Antimicrobial and Antivirulence Activities of Carvacrol against Pathogenic Aeromonas hydrophila
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Agents and Bacterial Strain
2.2. Drug Sensitivity Tests
2.3. Synergistic Effect Assay
2.4. Biofilm Production Assay
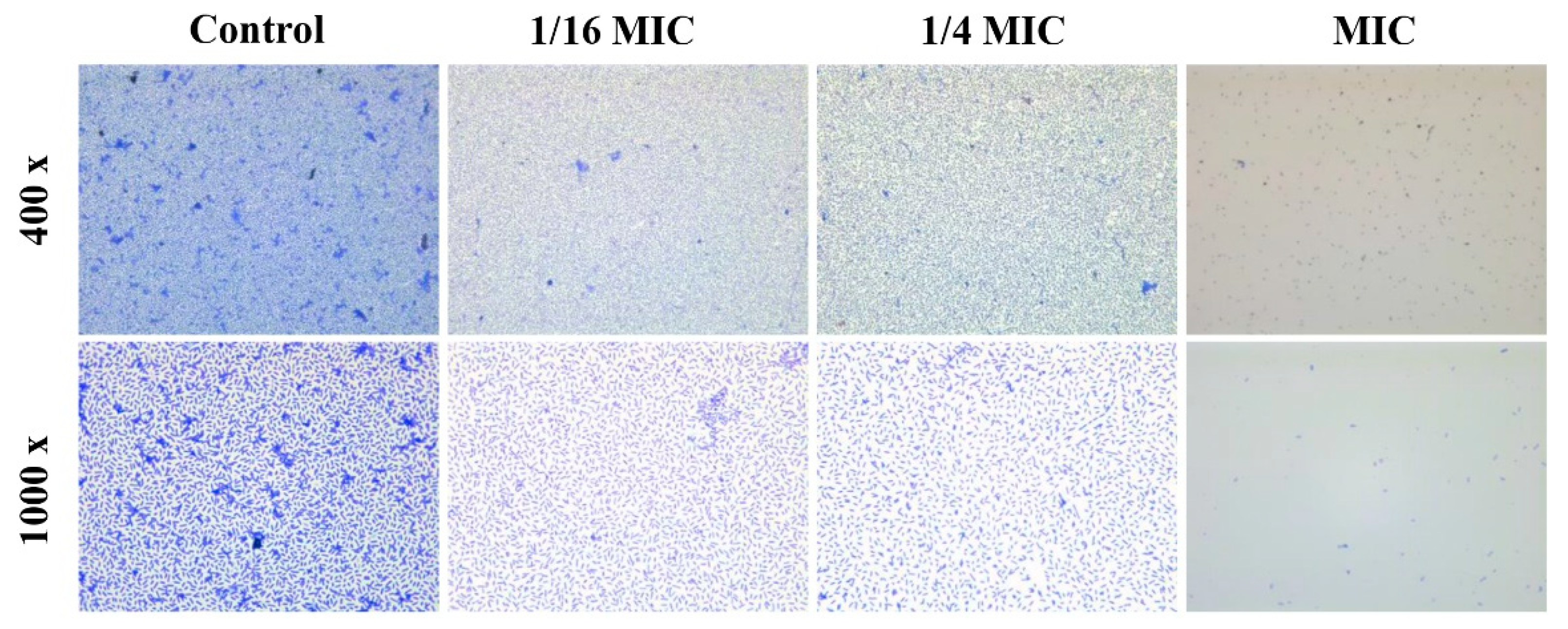
2.5. Microscopic Analysis of Biofilm Formation
2.6. Biofilm Eradication Assay
2.7. Exopolysaccharide (EPS) Production
2.8. Protease and Hemolysis Activity Assays
2.9. Quantitative Real-Time PCR
2.10. Challenge Test
2.11. Statistical Analysis
3. Results
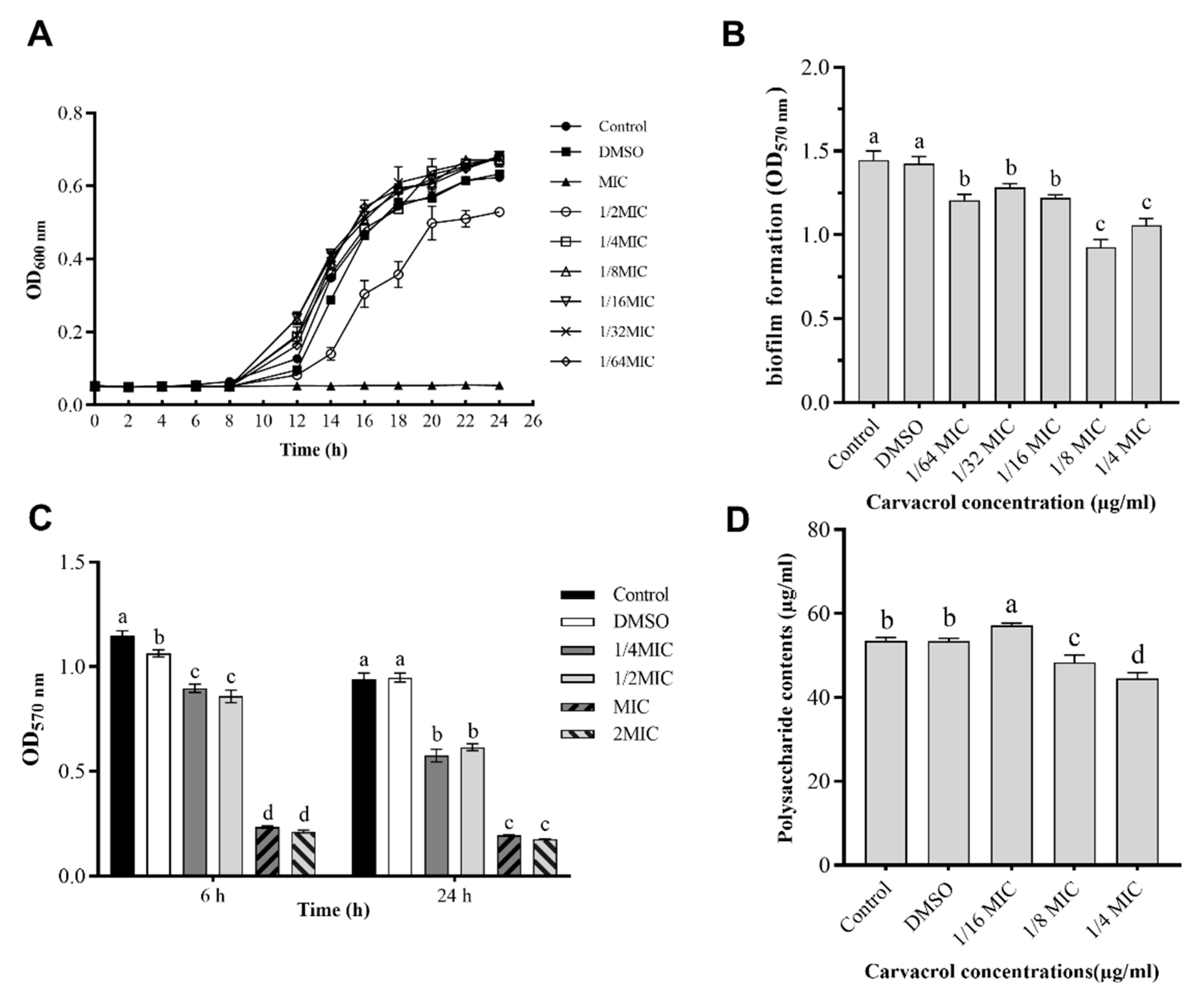
3.1. Inhibitory Effect of Carvacrol on A. hydrophila NJ-35
3.2. Synergistic Effect of Carvacrol Combined with Antibiotics
3.3. Antibiofilm Activity of Carvacrol
3.4. Quantification of EPS Production
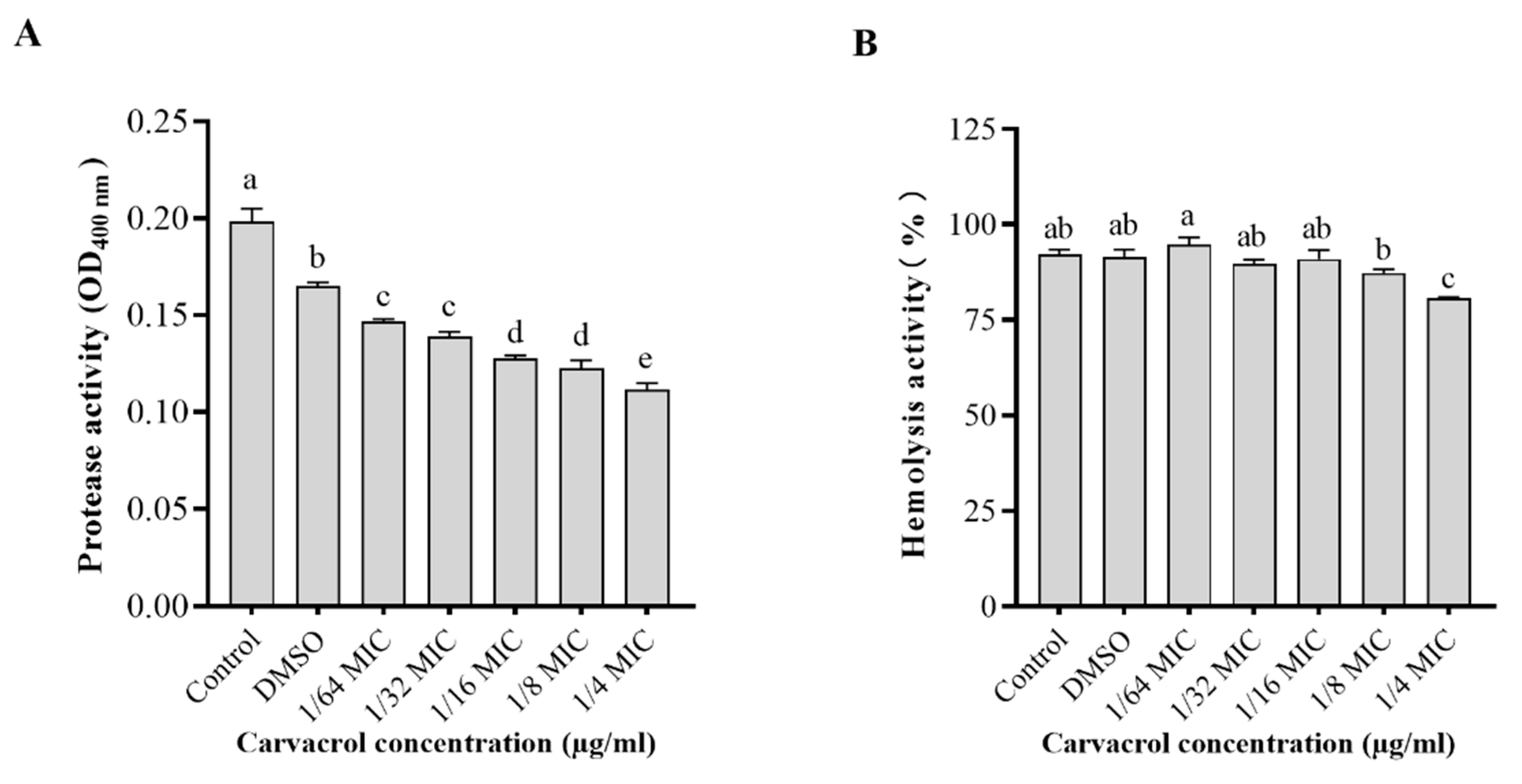
3.5. Effect of Carvacrol on Protease and Hemolytic Activities
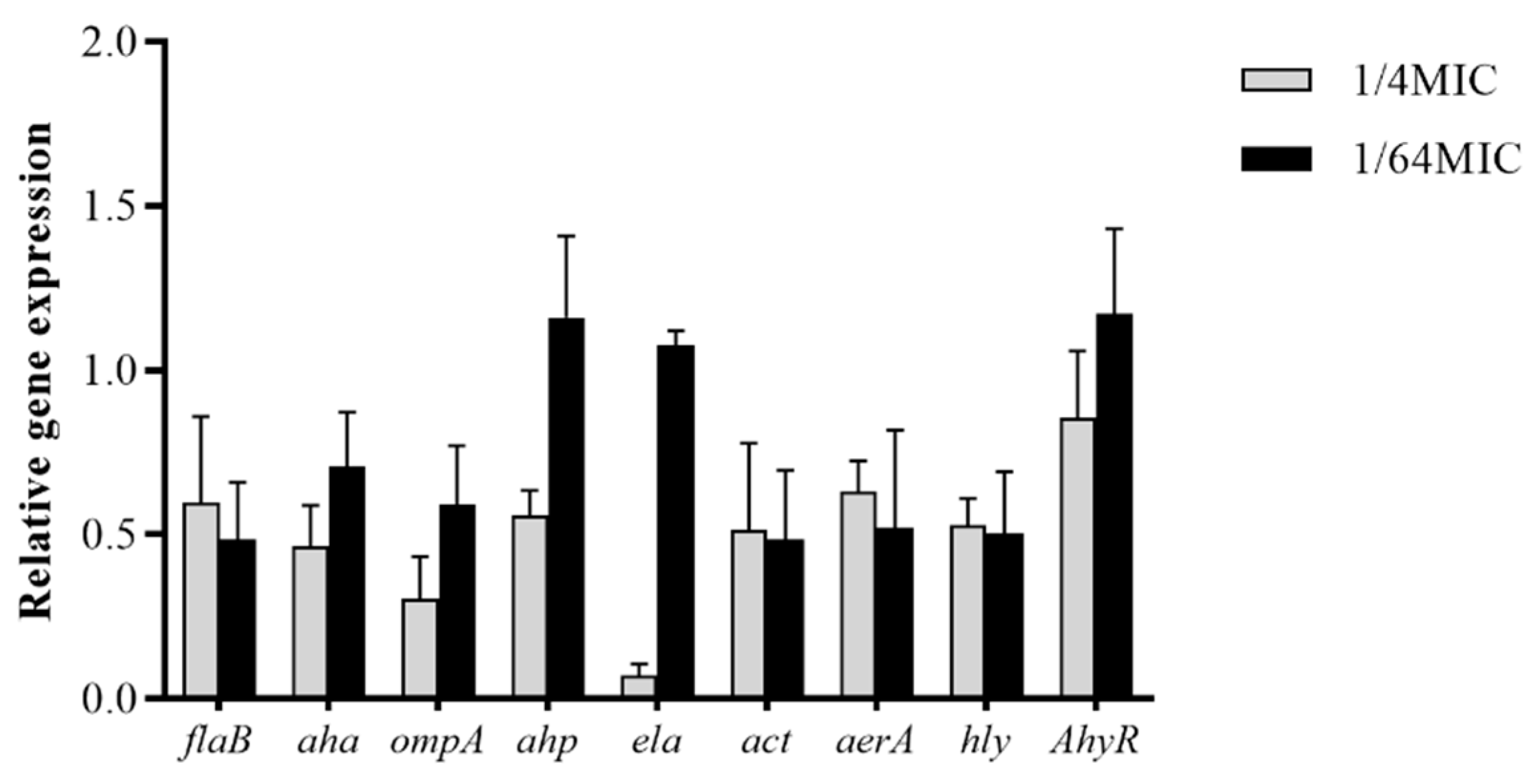
3.6. Modulation of A. hydrophila Virulence Gene Expression by Carvacrol
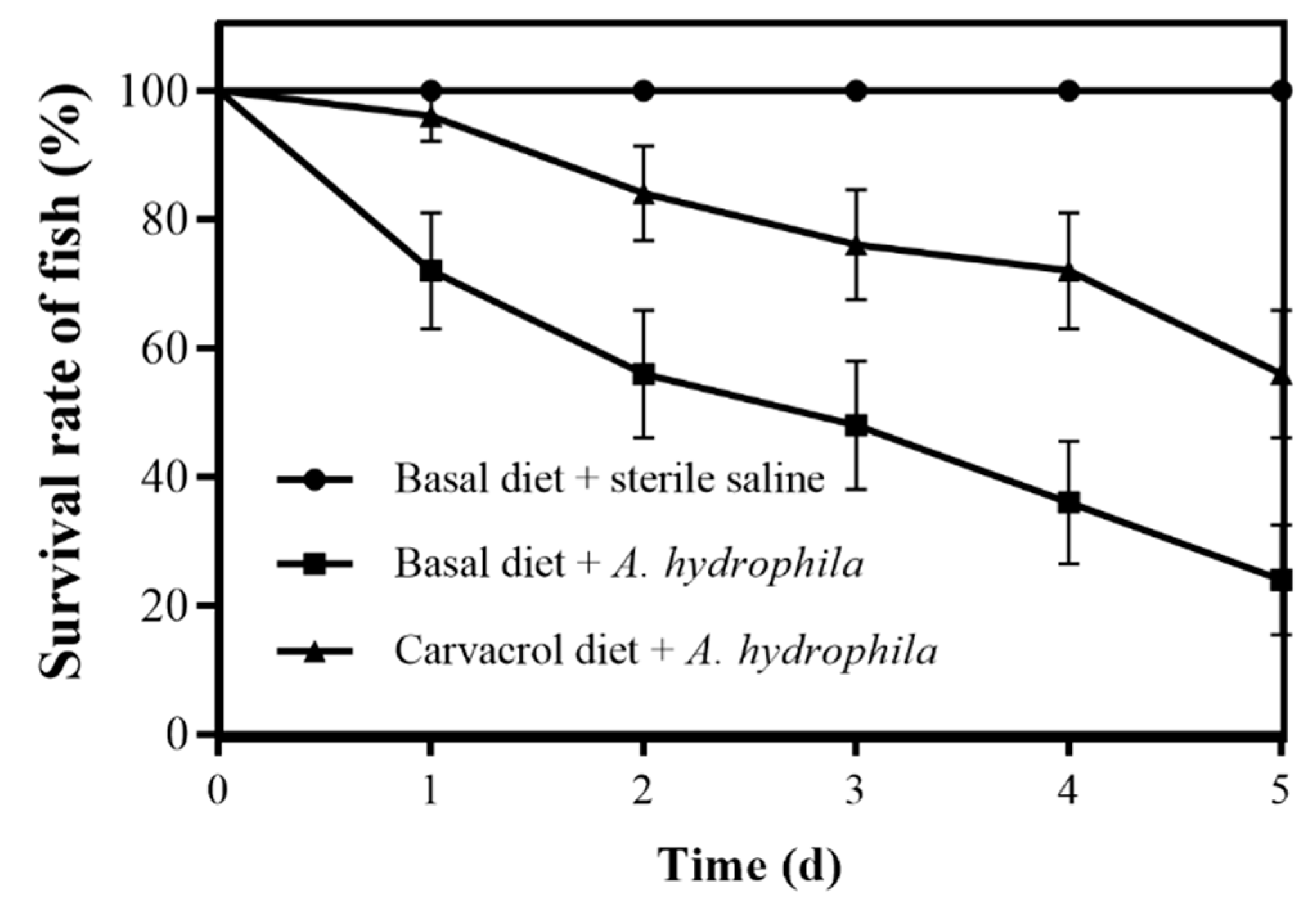
3.7. Protective Effects of Carvacrol on Grass Carp against A. hydrophila Infection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dhanapala, P.M.; Kalupahana, R.S.; Kalupahana, A.W.; Wijesekera, D.P.H.; Kottawatta, S.A.; Jayasekera, N.K.; Silva-Fletcher, A.; Jagoda, S.D.S. Characterization and antimicrobial resistance of environmental and clinical Aeromonas species isolated from fresh water ornamental fish and associated farming environment in sri lanka. Microorganisms 2021, 9, 2106. [Google Scholar] [CrossRef] [PubMed]
- Bardhan, A.; Abraham, T.J. antibiotic-resistance in motile Aeromonas spp. of indian major carps sold in retail markets of peri-urban kolkata, India. J. Aquat. Food Prod. Technol. 2021, 30, 786–793. [Google Scholar] [CrossRef]
- Hotinger, J.A.; Morris, S.T.; May, A.E. The case against antibiotics and for anti-virulence therapeutics. Microorganisms 2021, 9, 2049. [Google Scholar] [CrossRef]
- Topa, S.H.; Palombo, E.A.; Kingshott, P.; Blackall, L.L. Activity of cinnamaldehyde on quorum sensing and biofilm susceptibility to antibiotics in Pseudomonas aeruginosa. Microorganisms 2020, 8, 455. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, R.; Coppo, E.; Marchese, A.; Daglia, M.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Nabavi, S.M. Phytochemicals for human disease: An update on plant-derived compounds antibacterial activity. Microbiol. Res. 2017, 196, 44–68. [Google Scholar] [CrossRef] [PubMed]
