
|---|---|---|---|---|
| L. lactis LMG 7930 | Injection through inguinal glands | CD-1 mice | Staphylococcus chromogenes ↓ IL-1β ↑ TNF-α ↑ | [51] |
| E. mundtii H81 | Intramammary infusion | BALB/c mice | Pathogens ↓ NF-κB ↓ Inflammatory response ↓ | [52] |
| L. lactis DPC3147 | Infusion via streak canal | Holstein–Friesian cows | Lymphocytes ↑ Neutrophils ↑ Acute phase protein haptaglobin ↑ Milk amyloid A ↑ | [17] |
| L. lactis DPC3147 | Intramammary infusion | Holstein–Friesian, New Zealand Friesian, Norwegian Red, Normande and Montebelliards cows | Pathogens ↓ Intramammary response ↑ | [29] |
| L. lactis DPC 3147 | Intramammary infusion | Holstein Friesian cows | IL-8 ↑ Somatic cell count ↓ | [60] |
| L. rhamnosus GG | Intramammary inoculation | Multiparous water buffaloes (Bubalus bubalis) | Leukocytes ↑ Pseudomonas ↓ Somatic cell count ↓ | [55] |
| E. faecium SF68 | Intramammary infusion | Holstein cows | Innate immunity ↑ Metalloproteinase 9 ↑ Neutrophil infiltration ↓ | [7] |
| B. breve | Intramammary infusion | Holstein cows | Pathogens ↓ Innate immune response ↑ Somatic cell counts ↓ | [61] |
| B. breve | Intramammary Infusion | Holstein cows | Pathogens ↓ Innate immune response ↑ | [54] |
| L. lactis subsp. lactis CRL1655; L. perolens CRL1724 | Intramammary infusion | Holstein cows | Pathogens ↓ Innate immune response ↑ Somatic cell counts ↓ | [30] |
| Saccharomyces cerevisiae and Lactobacillus | Oral feed | Fleckvieh cows | Prevalence of subclinical mastitis ↓ | [58] |
| Saccharomyces cerevisiae and L. lactis | Oral feed | Holstein cows | Mammary gland inflammation ↓ Enterococcus ↓ Streptococcus ↓ Lactococcus ↑ | [48] |
| Bacillus subtilis C-3102 | Oral feed | Holstein cows | Inflammation ↓ Blood CD4+ T cells ↑ Blood CD11c+CD172ahigh dendritic cells ↑ Blood WC1+γδ+ T ↓ Blood CD8+γδ+ T cells ↓ | [59] |
5. In Vitro Studies of Probiotics for the Prevention or Treatment of Bovine Mastitis
| Species/Strains | Experimental Mode | Probiotics Effects | References |
|---|---|---|---|
| L. bulgaricus | In vitro (SDS-PAGE) | Inhibition S. aureus and S. agalactiae by bacteriocin production | [62] |
| L. acidophilus DSM 20079, L. plantarum ATCC 8014, L. casei ATCC 39392, L. reuteri ATCC 23272 | In vitro (Well diffusion and Co-culture) | Inhibition and antagonistic activity against S. aureus | [63] |
| L. helveticus | In vitro (Well diffusion and Co-culture) | Antimicrobial activity against S. aureus, S. haemolyticus, S. simulans, S. vitulinus | [64] |
| B. amyloliquefaciens, B. cereus, B. licheniformis, B. subtilis ATCC 21332 | In vitro (Agar diffusion) | Inhibition of Gram-positive bacteria | [65] |
| L. sakei EIR/BG-1 | In vitro (Well diffusion) | Antimicrobial and antibiofilm activity against S. agalactiae, S. dysgalactiae subsp. dysgalactiae, and methicillin resistant S. aureus | [66] |
| L. rhamnosus ATCC 7469, L. plantarum 2/37 | In vitro (Culture based method) | Antimicrobial activity against S. aureus, S. xylosus, and S. epidermidis by biofilm production | [67] |
| L. plantarum | In vitro (Phage mixture) | Antimicrobial activity against S. aureus | [68] |
| Enterococcus hirae CRL 1842, E. hirae B6.1B, Enterococcus hirae CRL 1846, Enterococcus hirae CRL 1847, Enterococcus hirae CRL 1848, Enterococcus hirae CRL 1837, Enterococcus hirae CRL 1834, Enterococcus hirae CRL 1835 | In vitro (Diffusion plate technique) | Inhibition of L. innocua, L. monocytogenes, and S. dysgalactiae by bacteriocin production | [69] |
| L. paracasei, L. plantarum, L. lactis, L. rhamnosus | BTCEC | Adhesion to teat canal epithelial cells and inhibition of the growth of S. aureus, S. epidermidis, S. xylosus, S. uberis, S. agalactiae, and E. coli | [30] |
| L. perolens, L. lactis subsp. Lactis CRL 1655 | BTCEC | Adhesion to teat canal epithelial cells and inhibition of the growth of S. dysgalactiae and S. aureus | [71] |
| L. casei BL23 | bMECs | Anti-inflammatory properties and inhibition of the internalization of S. aureus | [73] |
| L. lactis V7 | bMECs | Modulation of CXCL8 production and inhibition of cell invasion by S. aureus and E. coli | [74] |
| L. casei CIRM-BIA 667 | bMECs | Adhesion to teat canal epithelial cells and inhibition of the growth of S. aureus | [72] |
| L. brevis 1595, L. brevis 1597, L. plantarum 1610, L. casei 1542, L. lactis 1596, L. garvieae 1605 | bMECs | Anti-inflammatory properties (reduction of IL-8) against E. coli | [9] |
