Identification of Human Gut Microbiome Associated with Enterolignan Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Enterodiol-Glucuronide (EDGlu) and Enterolactone-Glucuronide (ELGlu)
2.2. Subject and Sample Collection
2.3. Analysis of Enterolignans in Serum
2.4. Gut Microbiome Analysis
2.5. Statistical Analysis
3. Results
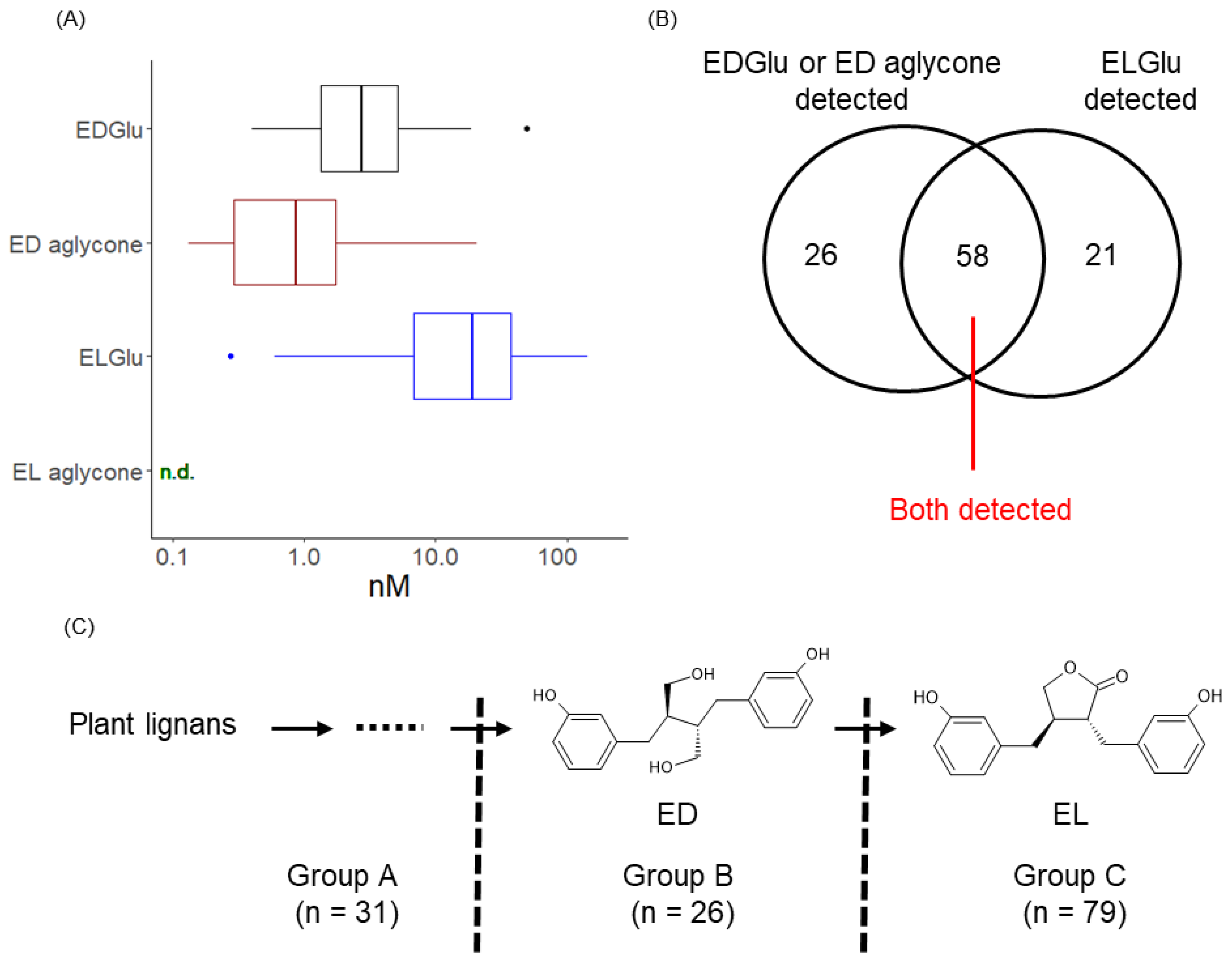
3.1. Detection of Enterolignans and Definition of Subject’s Enterolignan Metabolic Profile
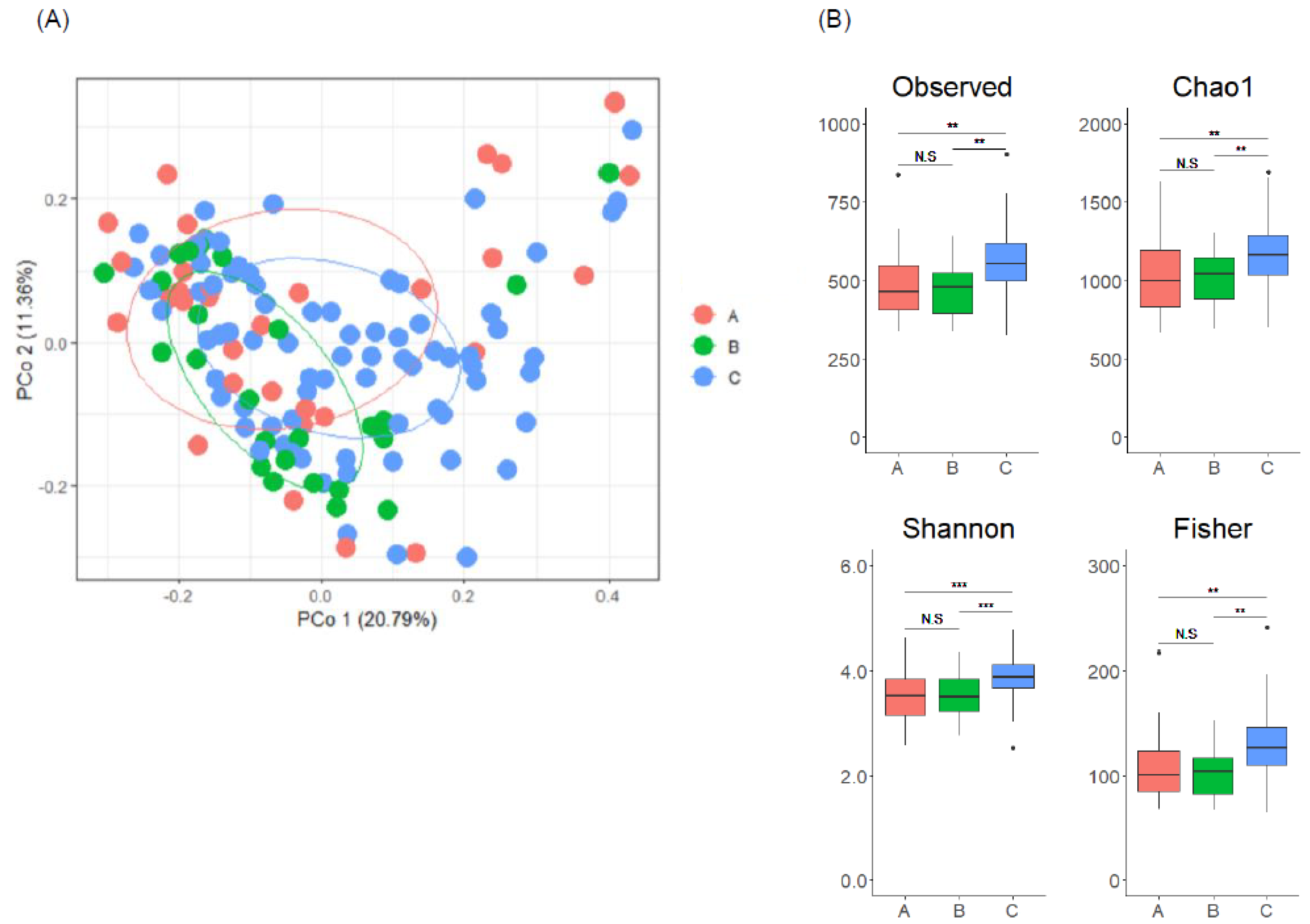
3.2. Gut Microbiome Composition Is Associated with Enterolignan Metabolic Profile
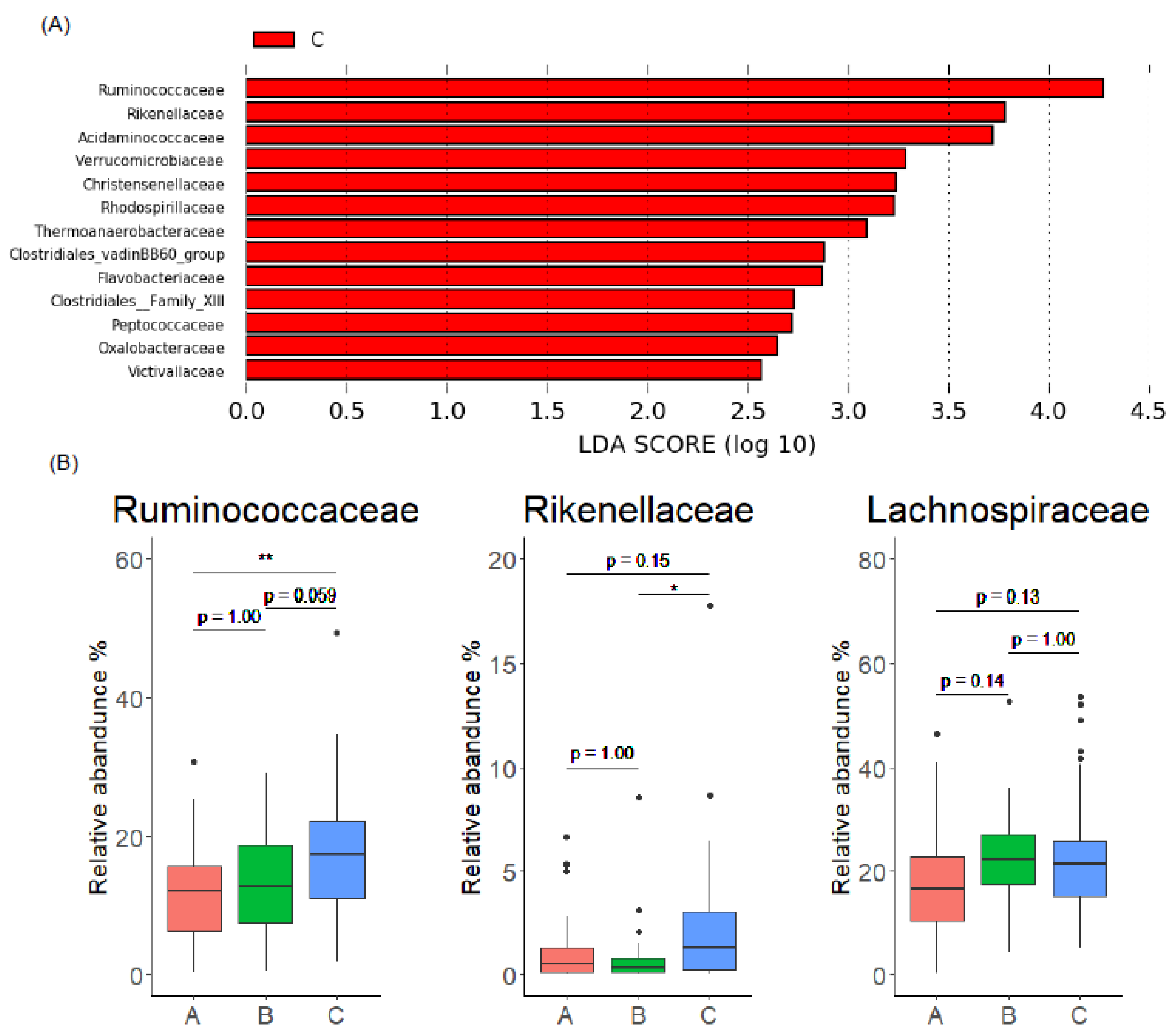
3.3. Gut Microbiome Characteristics of Enterolignan Producers
3.4. Gut Microbiome Characteristics of EL Producers
