The First Report of Coxiella burnetii as a Potential Neglected Pathogen of Acute Hepatitis of Unknown Causes in Egypt
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
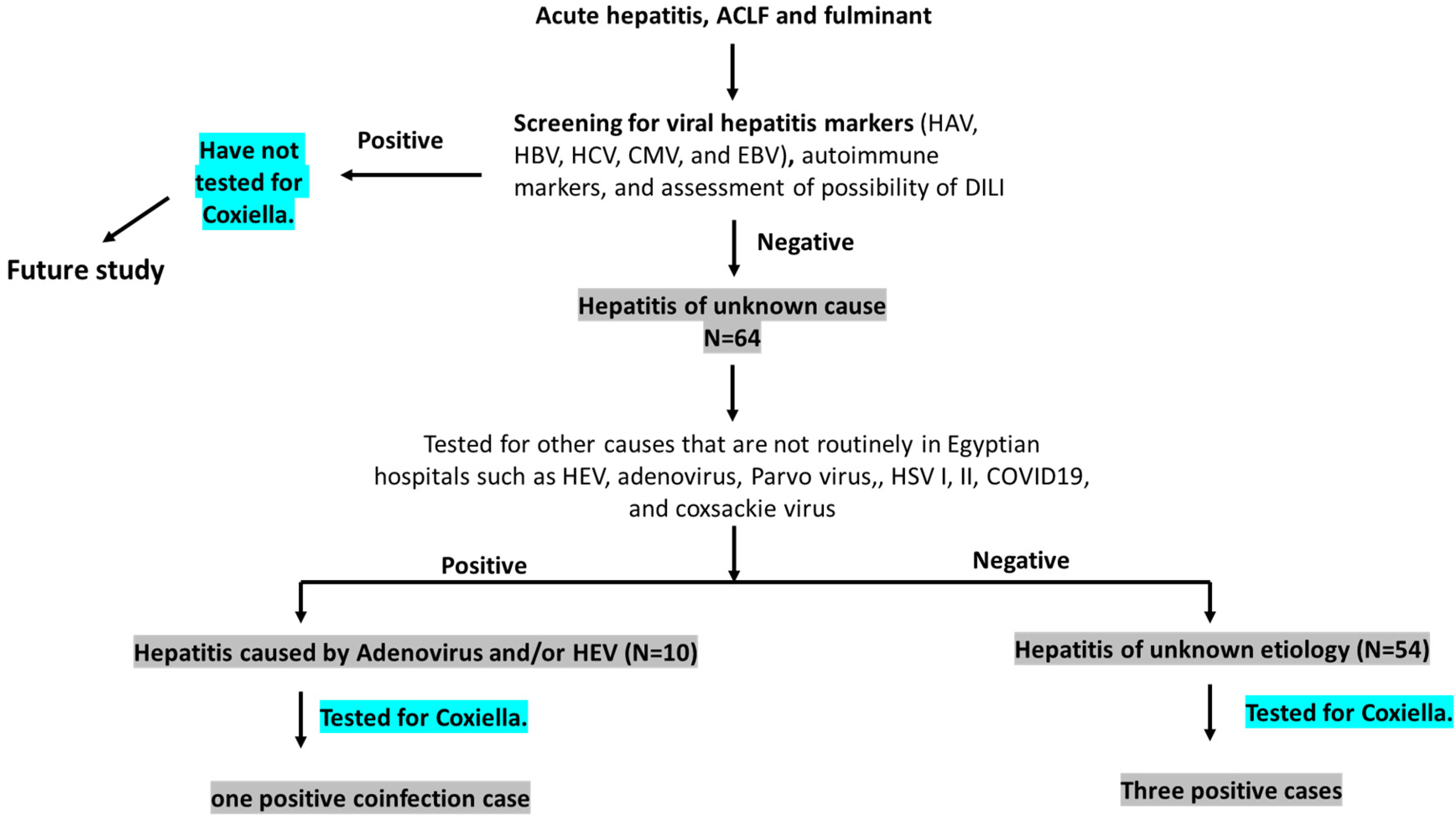
2.2. Definition of the Cases (Figure 1)
- (a)
- Hepatitis of unknown cause (HUC): Any acute hepatitis sample processed by Assiut University Hospital protocol and tested negative for viral hepatitis markers (HAV, HBV, HCV, CMV, and EBV), autoimmune makers, and DILI, which are routinely performed in the hospital.