| L. gasseri LA806 | bMECs | Reduction of proinflammatory cytokines (IL-8, IL-6, IL-1α, TNF-α) and prevention of S. aureus colonization | [13] |
| P. stilesii HOL36L1, L. lactis GIRO4S8, W. paramesenteroides GIR46L4, W. confusa GIR48L1, W. cibaria GIRO27L2, L. plantarum GUZ3L2, L. paracasei GIR53L1, S. lutetiensis HOL36L2 | MDA-MB-231 | Interference with adhesion and inhibition of S. aureus | [75] |
| L. lactis LMG 7930 | BME-UV1 | Reduction of the internalization of S. aureus, S. agalactiae, S. aureus, S. chromogenes, S. epidermidis, S. intermedius, and L. cremoris | [51] |
| L. lactis LL11 | BME-UV1 | Reduction of TNF-α against mastitis pathogen | [76] |
| L. rhamnosus GR-1 | bMECs | Ameliorates E. coli induced Inflammation | [77] |
| L. acidophilus CRL2074 | BME | Reduction of proinflammatory cytokines and chemokines (IL-1α, IL-1β, MCP-1, IL-8, CXCL3) induced by E. coli derived LPS | [3] |
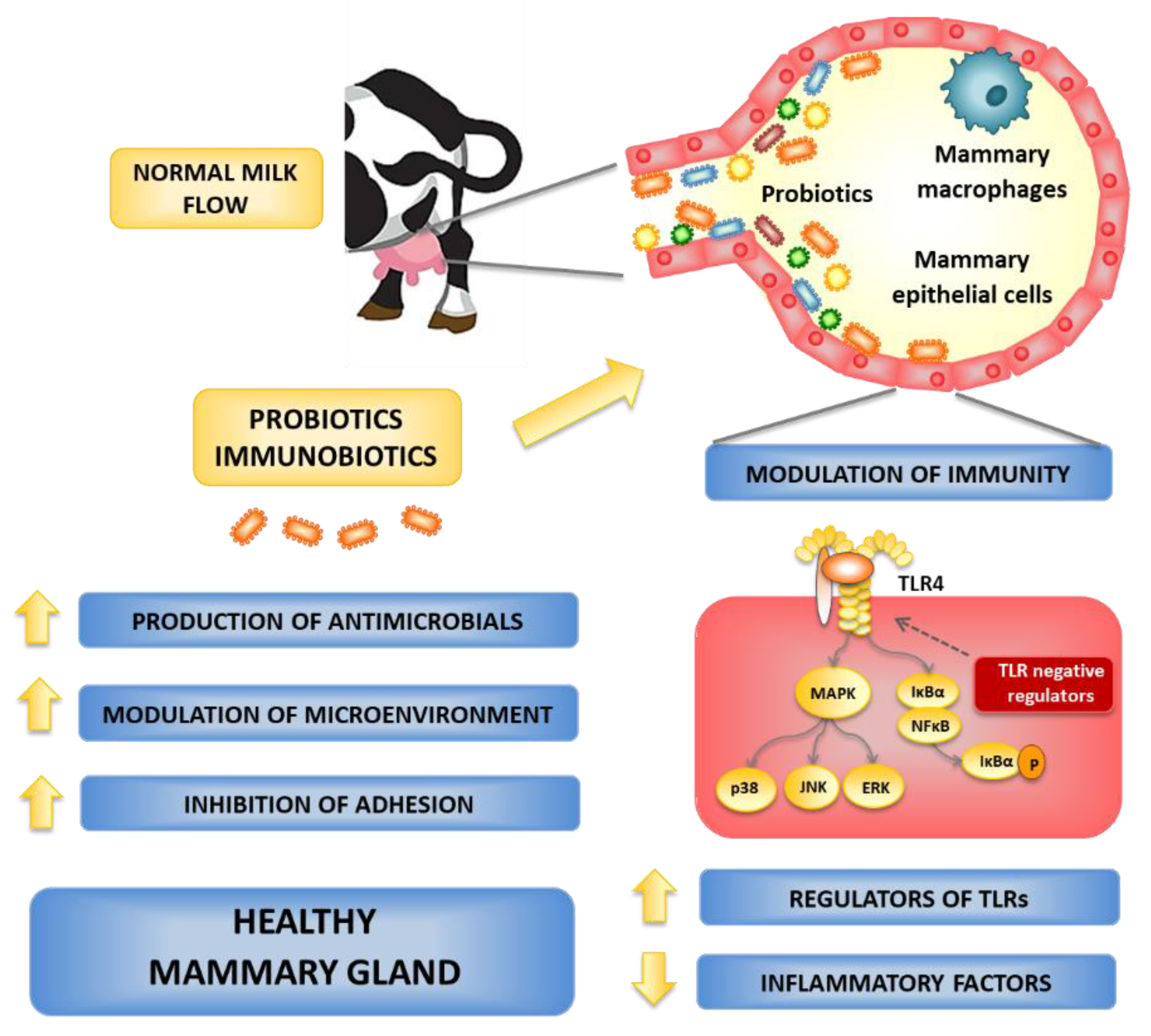
6. Possible Routes for Probiotics to Control the Bovine Mastitis
6.1. Local Administration of Probiotics
6.2. Oral Administration of Probiotics
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hasan, M.S.; Kober, A.K.M.H.; Rana, E.A.; Bari, M.S. Association of udder lesions with subclinical mastitis in dairy cows of Chattogram, Bangladesh. Adv. Anim. Vet. Sci. 2022, 10, 226–235. [Google Scholar]
- Islam, M.A.; Rony, S.A.; Kitazawa, H.; Rahman, A.K.M.A. Bayesian latent class evaluation of three tests for the screening of subclinical caprine mastitis in Bangladesh. Trop. Anim. Health Prod. 2020, 52, 2873–2881. [Google Scholar] [CrossRef] [PubMed]
- Fukuyama, K.; Islam, M.A.; Takagi, M.; Ikeda-Ohtsubo, W.; Kurata, S.; Aso, H.; Vignolo, G.; Villena, J.; Kitazawa, H. Evaluation of the immunomodulatory ability of lactic acid bacteria Isolated from feedlot cattle against mastitis Using a Bovine Mammary Epithelial Cells In Vitro Assay. Pathogens 2020, 9, 410. [Google Scholar] [CrossRef]
- Halasa, T.; Huijps, K.; Østerås, O.; Hogeveen, H. Economic effects of bovine mastitis and mastitis management: A review. Vet. Q. 2007, 29, 18–31. [Google Scholar] [CrossRef]
- Jensen, K.; Günther, J.; Talbot, R.; Petzl, W.; Zerbe, H.; Schuberth, H.J.; Seyfert, H.M.; Glass, E.J. Escherichia coli- and Staphylococcus aureus-induced mastitis differentially modulate transcriptional responses in neighbouring uninfected bovine mammary gland quarters. BMC Genom. 2013, 14, 36. [Google Scholar] [CrossRef] [PubMed]
- Schukken, Y.H.; Günther, J.; Fitzpatrick, J.; Fontaine, M.C.; Goetze, L.; Holst, O.; Leigh, J.; Petzl, W.; Schuberth, H.-J.; Sipka, A.; et al. Host-response patterns of intramammary infections in dairy cows. Vet. Immunol. Immunopathol. 2011, 144, 270–289. [Google Scholar] [CrossRef]
- Peng, H.Y.; Tiantong, A.; Chen, S.E.; Piamya, P.; Liu, W.B.; Peh, H.C.; Lee, J.W.; Chen, M.T.; Nagahata, H.; Chang, C.J. Ultrasonicated Enterococcus faecium SF68 enhances neutrophil free radical production and udder innate immunity of drying-off dairy cows. J. Dairy Res. 2013, 80, 349–359. [Google Scholar] [CrossRef]
- Kalmus, P.; Simojoki, H.; Orro, T.; Taponen, S.; Mustonen, K.; Halopainen, J.; Payörälä, S. Efficacy of 5-day parenteral versus intramammary benzylpenicillin for treatment of clinical mastitis caused by gram-positive bacteria susceptible to penicillin in vitro. J. Dairy Sci. 2014, 97, 2155–2164. [Google Scholar] [CrossRef]
- Bouchard, D.S.; Seridan, B.; Saraoui, T.; Rault, L.; Germon, P.; Gonzalez-Moreno, C.; Nader-Macias, F.M.; Baud, D.; François, P.; Chuat, V.; et al. Lactic acid bacteria isolated from bovine mammary microbiota: Potential allies against bovine mastitis. PLoS ONE 2015, 10, e0144831. [Google Scholar] [CrossRef]
- Bradley, A.J.; Breen, J.E.; Payne, B.; Green, M.J. A comparison of broad-spectrum and narrow-spectrum dry cow therapy used alone and in combination with a teat sealant. J. Dairy Sci. 2011, 94, 692–704. [Google Scholar] [CrossRef]
- Dalton, J.C. Antibiotic residue prevention in milk and dairy beef. West. Dairy News 2006, 6, 79. [Google Scholar]
- Saini, V.; McClure, J.T.; Léger, D.; Keefe, G.C.; Scholl, D.T.; Morck, D.W.; Barkema, H.W. Antimicrobial resistance profiles of common mastitis pathogens on Canadian dairy farms. J. Dairy Sci. 2012, 95, 4319–4332. [Google Scholar] [CrossRef] [PubMed]