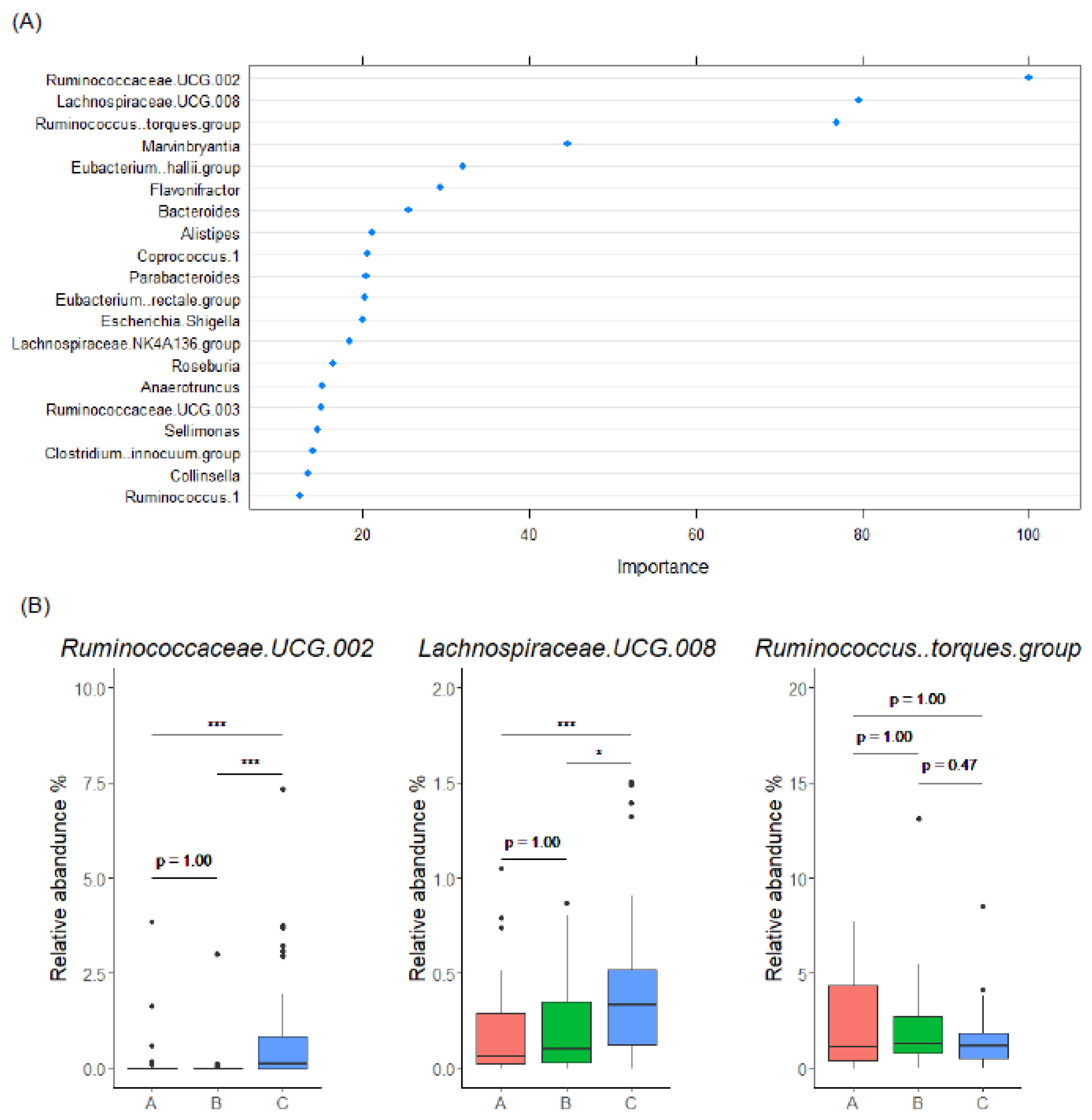
3.5. Gut Microbiome-Based Classification of Enterolignan Metabolic Profile
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the Gut Microbiota in Nutrition and Health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef] [PubMed]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut Microbiota Functions: Metabolism of Nutrients and Other Food Components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Aso, T.; Uchiyama, S.; Matsumura, Y.; Taguchi, M.; Nozaki, M.; Takamatsu, K.; Ishizuka, B.; Kubota, T.; Mizunuma, H.; Ohta, H. A Natural S-Equol Supplement Alleviates Hot Flushes and Other Menopausal Symptoms in Equol Nonproducing Postmenopausal Japanese Women. J. Women’s Health 2012, 21, 92–100. [Google Scholar] [CrossRef]