- Suntres, Z.E.; Coccimiglio, J.; Alipour, M. The bioactivity and toxicological actions of carvacrol. Crit. Rev. Food Sci. Nutr. 2015, 55, 304–318. [Google Scholar] [CrossRef]
- Liu, F.; Jin, P.; Sun, Z.; Du, L.; Wang, D.; Zhao, T.; Doyle, M.P. Carvacrol oil inhibits biofilm formation and exopolysaccharide production of Enterobacter cloacae. Food Control 2021, 119, 107473. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, F.; Ji, B.-P.; Pei, R.-S.; Xu, N. The antibacterial mechanism of carvacrol and thymol against Escherichia coli. Lett. Appl. Microbiol. 2008, 47, 174–179. [Google Scholar] [CrossRef]
- Tapia-Rodriguez, M.R.; Hernandez-Mendoza, A.; Gonzalez-Aguilar, G.A.; Martinez-Tellez, M.A.; Martins, C.M.; Ayala-Zavala, J.F. Carvacrol as potential quorum sensing inhibitor of Pseudomonas aeruginosa and biofilm production on stainless steel surfaces. Food Control 2017, 75, 255–261. [Google Scholar] [CrossRef]
- Das, S.; Chourashi, R.; Mukherjee, P.; Kundu, S.; Koley, H.; Dutta, M.; Mukhopadhyay, A.K.; Okamoto, K.; Chatterjee, N.S. Inhibition of growth and virulence of Vibrio cholerae by carvacrol, an essential oil component of Origanum spp. J. Appl. Microbiol. 2021, 131, 1147–1161. [Google Scholar] [CrossRef]
- Hao, Y.; Li, J.; Shi, L. A carvacrol-rich essential oil extracted from oregano (Origanum vulgare “Hot & Spicy”) exerts potent antibacterial effects against Staphylococcus aureus. Front. Microbiol. 2021, 12, 741861. [Google Scholar] [CrossRef] [PubMed]
- Burt, S.A.; Ojo-Fakunle, V.T.A.; Woertman, J.; Veldhuizen, E.J.A. The natural antimicrobial carvacrol inhibits quorum sensing in Chromobacterium violaceum and reduces bacterial biofilm formation at sub-lethal concentrations. PLoS ONE 2014, 9, e93414. [Google Scholar] [CrossRef] [PubMed]
- Mooyottu, S.; Kollanoor-Johny, A.; Flock, G.; Bouillaut, L.; Upadhyay, A.; Sonenshein, A.; Venkitanarayanan, K. Carvacrol and Trans.-cinnamaldehyde reduce Clostridium difficile toxin production and cytotoxicity in vitro. IJMS 2014, 15, 4415–4430. [Google Scholar] [CrossRef]