- Blanchet, F.; Rault, L.; Peton, V.; Le Loir, Y.; Blondeau, C.; Lenoir, L.; Dubourdeaux, M.; Even, S. Heat inactivation partially preserved barrier and immunomodulatory effects of Lactobacillus gasseri LA806 in an in vitro model of bovine mastitis. Benef. Microbes 2021, 12, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Chaimanee, V.; Sakulsingharoj, C.; Deejing, S.; Seetakoses, P.; Niamsup, P. Screening and characterization of bacteriocin-producing bacteria capable of inhibiting the growth of bovine mastitis. Maejo Int. J. Sci. Technol. 2009, 3, 43–52. [Google Scholar]
- Espeche, M.C.; Otero, M.C.; Sesma, F.; Nader-Macias, M.E.F. Screening of surface properties and antagonistic substances production by lactic acid bacteria isolated from the mammary gland of healthy and mastitic cows. Vet. Microbiol. 2009, 135, 346–357. [Google Scholar] [CrossRef]
- Crispie, F.; Alonso-Gomez, M.; O’Loughlin, C.; Klostermann, K.; Flynn, J.; Arkins, S.; Meaney, W.; Ross, R.P.; Hill, C. Intramammary infusion of a live culture for treatment of bovine mastitis: Effect of live lactococci on the mammary immune response. J. Dairy Res. 2008, 75, 374–384. [Google Scholar] [CrossRef]
- Beecher, C.; Daly, M.; Berry, D.P.; Klostermann, K.; Flynn, J.; Meaney, W.; Hill, C.; McCarthy, T.V.; Ross, R.P.; Giblin, L. Administration of a live culture of Lactococcus lactis DPC 3147 into the bovine mammary gland stimulates the local host immune response, particularly IL-1β and IL-8 gene expression. J. Dairy Res. 2009, 76, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Sharun, K.; Dhama, K.; Tiwari, R.; Gugjoo, M.B.; Yatoo, M.I.; Patel, S.K.; Pathak, M.; Karthik, K.; Khurana, S.K.; Singh, R.; et al. Advances in therapeutic and management approaches of bovine mastitis: A comprehensive review. Vet. Q. 2021, 41, 107–136. [Google Scholar] [CrossRef]
- Villena, J.; Aso, H.; Rutten, V.P.M.G.; Takahashi, H.; van Eden, W.; Kitazawa, H. Immunobiotics for the Bovine Host: Their Interaction with Intestinal Epithelial Cells and Their Effect on Antiviral Immunity. Front. Immunol. 2018, 9, 326. [Google Scholar] [CrossRef]
- Mansilla, F.; Takagi, M.; Garcia-Castillo, V.; Aso, H.; Nader-Macias, M.E.; Vignolo, G.; Kitazawa, H.; Villena, J. Modulation of Toll-like receptor-mediated innate immunity in bovine intestinal epithelial cells by lactic acid bacteria isolated from feedlot cattle. Benef. Microbes 2020, 11, 269–282. [Google Scholar] [CrossRef]
- Francoz, D.; Wellemans, V.; Dupre, J.P.; Roy, J.P.; Labelle, F.; Lacasse, P.; Dufour, S. Invited review: A systematic review and qualitative analysis of treatments other than conventional antimicrobials for clinical mastitis in dairy cows. J. Dairy Sci. 2017, 100, 7751–7770. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.; Barkema, H.W.; Brito, L.F.; Narayana, S.G.; Miglior, F. Symposium review: Novel strategies to genetically improve mastitis resistance in dairy cattle. J. Dairy Sci. 2018, 101, 2724–2736. [Google Scholar] [CrossRef] [PubMed]
- Deb, R.; Kumar, A.; Chakraborty, S.; Verma, A.K.; Tiwari, R.; Dhama, K.; Singh, U.; Kumar, S. Trends in diagnosis and control of bovine mastitis: A review. Pak. J. Biol. Sci. 2013, 16, 1653–1661. [Google Scholar] [CrossRef]
- Bradley, A.J.; Breen, J.E.; Payne, B.; White, V.; Green, M.J. An investigation of the efficacy of a polyvalent mastitis vaccine using different vaccination regimens under field conditions in the United Kingdom. J. Dairy Sci. 2015, 98, 1706–1720. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, R.; Martin, V.; Maldonado, A.; Jimenez, E.; Fernandez, L.; Rodriguez, J.M. Treatment of infectious mastitis during lactation: Antibiotics versus oral administration of Lactobacilli isolated from breast milk. Clin. Infect. Dis. 2010, 50, 1551–1558. [Google Scholar] [CrossRef]
- Klostermann, K.; Crispie, F.; Flynn, J.; Ross, R.P.; Hill, C.; Meaney, W.J. Intramammary infusion of a live culture of Lactococcus lactis for treatment of bovine mastitis: Comparison of antibiotic treatment in field trials. J. Dairy Res. 2008, 75, 365–373. [Google Scholar] [CrossRef]
- Islam, M.A.; Takagi, M.; Fukuyama, K.; Komatsu, R.; Albarracin, L.; Nochi, T.; Suda, Y.; Ikeda-Ohtsubo, W.; Rutten, V.; Eden, W.V.; et al. Transcriptome analysis of the inflammatory responses of bovine mammary epithelial cells: Exploring immunomodulatory target genes for bovine mastitis. Pathogens 2020, 9, 200. [Google Scholar] [CrossRef]