- (b)
- Acute hepatitis of unknown etiology (AHUE): HUC samples that tested negative for other viral pathogens including adenovirus, COVID-19, Parvovirus, HSV, coxsackie virus, and HEV, which are not routinely performed in the hospital.
- (c)
- Hepatitis caused by less common viral pathogens: HUC samples that tested positive for one or more of the following viral pathogens: Adenovirus, COVID-19, Parvovirus, HSV, coxsackie virus, and HEV.
2.3. Screening of Less Common Viral Pathogens
2.4. Molecular Assessment of C. burnetii
2.5. Statistics
3. Results
3.1. Molecular Prevalence of Coxiella burnetii Infection in Acute Hepatitis of Unknown (AHUE) Patients
3.2. Clinical Characteristics of Acute Q-Fever Hepatitis among AHUE Patients
3.3. Molecular Prevalence and Clinical Characteristics of Coxiella burnetii Infection in Acute Hepatitis Caused by Less Common Viral Pathogens
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Multi-Country–Acute, Severe Hepatitis of Unknown Origin in Children. Disease Outbreak News. 12 July 2022. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON400 (accessed on 1 October 2022).
- World Health Organization. Acute Hepatitis of Unknown Aetiology in Children-Multi-Country. Disease Outbreak News. 27 May 2022. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON389 (accessed on 1 October 2022).
- Sayed, I.M.; El-Mokhtar, M.A.; Mahmoud, M.A.R.; Elkhawaga, A.A.; Gaber, S.; Seddek, N.H.; Abdel-Wahid, L.; Ashmawy, A.M.; Alkareemy, E.A.R. Clinical Outcomes and Prevalence of Hepatitis E Virus (HEV) among Non-A-C Hepatitis Patients in Egypt. Infect. Drug Resist. 2021, 14, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Eldin, C.; Mélenotte, C.; Mediannikov, O.; Ghigo, E.; Million, M.; Edouard, S.; Mege, J.-L.; Maurin, M.; Raoult, D. From Q Fever to Coxiella burnetii Infection: A Paradigm Change. Clin. Microbiol. Rev. 2017, 30, 115–190. [Google Scholar] [CrossRef] [PubMed]
- Devaux, C.A.; Osman, I.O.; Million, M.; Raoult, D. Coxiella burnetii in Dromedary Camels (Camelus dromedarius): A Possible Threat for Humans and Livestock in North Africa and the Near and Middle East? Front. Vet. Sci. 2020, 7, 558481. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, G.; Colitti, B.; Pagnini, U.; D’Angelo, D.; Iovane, G.; Rosati, S.; Montagnaro, S. Serological Evidence of Q Fever among Dairy Cattle and Buffalo Populations in the Campania Region, Italy. Pathogens 2022, 11, 901. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.; Lorber, B. Diseases Transmitted by Man’s Best Friend: The Dog. Microbiol. Spectr. 2015, 3. [Google Scholar] [CrossRef]
- Horton, K.C.; Wasfy, M.; Samaha, H.; Abdel-Rahman, B.; Safwat, S.; Fadeel, M.A.; Mohareb, E.; Dueger, E. Serosurvey for Zoonotic Viral and Bacterial Pathogens among Slaughtered Livestock in Egypt. Vector-Borne Zoonotic Dis. 2014, 14, 633–639. [Google Scholar] [CrossRef]
- Abbass, H.; Selim, S.A.K.; Sobhy, M.M.; El-Mokhtar, M.A.; ElHariri, M.; Abd-Elhafeez, H.H. High prevalence of Coxiella burnetii infection in humans and livestock in Assiut, Egypt: A serological and molecular survey. Vet. World 2020, 13, 2578–2586. [Google Scholar] [CrossRef]