- Milos, M.; Makota, D. Investigation of antioxidant synergisms and antagonisms among thymol, carvacrol, thymoquinone and p-cymene in a model system using the briggs–rauscher oscillating reaction. Food Chem. 2012, 131, 296–299. [Google Scholar] [CrossRef]
- Sánchez, C.; Aznar, R.; Sánchez, G. The effect of carvacrol on enteric viruses. Int. J. Food Microbiol. 2015, 192, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Jiang, S.; Yang, Y.; Fan, L.; Su, F.; Ye, M. Synthesis and antifungal activity of carvacrol and thymol esters with heteroaromatic carboxylic acids. Nat. Prod. Res. 2019, 33, 1924–1930. [Google Scholar] [CrossRef]
- Cicalău, G.; Babes, P.; Calniceanu, H.; Popa, A.; Ciavoi, G.; Iova, G.; Ganea, M.; Scrobotă, I. Anti-inflammatory and antioxidant properties of carvacrol and magnolol, in periodontal disease and diabetes mellitus. Molecules 2021, 26, 6899. [Google Scholar] [CrossRef]
- Fonseca, L.M.; Radünz, M.; Crizel, R.L.; Camargo, T.M.; Gandra, E.A.; Dias, A.R.G. Effect of carvacrol encapsulation in starch-based nanofibers: Thermal resistance and antioxidant and antimicrobial properties. J. Food Process. Preserv. 2021, 45, e15409. [Google Scholar] [CrossRef]
- Pang, M.; Jiang, J.; Xie, X.; Wu, Y.; Dong, Y.; Kwok, A.H.Y.; Zhang, W.; Yao, H.; Lu, C.; Leung, F.C.; et al. Novel insights into the pathogenicity of epidemic Aeromonas hydrophila ST251 clones from comparative genomics. Sci. Rep. 2015, 5, 9833. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Prakash, A.; Meletiadis, J.; Sharma, C.; Chowdhary, A. Comparison of EUCAST and CLSI reference microdilution MICs of eight antifungal compounds for Candida auris and associated tentative epidemiological cutoff values. Antimicrob. Agents Chemother. 2017, 61, e00485-17. [Google Scholar] [CrossRef]
- Yin, L.; Chen, J.; Wang, K.; Geng, Y.; Lai, W.; Huang, X.; Chen, D.; Guo, H.; Fang, J.; Chen, Z.; et al. Study the antibacterial mechanism of cinnamaldehyde against drug-resistant Aeromonas hydrophila in vitro. Microb. Pathog. 2020, 145, 104208. [Google Scholar] [CrossRef] [PubMed]
- Palaniappan, K.; Holley, R.A. Use of natural antimicrobials to increase antibiotic susceptibility of drug resistant bacteria. Int. J. Food Microbiol. 2010, 140, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Moody, J. Synergism testing: Broth microdilution checkerboard and broth macrodilution method. In Clinical Microbiology Procedures Handbook; American Society for Microbiology: Washington, DC, USA, 2004; pp. 1–28. [Google Scholar]