- Kober, A.K.M.H.; Riaz Rajoka, M.S.; Mehwish, H.M.; Villena, J.; Kitazawa, H. Immunomodulation potential of probiotics: A novel strategy for improving livestock health, immunity, and productivity. Microorganisms 2022, 10, 388. [Google Scholar] [CrossRef]
- Pellegrino, M.; Berardo, N.; Giraudo, J.; Nader-Macias, M.E.F.; Bogni, C. Bovine mastitis prevention: Humoral and cellular response of dairy cows inoculated with lactic acid bacteria at the dry-off period. Benef. Microbes 2017, 8, 589–596. [Google Scholar] [CrossRef]
- Diepers, A.; Krömker, V.; Zinke, C.; Wente, N.; Pan, L.; Paulsen, K.; Paduch, J.H. In vitro ability of lactic acid bacteria to inhibit mastitis-causing pathogens. Sustain. Chem. Pharm. 2017, 5, 84–92. [Google Scholar] [CrossRef]
- Bardhan, D. Estimates of economic losses due to clinical mastitis in organized dairy farms. Indian J. Dairy Sci. 2013, 66, 168–172. [Google Scholar]
- Bari, M.; Alam, M.; Uddin, M.; Rahman, M. Prevalence and associated risk factors of bovine clinical mastitis in Patiyaupazila under Chittagong district of Bangladesh. Int. J. Nat. Sci. 2016, 4, 5–9. [Google Scholar] [CrossRef]
- Iweka, P.; Kawamura, S.; Mitani, T.; Kawaguchi, T.; Koseki, S. Online milk quality assessment during milking using near-infrared spectroscopic sensing system. Environ. Control Biol. 2020, 58, 1–6. [Google Scholar] [CrossRef]
- Takahashi, H. Early diagnosis and cytokine therapy of subclinical mastitis in dairy cows. Proc. Jpn. Soc. Anim. Nutr. Met. 2005, 49, 59–70. [Google Scholar]
- Aghamohammadi, M.; Haine, D.; Kelton, D.F.; Barkema, H.W.; Hogeveen, H.; Keefe, G.P.; Dufour, S. Herd-level mastitis-associated costs on Canadian dairy farms. Front. Vet. Sci. 2018, 5, 100. [Google Scholar] [CrossRef]
- Liang, D.; Arnold, L.M.; Stowe, C.J.; Harmon, R.J.; Bewley, J.M. Estimating US dairy clinical disease costs with stochastic simulation model. J. Dairy Sci. 2017, 100, 1472–1486. [Google Scholar] [CrossRef]
- Romero, J.; Benavides, E.; Meza, C. Assessing financial impacts of subclinical mastitis on Colombian dairy farms. Front. Vet. Sci. 2018, 5, 273. [Google Scholar] [CrossRef]
- Hogeveen, H.; Huijps, K.; Lam, T.J. Economic aspects of mastitis: New developments. N. Z. Vet. J. 2011, 59, 16–23. [Google Scholar] [CrossRef]
- Hillerton, J.E.; Berry, E.A. Treating mastitis in the cow—A tradition or an archaism. J. Appl. Microbiol. 2005, 98, 1250–1255. [Google Scholar] [CrossRef]
- Dego, O.K. Bovine Mastitis: Part I. In Animal Reproduction in Veterinary Medicine; Aral, F., Payan-Carreira, R., Quaresma, M., Eds.; IntechOpen: London, UK, 2020. [Google Scholar]
- Rollin, E.; Dhuyvetter, K.C.; Overton, M.W. The cost of clinical mastitis in the first 30 days of lactation: An economic modeling tool. Prev. Vet. Med. 2015, 122, 257–264. [Google Scholar] [CrossRef]
- Huijps, K.; Lam, T.J.; Hogeveen, H. Costs of mastitis: Facts and perception. J. Dairy Res. 2008, 75, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Babra, C.; Tiwari, J.G.; Pier, G.; Thein, T.H.; Sunagar, R.; Sundareshan, S.; Isloor, S.; Hegde, N.R.; de Wet, S.; Deighton, M.; et al. The persistence of biofilm-associated antibiotic resistance of Staphylococcus aureus isolated from clinical bovine mastitis cases in Australia. Folia Microbiol. 2013, 58, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.K.; Fox, L.K.; Hancock, D.D.; McMahan, W.; Park, Y.H. Prevalence and antibiotic resistance of mastitis pathogens isolated from dairy herds transitioning to organic management. J. Vet. Sci. 2012, 13, 103–105. [Google Scholar] [CrossRef]
- Wilson, D.J.; Mallard, B.A.; Burton, J.L.; Schukken, Y.H.; Grohn, Y.T. Association of Escherichia coli J5-specific serum antibody responses with clinical mastitis outcome for J5 vaccinate and control dairy cattle. Clin. Vaccine Immunol. 2009, 16, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Collado, R.; Prenafeta, A.; González-González, L.; Pérez-Pons, J.A.; Sitjà, M. Probing vaccine antigens against bovine mastitis caused by Streptococcus uberis. Vaccine 2016, 34, 3848–3854. [Google Scholar] [CrossRef]