- Mathews, K.O.; Savage, C.; Norris, J.M.; Phalen, D.; Malikides, N.; Sheehy, P.A.; Bosward, K.L. Risk factors associated with self-reported Q fever in Australian wildlife rehabilitators: Findings from an online survey. Zoonoses Public Health 2022. [Google Scholar] [CrossRef]
- Beaudeau, F.; Pouquet, M.; Guatteo, R.; Bareille, N.; Moret, L. Risk of seropositivity to Coxiella burnetii in humans living in areas with endemically infected cattle: No way for specific prevention. Zoonoses Public Health 2021, 68, 144–152. [Google Scholar] [CrossRef]
- Anastácio, S.; Carolino, N.; Sidi-Boumedine, K.; da Silva, G.J. Q Fever Dairy Herd Status Determination Based on Serological and Molecular Analysis of Bulk Tank Milk. Transbound. Emerg. Dis. 2014, 63, e293–e300. [Google Scholar] [CrossRef]
- Szymańska-Czerwińska, M.; Galińska, E.M.; Niemczuk, K.; Knap, J.P. Prevalence of Coxiella burnetii Infection in Humans Occupationally Exposed to Animals in Poland. Vector-Borne Zoonotic Dis. 2015, 15, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, N.O.; Elhofy, F.I.; Fahmy, A.H.; Sobhy, M.M.; Agag, M. Seroprevalence and molecular detection of Coxiella burnetii infection in sheep, goats and human in Egypt. ISOI J. Microbiol. Biotechnol. Food Sci. 2016, 2, 1–7. [Google Scholar]
- Melenotte, C.; Protopopescu, C.; Million, M.; Edouard, S.; Carrieri, M.P.; Eldin, C.; Angelakis, E.; Djossou, F.; Bardin, N.; Fournier, P.-E.; et al. Clinical Features and Complications of Coxiella burnetii Infections from the French National Reference Center for Q Fever. JAMA Netw. Open 2018, 1, e181580. [Google Scholar] [CrossRef] [PubMed]
- Raoult, D.; Tissot-Dupont, H.; Foucault, C.; Gouvernet, J.; Fournier, P.E.; Bernit, E.; Stein, A.; Nesri, M.; Harle, J.R.; Weiller, P.J. Q fever 1985–1998. Clinical and epidemiologic features of 1383 infections. Medicine 2000, 79, 109–123. [Google Scholar] [CrossRef]
- Domingo, P.; Muñoz-Batet, C.; Franquet, T.; Gurguí, M.; Sancho, F.; Vazquez, G. Acute Q Fever in Adult Patients: Report on 63 Sporadic Cases in an Urban Area. Clin. Infect. Dis. 1999, 29, 874–879. [Google Scholar] [CrossRef]
- Chang, K.; Yan, J.-J.; Lee, H.-C.; Liu, K.H.; Lee, N.Y.; Ko, W.-C. Acute hepatitis with or without jaundice: A predominant presentation of acute Q fever in southern Taiwan. J. Microbiol. Immunol. Infect. 2004, 37, 103–108. [Google Scholar]
- Heinrich, R.; Naujoks-Heinrich, S.; Saebisch, R.; Seuffer, R.; Grauer, W.; Jacob, R.; Schomerus, H. Seroprevalence of Q fever in an endemic area of southern Germany. Dtsch. Med. Wochenschr. 1983, 108, 1318–1324. [Google Scholar] [CrossRef]
- Anderson, A.; Bijlmer, H.; Fournier, P.E.; Graves, S.; Hartzell, J.; Kersh, G.J.; Limonard, G.; Marrie, T.J.; Massung, R.F.; McQuiston, J.H.; et al. Diagnosis and management of Q fever—United States, 2013: Recommendations from CDC and the Q Fever Working Group. MMWR Recomm. Rep. 2013, 62, 1–30. [Google Scholar]