- Kot, B.; Kwiatek, K.; Janiuk, J.; Witeska, M.; Pękala-Safińska, A. Antibacterial activity of commercial phytochemicals against Aeromonas species isolated from fish. Pathogens 2019, 8, 142. [Google Scholar] [CrossRef]
- Felix e Silva, A.; Pires, I.C.; Costa, M.M.; Melo, J.F.B.; Lorenzo, V.P.; Melo, F.V.S.T.; Copatti, C.E. Antibacterial and antibiofilm activities and synergism with florfe.enicol from the essential oils of Lippia sidoides and Cymbopogon citratus against Aeromonas hydrophila. J. Appl. Microbiol. 2022, 132, 1802–1812. [Google Scholar] [CrossRef] [PubMed]
- Magi, G.; Marini, E.; Facinelli, B. Antimicrobial activity of essential oils and carvacrol, and synergy of carvacrol and erythromycin, against clinical, erythromycin-resistant group A streptococci. Front. Microbiol. 2015, 6, 165. [Google Scholar] [CrossRef]
- Bazargani, M.M.; Rohloff, J. Antibiofilm activity of essential oils and plant extracts against Staphylococcus aureus and Escherichia coli biofilms. Food Control 2016, 61, 156–164. [Google Scholar] [CrossRef]
- Alexpandi, R.; Abirami, G.; Satish, L.; Swasthikka, R.P.; Krishnaveni, N.; Jayakumar, R.; Pandian, S.K.; Veera Ravi, A. Tocopherol and phytol possess anti-quorum sensing mediated anti-infective behavior against Vibrio campbellii in aquaculture: An in vitro and in vivo study. Microb. Pathog. 2021, 161, 105221. [Google Scholar] [CrossRef]
- Dong, J.; Zhang, D.; Li, J.; Liu, Y.; Zhou, S.; Yang, Y.; Xu, N.; Yang, Q.; Ai, X. Genistein inhibits the pathogenesis of Aeromonas hydrophila by disrupting quorum sensing mediated biofilm formation and aerolysin production. Front. Pharmacol. 2021, 12, 753581. [Google Scholar] [CrossRef]
- Vollaro, A.; Esposito, A.; Esposito, E.P.; Zarrilli, R.; Guaragna, A.; De Gregorio, E. PYED-1 inhibits biofilm formation and disrupts the preformed biofilm of Staphylococcus aureus. Antibiotics 2020, 9, 240. [Google Scholar] [CrossRef]
- Gao, J.; Xi, B.; Chen, K.; Song, R.; Qin, T.; Xie, J.; Pan, L. The stress hormone norepinephrine increases the growth and virulence of Aeromonas hydrophila. MicrobiologyOpen 2019, 8, e00664. [Google Scholar] [CrossRef]