- Côté-Gravel, J.; Malouin, F. Symposium review: Features of Staphylococcus aureus mastitis pathogenesis that guide vaccine development strategies. J. Dairy Sci. 2019, 102, 4727–4740. [Google Scholar] [CrossRef]
- Maity, S.; Ambatipudi, K. Mammary microbial dysbiosis leads to the zoonosis of bovine mastitis: A One-Health perspective. FEMS Microbiol Ecol. 2020, 97, fiaa241. [Google Scholar] [CrossRef]
- Derakhshani, H.; Fehr, K.B.; Sepehri, S.; Francoz, D.; De Buck, J.; Barkema, H.W.; Plaizier, J.C.; Khafipour, E. Invited review: Microbiota of the bovine udder: Contributing factors and potential implications for udder health and mastitis susceptibility. J. Dairy Sci. 2018, 101, 10605–10625. [Google Scholar] [CrossRef]
- Bronzo, V.; Lopreiato, V.; Riva, F.; Amadori, M.; Curone, G.; Addis, M.F.; Cremonesi, P.; Moroni, P.; Trevisi, E.; Castiglioni, B. The Role of Innate Immune Response and Microbiome in Resilience of Dairy Cattle to Disease: The Mastitis Model. Animals 2020, 10, 1397. [Google Scholar] [CrossRef]
- Camperio, C.; Armas, F.; Biasibetti, E.; Frassanito, P.; Giovannelli, C.; Spuria, L.; D’Agostino, C.; Tait, S.; Capucchio, M.T.; Marianelli, C. A mouse mastitis model to study the effects of the intramammary infusion of a food-grade Lactococcus lactis strain. PLoS ONE 2017, 12, e0184218. [Google Scholar] [CrossRef]
- Qiu, M.; Feng, L.; Yu, Z.; Zhao, C.; Gao, S.; Bao, L.; Zhang, N.; Fu, Y.; Hu, X. Probiotic Enterococcus mundtii H81inhibits the NF-κB signaling pathway to ameliorate Staphylococcus aureus-induced mastitis in mice. Microb. Pathog. 2022, 164, 105414. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Liu, Y.C.; Wang, Y.; Li, H.; Wang, X.M.; Wu, Y.; Zhang, D.R.; Gao, S.; Qi, Z.L. Impact of yeast and lactic acid bacteria on mastitis and milk microbiota composition of dairy cows. AMB Express 2020, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Nagahata, H.; Mukai, T.; Natsume, Y.; Okuda, M.; Ando, T.; Hisaeda, K.; Gondaira, S.; Higuchi, H. Effects of intramammary infusion of Bifidobacterium breve on mastitis pathogens and somatic cell response in quarters from dairy cows with chronic subclinical mastitis. Anim. Sci. J. 2020, 91, e13406. [Google Scholar] [CrossRef]
- Catozzi, C.; Cuscó, A.; Lecchi, C.; De Carlo, E.; Vecchio, D.; Martucciello, A.; D’Angelo, L.; Francino, O.; Sanchez Bonastre, A.; Ceciliani, F. Impact of intramammary inoculation of inactivated Lactobacillus rhamnosus and antibiotics on the milk microbiota of water buffalo with subclinical mastitis. PLoS ONE 2019, 14, e0210204. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jiang, N.; Zhang, W.; Lv, Z.; Liu, J.; Shi, H. Bacillus amyloliquefaciens-9 Reduces Somatic Cell Count and Modifies Fecal Microbiota in Lactating Goats. Mar. Drugs 2021, 19, 404. [Google Scholar] [CrossRef] [PubMed]
- Lamari, I.; Mimoune, N.; Khelef, D. Učinak dodatka prehrani na subklinički mastitis krava. Vet. Stanica 2021, 52, 445–460. [Google Scholar] [CrossRef]
- Saha, S.; Singha, S.; Ahmed, S.S.; Toledo-Alvarado, H.; Khan, M.M.H. Effects of yeast (Saccharomyces cerevisiae type boulardii CNCM I-1079) supplementation on growth performance and blood metabolites in Black Bengal goat kids. Vet. Arh. 2018, 88, 661–672. [Google Scholar] [CrossRef]
- Urakawa, M.; Zhuang, T.; Sato, H.; Takanashi, S.; Yoshimura, K.; Endo, Y.; Katsura, T.; Umino, T.; Tanaka, K.; Watanabe, H.; et al. Prevention of mastitis in multiparous dairy cows with a previous history of mastitis by oral feeding with probiotic Bacillus subtilis. Anim. Sci. J. 2022, 93, e13764. [Google Scholar] [CrossRef]
- Kitching, M.; Mathur, H.; Flynn, J.; Byrne, N.; Dillon, P.; Sayers, R.; Rea, M.C.; Hill, C.; Ross, R.P. A Live Bio-Therapeutic for Mastitis, Containing Lactococcus lactis DPC3147 with Comparable Efficacy to Antibiotic Treatment. Front. Microbiol. 2019, 10, 2220. [Google Scholar] [CrossRef]
- Nagahata, H.; Kine, M.; Watanabe, H.; Tanaka, A.; Takahashi, A.; Gondaira, S.; Higuchi, H. Somatic cell and innate immune responses in mammary glands of lactating cows to intramammary infusion of Bifidobacterium breve at pre-drying off period. J. Vet. Med. Sci. 2021, 83, 1845–1851. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, J.H.; Son, J.H.; Seo, H.J.; Park, S.J.; Paek, N.S.; Kim, S.K. Characterization of bacteriocin produced by Lactobacillus bulgaricus. J. Microbiol. Biotechol. 2004, 14, 503–508. [Google Scholar]