- Mobarez, A.M.; Amiri, F.B.; Esmaeili, S. Seroprevalence of Q fever among human and animal in Iran; A systematic review and meta-analysis. PLOS Negl. Trop. Dis. 2017, 11, e0005521. [Google Scholar] [CrossRef]
- Abdel-Moein, K.; Hamza, D. Rat as an overlooked reservoir for Coxiella burnetii: A public health implication. Comp. Immunol. Microbiol. Infect. Dis. 2018, 61, 30–33. [Google Scholar] [CrossRef]
- Abdel-Moein, K.A.; Zaher, H.M. Parturient Cat As a Potential Reservoir for Coxiella burnetii: A Hidden Threat to Pet Owners. Vector-Borne Zoonotic Dis. 2021, 21, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Abushahba, M.F.N.; Abdelbaset, A.E.; Rawy, M.S.; Ahmed, S.O. Cross-sectional study for determining the prevalence of Q fever in small ruminants and humans at El Minya Governorate, Egypt. BMC Res. Notes 2017, 10, 538. [Google Scholar] [CrossRef] [PubMed]
- Gwida, M.; El-Ashker, M.; El-Diasty, M.; Engelhardt, C.; Khan, I.; Neubauer, H. Q fever in cattle in some Egyptian Governorates: A preliminary study. BMC Res. Notes 2014, 7, 881. [Google Scholar] [CrossRef] [PubMed]
- Klemmer, J.; Njeru, J.; Emam, A.; El-Sayed, A.; Moawad, A.A.; Henning, K.; Elbeskawy, M.A.; Sauter-Louis, C.; Straubinger, R.K.; Neubauer, H.; et al. Q fever in Egypt: Epidemiological survey of Coxiella burnetii specific antibodies in cattle, buffaloes, sheep, goats and camels. PLoS ONE 2018, 13, e0192188. [Google Scholar] [CrossRef]
- El-Mokhtar, M.A.; Ramadan, H.K.-A.; Thabet, M.M.; Abd-Elkader, A.S.; Fouad, M.; Sallam, M.M.; Elgohary, E.A.; El-Hafeez, A.A.A.; Mohamed, M.E.; Sayed, I.M. The Unmet Needs of Hepatitis E Virus Diagnosis in Suspected Drug-Induced Liver Injury in Limited Resource Setting. Front. Microbiol. 2021, 12, 2851. [Google Scholar] [CrossRef]
- Heim, A.; Ebnet, C.; Harste, G.; Pring-Åkerblom, P. Rapid and quantitative detection of human adenovirus DNA by real-time PCR. J. Med. Virol. 2003, 70, 228–239. [Google Scholar] [CrossRef]
- El-Mokhtar, M.A.; Ramadan, H.K.-A.; Hameed, M.R.A.; Kamel, A.M.; Mandour, S.A.; Ali, M.; Abdel-Malek, M.A.Y.; El-Kareem, D.M.A.; Adel, S.; Salama, E.H.; et al. Evaluation of hepatitis E antigen kinetics and its diagnostic utility for prediction of the outcomes of hepatitis E virus genotype 1 infection. Virulence 2021, 12, 1334–1344. [Google Scholar] [CrossRef]
- El-Mokhtar, M.A.; Seddik, M.I.; Osman, A.O.; Mahmoud, A.A.; Mandour, S.A.; Radwan, E.; Ali, M.; E Ismael, A.; Twisy, H.O.; Ramadan, H.K.-A.; et al. No evidence of HEV genotype 1 infections harming the male reproductive system. Virology 2020, 554, 37–41. [Google Scholar] [CrossRef]
- Lacout, A.; Mas, M.; Pajaud, J.; Perronne, V.; Lequette, Y.; Franck, M.; Perronne, C. Real time micro-organisms PCR in 104 patients with polymorphic signs and symptoms that may be related to a tick bite. Eur. J. Microbiol. Immunol. 2021, 11, 62–75. [Google Scholar] [CrossRef]