- Luo, G.; Huang, L.; Su, Y.; Qin, Y.; Xu, X.; Zhao, L.; Yan, Q. Flra, flrb and flrc regulate adhesion by controlling the expression of critical virulence genes in Vibrio alginolyticus. Emerg. Microbes Infect. 2016, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bandeira Junior, G.; Sutili, F.J.; Gressler, L.T.; Ely, V.L.; Silveira, B.P.; Tasca, C.; Reghelin, M.; Matter, L.B.; Vargas, A.P.C.; Baldisserotto, B. Antibacterial potential of phytochemicals alone or in combination with antimicrobials against fish pathogenic bacteria. J. Appl. Microbiol. 2018, 125, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Antibacterial plant compounds, extracts and essential oils: An updated review on their effects and putative mechanisms of action. Phytomedicine 2021, 90, 153626. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Wang, Y.; Chen, S.; Zhu, J. Efficacy of ten structurally related essential oil components on anti-biofilm and anti-quorum sensing against fish spoilers Pseudomonas and Aeromonas. J. Aquat. Food Prod. Technol. 2021, 30, 462–473. [Google Scholar] [CrossRef]
- Kachur, K.; Suntres, Z. The antibacterial properties of phenolic isomers, carvacrol and thymol. Crit. Rev. Food Sci. Nutr. 2020, 60, 3042–3053. [Google Scholar] [CrossRef]
- Ahmad, A.; Elisha, I.L.; van Vuuren, S.; Viljoen, A. Volatile phenolics: A comprehensive review of the anti-infective properties of an important class of essential oil constituents. Phytochemistry 2021, 190, 112864. [Google Scholar] [CrossRef]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial activity of terpenes and terpenoids present in essential oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef]
- Cristani, M.; D’Arrigo, M.; Mandalari, G.; Castelli, F.; Sarpietro, M.G.; Micieli, D.; Venuti, V.; Bisignano, G.; Saija, A.; Trombetta, D. Interaction of four monoterpenes contained in essential oils with model membranes: Implications for their antibacterial activity. J. Agric. Food Chem. 2007, 55, 6300–6308. [Google Scholar] [CrossRef]
- Dong, J.; Zhang, L.; Liu, Y.; Xu, N.; Zhou, S.; Yang, Q.; Yang, Y.; Ai, X. Thymol protects channel catfish from Aeromonas hydrophila infection by inhibiting aerolysin expression and biofilm formation. Microorganisms 2020, 8, 636. [Google Scholar] [CrossRef]