- Nazila, A.S.; Rooha, K.K.; Bagher, Y.; Taher, N.S. Antagonistic activity of probiotic lactobacilli against Staphylococcus aureus isolated from bovine mastitis. Afr. J. Microbiol. Res. 2010, 4, 2169–2173. [Google Scholar]
- Gulbe, G.; Valdovska, A.; Saulite, V.; Jermolajevs, J. In vitro assessment for antimicrobial activity of Lactobacillus helveticus and its natural glycopeptides against mastitis causing pathogens in dairy cattle. Open Biotechnol. J. 2015, 9, 61–66. [Google Scholar] [CrossRef][Green Version]
- Al-Qumber, M.; Tagg, J.R. Commensal bacilli inhibitory to mastitis pathogens isolated from the udder microbiota of healthy cows. J. Appl. Microbiol. 2006, 101, 1152–1160. [Google Scholar] [CrossRef] [PubMed]
- Sevin, S.; Karaca, B.; Haliscelik, O.; Kibar, H.; OmerOglou, E.; Kiran, F. Postbiotics secreted by Lactobacillus sakei EIR/CM-1 isolated from cow milk microbiota, display antibacterial and antibiofilm activity against ruminant mastitis-causing pathogens. Ital. J. Anim. Sci. 2021, 20, 1302–1316. [Google Scholar] [CrossRef]
- Wallis, J.K.; Krömker, V.; Paduch, J.H. Biofilm challenge: Lactic acid bacteria isolated from bovine udders versus Staphylococci. Foods 2019, 8, 79. [Google Scholar] [CrossRef]
- Titze, I.; Krömker, V. Antimicrobial activity of a phage mixture and a lactic acid bacterium against Staphylococcus aureus from bovine mastitis. Vet. Sci. 2020, 7, 31. [Google Scholar] [CrossRef]
- Espeche, M.C.; Pellegrino, M.; Frola, I.; Larriestra, A.; Bogni, C.; Nader-Macías, M.F. Lactic acid bacteria from raw milk as potentially beneficial strains to prevent bovine mastitis. Anaerobe 2012, 18, 103–109. [Google Scholar] [CrossRef]
- Jiao, X.; Li, K.; Geng, M.; Li, K.; Liang, W.; Zhang, J.; Zhang, Q.; Gao, H.; Wei, X.; Yang, J. Activated T cells are the cellular source of IL-22 that enhances proliferation and survival of lymphocytes in Nile tilapia. Fish Shellfish Immunol. 2022, 128, 216–227. [Google Scholar] [CrossRef]
- Pellegrino, M.S.; Frola, I.D.; Natanael, B.; Gobelli, D.; Nader-Macias, M.E.; Bogni, C.I. In vitro characterization of lactic acid bacteria isolated from bovine milk as potential probiotic strains to prevent bovine mastitis. Probiotics Antimicob. Proteins 2019, 11, 74–84. [Google Scholar] [CrossRef]
- Bouchard, D.S.; Rault, L.; Berkova, N.; Le Loir, Y.; Even, S. Inhibition of Staphylococcus aureus invasion into bovine mammary epithelial cells by contact with live Lactobacillus casei. Appl. Environ. Microbiol. 2013, 79, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.F.S.; Rault, L.; Seyffert, N.; Azevedo, V.; Le Loir, Y.; Even, S. Lactobacillus casei BL23 modulates the innate immune response in Staphylococcus aureus-stimulated bovine mammary epithelial cells. Benef. Microbes 2018, 9, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Assis, B.S.; Germon, P.; Silva, A.M.; Even, S.; Nicoli, J.R.; Loir, Y.L. Lactococcus lactis V7 inhibits the cell invasion of bovine mammary epithelial cells by Escherichia coli and Staphylococcus aureus. Benef. Microbes 2015, 6, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, R.S.; Silva, L.C.; de Souza, M.R.; Reis, R.B.; Bicalho, A.F.; Nunes, J.P.; Dias, A.A.; Nicoli, J.R.; Neumann, E.; Nunes, Á.C. Prospecting of potentially probiotic lactic acid bacteria from bovine mammary ecosystem: Imminent partners from bacteriotherapy against bovine mastitis. Int. Microbiol. 2022, 25, 189–206. [Google Scholar] [CrossRef] [PubMed]
- Malvisi, M.; Stuknytė, M.; Magro, G.; Minozzi, G.; Giardini, A.; De Noni, I.; Piccinini, R. Antibacterial activity and immunomodulatory effects on a bovine mammary epithelial cell line exerted by nisin A-producing Lactococcus lactis strains. J. Dairy Sci. 2016, 99, 2288–2296. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, M.C.; Yang, J.; Wang, J.F.; Zhu, Y.H. Lactobacillus rhamnosus GR-1 Ameliorates Escherichia coli-Induced Inflammation and Cell Damage via Attenuation of ASC-Independent NLRP3 Inflammasome Activation. Appl. Environ. Microbiol. 2015, 82, 1173–1182. [Google Scholar] [CrossRef]
- Paape, M.; Mehrzad, J.; Zhao, X.; Detilleux, J.; Burvenich, C. Defense of the bovine mammary gland by polymorphonuclear neutrophil leukocytes. J. Mammary Gland Biol. Neoplasia 2002, 7, 109–121. [Google Scholar] [CrossRef]