- Selim, A.; Ali, A.-F.; Moustafa, S.M.; Ramadan, E. Molecular and serological data supporting the role of Q fever in abortions of sheep and goats in northern Egypt. Microb. Pathog. 2018, 125, 272–275. [Google Scholar] [CrossRef]
- Leone, M.; Honstettre, A.; Lepidi, H.; Capo, C.; Bayard, F.; Raoult, D.; Mege, J.L. Effect of sex on Coxiella burnetii infection: Protective role of 17beta-estradiol. J. Infect. Dis. 2004, 189, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Zaibaq-Krill, J.; Weber, F.H. 2434 Cholestatic Jaundice: A Rare Presentation of Coxiella burnetii. Am. J. Gastroenterol. 2019, 114, S1348–S1349. [Google Scholar] [CrossRef]
- Pape, M.; Xanthis, A.; Hatzitolios, A.; Mandraveli, K.; Savopoulos, C.; Alexiou-Daniel, S. Acute hepatitis associated with Q fever in a man in Greece: A case report. J. Med. Case Rep. 2007, 1, 154. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Jang, J.J.; Kim, Y.S.; Lee, S.-O.; Choi, S.-H.; Kim, S.-H.; Yu, E. Clinicopathologic Features of Q Fever Patients with Acute Hepatitis. Korean J. Pathol. 2012, 46, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Amitai, Z.; Bromberg, M.; Bernstein, M.; Raveh, D.; Keysary, A.; David, D.; Pitlik, S.; Swerdlow, D.; Massung, R.; Rzotkiewicz, S.; et al. A Large Q Fever Outbreak in an Urban School in Central Israel. Clin. Infect. Dis. 2010, 50, 1433–1438. [Google Scholar] [CrossRef]
- El-Mokhtar, M.A.; Elkhawaga, A.A.; Sayed, I.M. Assessment of hepatitis E virus (HEV) in the edible goat products pointed out a risk for human infection in Upper Egypt. Int. J. Food Microbiol. 2020, 330, 108784. [Google Scholar] [CrossRef]
- Sayed, I.M.; Elkhawaga, A.A.; El-Mokhtar, M.A. Circulation of hepatitis E virus (HEV) and/or HEV-like agent in non-mixed dairy farms could represent a potential source of infection for Egyptian people. Int. J. Food Microbiol. 2019, 317, 108479. [Google Scholar] [CrossRef]
- Sayed, I.M.; Hammam, A.R.A.; Elfaruk, M.S.; Alsaleem, K.A.; Gaber, M.A.; Ezzat, A.A.; Salama, E.H.; Elkhawaga, A.A.; El-Mokhtar, M.A. Enhancement of the Molecular and Serological Assessment of Hepatitis E Virus in Milk Samples. Microorganisms 2020, 8, 1231. [Google Scholar] [CrossRef]
- Lai, C.-H.; Chin, C.; Chung, H.-C.; Huang, C.-K.; Chen, W.-F.; Yang, Y.-T.; Lin, H.-H.; Chen, W.; Chen, W.-F. Acute Q Fever Hepatitis in Patients with and without Underlying Hepatitis B or C Virus Infection. Clin. Infect. Dis. 2007, 45, e52–e59. [Google Scholar] [CrossRef][Green Version]
- Hsu, J.-Y.; Tsai, C.-C.; Tseng, K.-C. Fulminant hepatic failure and acute renal failure as manifestations of concurrent Q fever and cytomegalovirus infection: A case report. BMC Infect. Dis. 2014, 14, 651. [Google Scholar] [CrossRef]
- Alkan, W.J.; Evenchik, Z.; Eshchar, J. Q fever and infectious hepatitis. Am. J. Med. 1965, 38, 54–61. [Google Scholar] [CrossRef]
- Aguilar-Olivos, N.; Manzano-Robleda, M.D.C.; Gutiérrez-Grobe, Y.; Chablé-Montero, F.; Albores-Saavedra, J.; López-Méndez, E. Granulomatous hepatitis caused by Q fever: A differential diagnosis of fever of unknown origin. Ann. Hepatol. 2013, 12, 138–141. [Google Scholar] [CrossRef]