- Arnaouteli, S.; Bamford, N.C.; Stanley-Wall, N.R.; Kovács, Á.T. Bacillus subtilis biofilm formation and social interactions. Nat. Rev. Microbiol. 2021, 19, 600–614. [Google Scholar] [CrossRef]
- Wu, H.; Moser, C.; Wang, H.-Z.; Høiby, N.; Song, Z.-J. Strategies for combating bacterial biofilm infections. Int. J. Oral Sci. 2015, 7, 1–7. [Google Scholar] [CrossRef]
- Sharahi, J.Y.; Azimi, T.; Shariati, A.; Safari, H.; Tehrani, M.K.; Hashemi, A. Advanced strategies for combating bacterial biofilms. J. Cell. Physiol. 2019, 234, 14689–14708. [Google Scholar] [CrossRef]
- Gutierrez-Pacheco, M.M.; Gonzalez-Aguilar, G.A.; Martinez-Tellez, M.A.; Lizardi-Mendoza, J.; Madera-Santana, T.J.; Bernal-Mercado, A.T.; Vazquez-Armenta, F.J.; Ayala-Zavala, J.F. Carvacrol inhibits biofilm formation and production of extracellular polymeric substances of Pectobacterium carotovorum subsp. Carotovorum. Food Control 2018, 89, 210–218. [Google Scholar] [CrossRef]
- Selvaraj, A.; Valliammai, A.; Muthuramalingam, P.; Priya, A.; Suba, M.; Ramesh, M.; Karutha Pandian, S. Carvacrol targets sara and crtm of methicillin-resistant Staphylococcus aureus to mitigate biofilm formation and staphyloxanthin synthesis: An in vitro and in vivo approach. ACS Omega 2020, 5, 31100–31114. [Google Scholar] [CrossRef]
- Qin, Y.X.; Yan, Q.P.; Mao, X.X.; Chen, Z.; Su, Y.Q. Role of MshQ in MSHA pili biosynthesis and biofilm formation of Aeromonas hydrophila. Genet. Mol. Res. 2014, 13, 8982–8996. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Li, Z.; He, L.; He, L.; Chen, F.; Song, S.; Zhang, H.; Sun, W.; Bao, X.; Zhang, H.; Li, T.; et al. Anti-bacterial mechanism of the P-3 against Aeromonas hydrophila NJ-35 extracted from the Potentilla chinensis ser. (Wei Ling Cai). Aquaculture 2021, 541, 736746. [Google Scholar] [CrossRef]
- Defoirdt, T. Virulence mechanisms of bacterial aquaculture pathogens and antivirulence therapy for aquaculture. Rev. Aquac. 2014, 6, 100–114. [Google Scholar] [CrossRef]
- Maiti, B.; Shetty, M.; Shekar, M.; Karunasagar, I.; Karunasagar, I. Evaluation of two outer membrane proteins, Aha1 and OmpW of Aeromonas hydrophila as vaccine candidate for common carp. Vet. Immunol. Immunopathol. 2012, 149, 298–301. [Google Scholar] [CrossRef]