- Burvenich, C.; Van Merris, V.; Mehrzad, J.; Diez-Fraile, A.; Duchateau, L. Severity of E. coli mastitis is mainly determined by cow factors. Vet. Res. 2003, 34, 521–564. [Google Scholar] [CrossRef]
- Mitterhuemer, S.; Petzl, W.; Krebs, S.; Mehne, D.; Klanner, A.; Wolf, E.; Zerbe, H.; Blum, H. Escherichia coli infection induces distinct local and systemic transcriptome responses in the mammary gland. BMC Genom. 2010, 11, 138. [Google Scholar] [CrossRef]
- Rose, M.T.; Aso, H.; Yonekura, S.; Komatsu, T.; Hagino, A.; Ozutsumi, K.; Obara, Y. In vitro differentiation of a cloned bovine mammary epithelial cell. J. Dairy Res. 2002, 69, 345–355. [Google Scholar] [CrossRef]
- Maldonado, N.C.; Ficoseco, C.A.; Mansilla, F.A.; Melian, C.; Hebert, E.M.; Vignolo, G.M.; Nader-Macias, M.E.F. Identification, characterization and selection of autochthonous lactic acid bacteria as probiotic for feedlot cattle. Livest. Sci. 2018, 212, 99–110. [Google Scholar] [CrossRef]
- Oviedo-Boyso, J.; Valdez-Alarcón, J.J.; Cajero-Juárez, M.; Ochoa-Zarzosa, A.; López-Meza, J.E.; Bravo-Patiño, A.B.; Baizabal-Aguirre, V.M. Innate immune response of bovine mammary gland to pathogenic bacteria responsible for mastitis. J. Infect. 2007, 54, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Oviedo-Boyso, J.; Valdez-Alarcón, J.J.; Cajero-Juárez, M.; Ochoa-Zarzosa, A.; López-Meza, J.E.; Bravo-Patiño, A.B.; Baizabal-Aguirre, V.M. The capacity of bovine endothelial cells to eliminate intracellular Staphylococcus aureus and Staphylococcus epidermidis is increased by the proinflammatory cytokines TNF-α and IL-1β. FEMS Immunol. Med. Microbiol. 2008, 54, 53–59. [Google Scholar] [CrossRef]
- Paulrud, C.O. Basic concepts of the bovine teat canal. Vet. Res. Commun. 2005, 29, 215–245. [Google Scholar] [CrossRef] [PubMed]
- Huxley, J.N.; Helps, C.R.; Bradley, A.J. Identification of Corynebacterium bovis by endonuclease restriction analysis of the 16S rRNA gene sequence. J. Dairy Sci. 2004, 87, 38–45. [Google Scholar] [CrossRef]
- Rault, L.; Lévêque, P.A.; Barbey, S.; Launay, F.; Larroque, H.; Le Loir, Y.; Germon, P.; Guinard-Flament, J.; Even, S. Bovine teat cistern microbiota composition and richness are associated with the immune and microbial responses during transition to once-daily milking. Front. Microbiol. 2020, 11, 602404. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.M.; Souza, M.T.; Blagitz, M.G.; Souza, F.N.; Batista, C.F.; Alves, A.J.; Fernandes, A.C.C.; Sanchez, E.M.R.; Ordinola-Ramirez, C.M.; da Costa, L.; et al. Milk lymphocyte profile and macrophage functions: New insights into the immunity of the mammary gland in quarters infected with Corynebacterium bovis. BMC Vet. Res. 2021, 17, 282. [Google Scholar] [CrossRef] [PubMed]
- Braem, S.; Abrahamse, E.L.; Duthoo, W.; Notebaert, W. What determines the specificity of conflict adaptation? A review, critical analysis, and proposed synthesis. Front. Psychol. 2014, 5, 1134. [Google Scholar] [CrossRef]
- Kanmani, P.; Clua, P.; Vizoso-Pinto, M.G.; Rodriguez, C.; Alvarez, S.; Melnikov, V.; Takahashi, H.; Kitazawa, H.; Villena, J. Respiratory commensal bacteria Corynebacterium pseudodiphtheriticum improves resistance of infant mice to respiratory syncytial virus and Streptococcus pneumoniae superinfection. Front. Microbiol. 2017, 8, 1613. [Google Scholar] [CrossRef]
- Dentice Maidana, S.; Ortiz Moyano, R.; Vargas, J.M.; Fukuyama, K.; Kurata, S.; Melnikov, V.; Jure, M.Á.; Kitazawa, H.; Villena, J. Respiratory commensal bacteria increase protection against hypermucoviscous carbapenem-resistant Klebsiella pneumoniae ST25 infection. Pathogens 2022, 11, 1063. [Google Scholar] [CrossRef]
- Ortiz Moyano, R.; Raya Tonetti, F.; Tomokiyo, M.; Kanmani, P.; Vizoso-Pinto, M.G.; Kim, H.; Quilodrán-Vega, S.; Melnikov, V.; Alvarez, S.; Takahashi, H.; et al. The ability of respiratory commensal bacteria to beneficially modulate the lung innate immune response is a strain dependent characteristic. Microorganisms 2020, 8, 727. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Huang, W.; Hou, Q.; Kwok, L.; Sun, Z.; Ma, H.; Zhao, F.; Lee, Y.K.; Zhang, H. The effects of probiotics administration on the milk production, milk components and fecal bacteria microbiota of dairy cows. Sci. Bull. 2017, 62, 767–774. [Google Scholar] [CrossRef]