- Gerstl, B.; Movitt, E.R.; Skahen, J.R. Liver function and morphology in Q fever. Gastroenterology 1956, 30, 813–819. [Google Scholar] [CrossRef]
| Parameter | Acute Hepatitis of Unknown Etiology (AHUE) (N = 54) (n, %) |
|---|---|
| Age (years) * | 38 (27–45) |
| Gender | |
| Male | 35 (64.8%) |
| Female | 19 (35.2%) |
| Clinical symptoms | |
| Jaundice | 51 (94.4%) |
| Fever | 20 (37.03%) |
| Vomiting | 32 (59.26%) |
| Abdominal pain | 32 (59.26%) |
| Diarrhea | 21 (38.9%) |
| Dark urine | 25 (46.3%) |
| Ascites $ | 4 (7.4%) |
| Encephalopathy | 6 (11.1%) |
| Liver functions (LF) * | |
| ALT (IU/L) | 106 (72–303) |
| AST (IU/L) | 107 (67–347) |
| Total bilirubin (μmol/L) | 230 (142–272) |
| Direct bilirubin (μmol/L) | 153 (69–198) |
| Albumin (g/L) | 34 (29–38) |
| Abdominal Ultrasound | |
| Normal | 39 (72.2%) |
| Fatty Liver | 5 (9.26%) |
| Hepatomegaly | 3 (5.56%) |
| Hepatosplenomegaly | 3(5.56%) |
| Cirrhosis | 4 (7.4%) |
| Course of hepatitis | |
| Acute hepatitis (AH) | 43 (79.63%) |
| Acute on top of chronic liver failure (ACLF) | 4 (7.4%) |
| Fulminant hepatitis (FH) | 7 (12.96) |
| Patient #1 | Patient #2 | Patient #3 | |
|---|---|---|---|
| Age | 43 | 45 | 64 |
| Sex | Male | Male | Male |
| Clinical symptoms | |||
| Jaundice | Yes | Yes | Yes |
| Fever | No | Yes | No |
| Vomiting | No | Yes | No |
| Abdominal pain | Yes | Yes | No |
| Diarrhea | No | Yes | No |
| Dark urine | Yes | No | Yes |
| Ascites | No | No | Yes |
| Encephalopathy | No | No | Yes |
| Liver functions (LF) | |||
| ALT (IU/L) | 92 | 67 | 1354 |
| AST (IU/L) | 61 | 88 | 630 |
| Total bilirubin (μmol/L) | 263 | 235 | 233 |
| Direct bilirubin (μmol/L) | 155 | 156 | 181 |
| Albumin (g/L) | 31 | 33 | 24 |
| Abdominal Ultrasound | Normal | Normal | Cirrhosis |
| Course of hepatitis | Acute hepatitis | Acute hepatitis | ACLF |
| Blood pictures | |||
| WBCs (×1000/mm3) | 7.5 | 7.6 | 6.3 |
| Platelets (×1000/mm3) | 265 | 246 | 170 |
| International normalized ratio (INR) | 1.1 | 1.01 | 1.9 |
| Risk factor | |||
| Rural/urban | rural | Rural | rural |
| Q-Fever–Positive N = 3 | Q-Fever–Negative N = 51 | |
|---|---|---|
| Age mean ± SD | 50.67 ± 11.6 | 37.8 ± 12.73 |
| Sex | Male: 3/3 (100%) Female: 0/3 (0%) | Male: 32/51 (62.7%) Female: 19/51 (37.3%) |
| Clinical symptoms | ||
| Jaundice | 3/3 (100%) | 48/51 (94.1%) |
| Fever | 1/3 (33.3%) | 19/51(37.25%) |
| Vomiting | 1/3 (33.3%) | 31/51 (60.78%) |
| Abdominal pain | 2/3 (66.7%) | 30/51 (58.8%) |
| Diarrhea | 1/3 (33.3%) | 20/51 (39.2%) |
| Dark urine | 2/3 (66.7%) | 23/51 (45.1%) |
| Ascites | 1/3 (33.3%) | 3/51 (5.9%) |
| Encephalopathy | 1/3 (33.3%) | 5/51 (9.8%) |
| Liver functions (LF) * | ||
| ALT (IU/L) | 92 | 112 |
| AST (IU/L) | 88 | 108 |
| Total bilirubin (μmol/L) | 235 | 220.6 |
| Direct bilirubin (μmol/L) | 156 | 150.8 |
| Albumin (g/L) | 31 | 34 |
| Blood pictures | ||
| WBCs (×1000/mm3) | 7.13 ± 0.72 | 7.54 ± 2.8 |
| Platelets (×1000/mm3) | 227 ± 50.27 | 214 ± 80.22 |
| International normalized ratio (INR) | 1.34 ± 0.49 | 1.3 ± 0.64 |