- Ieva, R. Interfering with outer membrane biogenesis to fight gram-negative bacterial pathogens. Virulence 2017, 8, 1049–1052. [Google Scholar] [CrossRef][Green Version]
- Esteve, C.; Birbeck, T.H. Secretion of haemolysins and proteases by Aeromonas hydrophila EO63: Separation and characterization of the serine protease (caseinase) and the metalloprotease (elastase). J. Appl. Microbiol. 2004, 96, 994–1001. [Google Scholar] [CrossRef]
- Cascón, A.; Fregeneda, J.; Aller, M.; Yugueros, J.; Temprano, A.; Hernanz, C.; Sánchez, M.; Rodríguez-Aparicio, L.; Naharro, G. Cloning, characterization, and insertional inactivation of a major extracellular serine protease gene with elastolytic activity from Aeromonas hydrophila. J. Fish. Dis. 2000, 23, 49–59. [Google Scholar] [CrossRef]
- LaSarre, B.; Federle, M.J. Exploiting quorum sensing to confuse bacterial pathogens. Microbiol. Mol. Biol. Rev. 2013, 77, 73–111. [Google Scholar] [CrossRef]
- Kirke, D.F.; Swift, S.; Lynch, M.J.; Williams, P. The Aeromonas hydrophila LuxR homologue AhyR regulates the N -Acyl homoserine lactone synthase, AhyI positively and negatively in a growth phase-dependent manner. FEMS Microbiol. Lett. 2004, 241, 109–117. [Google Scholar] [CrossRef]
- Silva, J.M.D.; Paz, A.D.L.; Val, A.L. Effect of carvacrol on the haemato-immunological parameters, growth and resistance of Colossoma macropomum (characiformes: Serrasalmidae) infected by Aeromonas hydrophila. Aquac. Res. 2021, 52, 3291–3300. [Google Scholar] [CrossRef]
- Zheng, Z.L.; Tan, J.Y.W.; Liu, H.Y.; Zhou, X.H.; Xiang, X.; Wang, K.Y. Evaluation of oregano essential oil (Origanum heracleoticum L.) on growth, antioxidant effect and resistance against Aeromonas hydrophila in channel catfish (Ictalurus punctatus). Aquaculture 2009, 292, 214–218. [Google Scholar] [CrossRef]
- Liu, W.; Guo, X.; Chen, Y.; Tang, Y.; Xiao, H.; Li, S. Carvacrol promotes intestinal health in pengze crucian carp, enhancing resistance to Aeromonas hydrophila. Aquac. Rep. 2020, 17, 100325. [Google Scholar] [CrossRef]
- Aguiar, F.C.; Solarte, A.L.; Tarradas, C.; Gómez-Gascón, L.; Astorga, R.; Maldonado, A.; Huerta, B. Combined effect of conventional antimicrobials with essential oils and their main components against resistant Streptococcus suis Strains. Lett. Appl. Microbiol. 2019, 68, 562–572. [Google Scholar] [CrossRef]