- Luo, S.; Wang, Y.; Kang, X.; Liu, P.; Wang, G. Research progress on the association between mastitis and gastrointestinal microbes in dairy cows and the effect of probiotics. Microb. Pathog. 2022, 173, 105809. [Google Scholar] [CrossRef] [PubMed]
- Young, W.; Hine, B.C.; Wallace, O.A.; Callaghan, M.; Bibiloni, R. Transfer of intestinal bacterial components to mammary secretions in the cow. PeerJ 2015, 3, e888. [Google Scholar] [CrossRef]
- Ma, C.; Sun, Z.; Zeng, B.; Huang, S.; Zhao, J.; Zhang, Y.; Su, X.; Xu, J.; Wei, H.; Zhang, H. Cow-to-mouse fecal transplantations suggest intestinal microbiome as one cause of mastitis. Microbiome 2018, 6, 200. [Google Scholar] [CrossRef]
- Hoque, M.N.; Rahman, M.S.; Islam, T.; Sultana, M.; Crandall, K.A.; Hossain, M.A. Induction of mastitis by cow-to-mouse fecal and milk microbiota transplantation causes microbiome dysbiosis and genomic functional perturbation in mice. Anim. Microbiome 2022, 4, 43. [Google Scholar] [CrossRef]
- Villena, J.; Kitazawa, H. The Modulation of mucosal antiviral immunity by immunobiotics: Could they offer any benefit in the SARS-CoV-2 pandemic? Front. Physiol. 2020, 11, 699. [Google Scholar] [CrossRef]
- Villena, J.; Li, C.; Vizoso-Pinto, M.G.; Sacur, J.; Ren, L.; Kitazawa, H. Lactiplantibacillus plantarum as a potential adjuvant and delivery system for the development of SARS-CoV-2 oral vaccines. Microorganisms 2021, 9, 683. [Google Scholar] [CrossRef]
- Zelaya, H.; Alvarez, S.; Kitazawa, H.; Villena, J. Respiratory antiviral immunity and immunobiotics: Beneficial effects on inflammation-coagulation interaction during influenza virus infection. Front. Immunol. 2016, 7, 633. [Google Scholar] [CrossRef]
- Rodríguez, J.M.; Fernández, L.; Verhasselt, V. The gut-breast axis: Programming health for life. Nutrients 2021, 13, 606. [Google Scholar] [CrossRef]
- Ahlstedt, S.; Carlsson, B.; Hanson, L.A.; Goldblum, R.M. Antibody production by human colostral cells. I. Immunoglobulin class, specificity, and quantity. Scand. J. Immunol. 1975, 4, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Goldblum, R.M.; Ahlstedt, S.; Carlsson, B.; Hanson, L.A.; Jodal, U.; Lidin-Janson, G.; Sohl-Akerlund, A. Antibody-forming cells in human colostrum after oral immunisation. Nature 1975, 257, 97–98. [Google Scholar] [CrossRef] [PubMed]
- Azagra-Boronat, I.; Tres, A.; Massot-Cladera, M.; Franch, À.; Castell, M.; Guardiola, F.; Pérez-Cano, F.J.; Rodríguez-Lagunas, M.J. Lactobacillus fermentum CECT5716 supplementation in rats during pregnancy and lactation affects mammary milk composition. J. Dairy Sci. 2020, 103, 2982–2992. [Google Scholar] [CrossRef] [PubMed]
- De Moreno de LeBlanc, A.; Galdeano, C.M.; Chaves, S.; Perdigón, G. Oral administration of L. casei CRL 431 increases immunity in bronchus and mammary glands. Eur. J. Inflamm. 2005, 3, 23–28. [Google Scholar] [CrossRef]
- Utz, V.E.M.; Perdigón, G.; de Moreno de LeBlanc, A. Milk fermented by Lactobacillus casei CRL431 modifies cytokine profiles associated to different stages of breast cancer development in mice. Benef. Microbes 2019, 10, 689–697. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kober, A.K.M.H.; Saha, S.; Islam, M.A.; Rajoka, M.S.R.; Fukuyama, K.; Aso, H.; Villena, J.; Kitazawa, H. Immunomodulatory Effects of Probiotics: A Novel Preventive Approach for the Control of Bovine Mastitis. Microorganisms 2022, 10, 2255. https://doi.org/10.3390/microorganisms10112255
Kober AKMH, Saha S, Islam MA, Rajoka MSR, Fukuyama K, Aso H, Villena J, Kitazawa H. Immunomodulatory Effects of Probiotics: A Novel Preventive Approach for the Control of Bovine Mastitis. Microorganisms. 2022; 10(11):2255. https://doi.org/10.3390/microorganisms10112255
Chicago/Turabian StyleKober, A. K. M. Humayun, Sudeb Saha, Md. Aminul Islam, Muhammad Shahid Riaz Rajoka, Kohtaro Fukuyama, Hisashi Aso, Julio Villena, and Haruki Kitazawa. 2022. "Immunomodulatory Effects of Probiotics: A Novel Preventive Approach for the Control of Bovine Mastitis" Microorganisms 10, no. 11: 2255. https://doi.org/10.3390/microorganisms10112255
APA StyleKober, A. K. M. H., Saha, S., Islam, M. A., Rajoka, M. S. R., Fukuyama, K., Aso, H., Villena, J., & Kitazawa, H. (2022). Immunomodulatory Effects of Probiotics: A Novel Preventive Approach for the Control of Bovine Mastitis. Microorganisms, 10(11), 2255. https://doi.org/10.3390/microorganisms10112255