| Parameter | Acute Hepatitis Caused by Less Common Viral Pathogens (N = 10) (n, %) |
|---|---|
| Age (years) * | 33 (21–39) |
| Gender | |
| Male | 6 (60%) |
| Female | 4 (40%) |
| Clinical symptoms | |
| Jaundice | 9 (90%) |
| Fever | 3 (30%) |
| Vomiting | 2 (20 %) |
| Abdominal pain | 3 (30%) |
| Diarrhea | 0 (0%) |
| Dark urine | 4 (40%) |
| Ascites | 4 (40 %) |
| Encephalopathy | 3 (30%) |
| Liver functions (LF) * | |
| ALT (IU/L) | 519 (250–994) |
| AST (IU/L) | 428 (236–817) |
| Total bilirubin (μmol/L) | 164 (86–287) |
| Direct bilirubin (μmol/L) | 132 (56–215) |
| Albumin (g/L) | 25 (21–33) |
| Abdominal Ultrasound | |
| Normal | 5 (50%) |
| Hepatomegaly | 1 (10%) |
| Cirrhosis | 4 (40%) |
| HEV Infection N = 4 | HEV/Coxiella N = 1 | |
|---|---|---|
| Age (minimum–maximum) | 21–37 | 19 |
| Sex | Male: 1/4 (25%) Female: 3/4 (75%) | Male |
| Clinical symptoms | ||
| Jaundice | 4/4 (100%) | No |
| Fever | 1/4 (25%) | Yes |
| Vomiting | 1/4 (25%) | Yes |
| Abdominal pain | 1/4 (66.7%) | No |
| Diarrhea | 0/4 (0%) | No |
| Dark urine | 1/4 (25%) | No |
| Ascites | 2/4 (50%) | No |
| Encephalopathy | 2/4 (50%) | No |
| Liver functions (LF) | ||
| ALT (IU/L) | 272–659 | 1544 |
| AST (IU/L) | 264–1141 | 410 |
| Total bilirubin (μmol/L) | 147–414 | 80 |
| Direct bilirubin (μmol/L) | 118–325 | 60 |
| Albumin (g/L) | 20–28 | 40 |
| Blood pictures | ||
| WBCs (×1000/mm3) | 4.1–12.7 | 3 |
| Platelets (×1000/mm3) | 92–264 | 144 |
| International normalized ratio (INR) | 1–2.5 | 1.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Mokhtar, M.A.; Sayed, I.M.; Kamel, A.M.; Mesalam, A.A.; Elgohary, E.A.; Khalaf, K.A.b.; Adel, S.; Elfadl, A.A.; Khalifa, W.A.; Ramadan, H.K.-A. The First Report of Coxiella burnetii as a Potential Neglected Pathogen of Acute Hepatitis of Unknown Causes in Egypt. Microorganisms 2022, 10, 2168. https://doi.org/10.3390/microorganisms10112168
El-Mokhtar MA, Sayed IM, Kamel AM, Mesalam AA, Elgohary EA, Khalaf KAb, Adel S, Elfadl AA, Khalifa WA, Ramadan HK-A. The First Report of Coxiella burnetii as a Potential Neglected Pathogen of Acute Hepatitis of Unknown Causes in Egypt. Microorganisms. 2022; 10(11):2168. https://doi.org/10.3390/microorganisms10112168
Chicago/Turabian StyleEl-Mokhtar, Mohamed A., Ibrahim M. Sayed, Ayat M. Kamel, Ahmed Atef Mesalam, Elsayed A. Elgohary, Khaled Abo bakr Khalaf, Sara Adel, Azza Abo Elfadl, Walaa A. Khalifa, and Haidi Karam-Allah Ramadan. 2022. "The First Report of Coxiella burnetii as a Potential Neglected Pathogen of Acute Hepatitis of Unknown Causes in Egypt" Microorganisms 10, no. 11: 2168. https://doi.org/10.3390/microorganisms10112168
APA StyleEl-Mokhtar, M. A., Sayed, I. M., Kamel, A. M., Mesalam, A. A., Elgohary, E. A., Khalaf, K. A. b., Adel, S., Elfadl, A. A., Khalifa, W. A., & Ramadan, H. K.-A. (2022). The First Report of Coxiella burnetii as a Potential Neglected Pathogen of Acute Hepatitis of Unknown Causes in Egypt. Microorganisms, 10(11), 2168. https://doi.org/10.3390/microorganisms10112168