- da Silva, E.G.; Bandeira Junior, G.; Cargnelutti, J.F.; Santos, R.C.V.; Gündel, A.; Baldisserotto, B. In vitro antimicrobial and antibiofilm activity of S-(-)-limonene and R-(+)-limonene against fish bacteria. Fishes 2021, 6, 32. [Google Scholar] [CrossRef]
- Pirog, T.P.; Kliuchka, I.V.; Kliuchka, L.V. Synergistic action of essential oils with the biocides on microorganisms. Biotechnol. Acta 2019, 12, 5–18. [Google Scholar] [CrossRef][Green Version]
- Bandeira Junior, G.; Souza, C.D.F.; Baldissera, M.D.; Descovi, S.N.; Silveira, B.P.D.; Tasca, C.; Mourão, R.H.V.; Vargas, A.P.C.D.; Baldisserotto, B. Plant essential oils against bacteria isolated from fish: An in vitro screening and in vivo efficacy of Lippia origanoides. Cienc. Rural 2019, 49, e20190064. [Google Scholar] [CrossRef]
- Ren, Y.; Li, S.; Wu, Z.; Zhou, C.; Zhang, D.; Chen, X. The influences of Bacillus subtilis on the virulence of Aeromonas hydrophila and expression of LuxS gene of both bacteria under co-cultivation. Curr. Microbiol. 2017, 74, 718–724. [Google Scholar] [CrossRef]
- Sun, B.; Luo, H.; Jiang, H.; Wang, Z.; Jia, A. Inhibition of quorum sensing and biofilm formation of esculetin on Aeromonas hydrophila. Front. Microbiol. 2021, 12, 737626. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Qin, T.; Chen, K.; Pan, L.; Xie, J.; Xi, B. Antimicrobial and Antivirulence Activities of Carvacrol against Pathogenic Aeromonas hydrophila. Microorganisms 2022, 10, 2170. https://doi.org/10.3390/microorganisms10112170
Wang J, Qin T, Chen K, Pan L, Xie J, Xi B. Antimicrobial and Antivirulence Activities of Carvacrol against Pathogenic Aeromonas hydrophila. Microorganisms. 2022; 10(11):2170. https://doi.org/10.3390/microorganisms10112170
Chicago/Turabian StyleWang, Junwei, Ting Qin, Kai Chen, Liangkun Pan, Jun Xie, and Bingwen Xi. 2022. "Antimicrobial and Antivirulence Activities of Carvacrol against Pathogenic Aeromonas hydrophila" Microorganisms 10, no. 11: 2170. https://doi.org/10.3390/microorganisms10112170
APA StyleWang, J., Qin, T., Chen, K., Pan, L., Xie, J., & Xi, B. (2022). Antimicrobial and Antivirulence Activities of Carvacrol against Pathogenic Aeromonas hydrophila. Microorganisms, 10(11), 2170. https://doi.org/10.3390/microorganisms10112170