- Pawlowski, J.W.; Martin, B.R.; McCabe, G.P.; McCabe, L.; Jackson, G.S.; Peacock, M.; Barnes, S.; Weaver, C.M. Impact of Equol-Producing Capacity and Soy-Isoflavone Profiles of Supplements on Bone Calcium Retention in Postmenopausal Women: A Randomized Crossover Trial1,2. Am. J. Clin. Nutr. 2015, 102, 695–703. [Google Scholar] [CrossRef]
- Yasuda, S.; Okahashi, N.; Tsugawa, H.; Ogata, Y.; Ikeda, K.; Suda, W.; Arai, H.; Hattori, M.; Arita, M. Elucidation of Gut Microbiota-Associated Lipids Using LC-MS/MS and 16S rRNA Sequence Analyses. iScience 2020, 23, 101841. [Google Scholar] [CrossRef]
- Kishino, S.; Takeuchi, M.; Park, S.B.; Hirata, A.; Kitamura, N.; Kunisawa, J.; Kiyono, H.; Iwamoto, R.; Isobe, Y.; Arita, M.; et al. Polyunsaturated Fatty Acid Saturation by Gut Lactic Acid Bacteria Affecting Host Lipid Composition. Proc. Natl. Acad. Sci. USA 2013, 110, 17808–17813. [Google Scholar] [CrossRef]
- Nagatake, T.; Kishino, S.; Urano, E.; Murakami, H.; Kitamura, N.; Konishi, K.; Ohno, H.; Tiwari, P.; Morimoto, S.; Node, E.; et al. Intestinal Microbe-Dependent ω3 Lipid Metabolite αKetoA Prevents Inflammatory Diseases in Mice and Cynomolgus Macaques. Mucosal Immunol. 2022, 15, 289–300. [Google Scholar] [CrossRef]
- Goecks, J.; Jalili, V.; Heiser, L.M.; Gray, J.W. How Machine Learning Will Transform Biomedicine. Cell 2020, 181, 92–101. [Google Scholar] [CrossRef]
- Berry, S.E.; Valdes, A.M.; Drew, D.A.; Asnicar, F.; Mazidi, M.; Wolf, J.; Capdevila, J.; Hadjigeorgiou, G.; Davies, R.; Al Khatib, H.; et al. Human Postprandial Responses to Food and Potential for Precision Nutrition. Nat. Med. 2020, 26, 964–973. [Google Scholar] [CrossRef]
- Subramanian, S.; Huq, S.; Yatsunenko, T.; Haque, R.; Mahfuz, M.; Alam, M.A.; Benezra, A.; Destefano, J.; Meier, M.F.; Muegge, B.D.; et al. Persistent Gut Microbiota Immaturity in Malnourished Bangladeshi Children. Nature 2014, 510, 417–421. [Google Scholar] [CrossRef]
- Gehrig, J.L.; Venkatesh, S.; Chang, H.W.; Hibberd, M.C.; Kung, V.L.; Cheng, J.; Chen, R.Y.; Subramanian, S.; Cowardin, C.A.; Meier, M.F.; et al. Effects of Microbiota-Directed Foods in Gnotobiotic Animals and Undernourished Children. Science 2019, 365, eaau4732. [Google Scholar] [CrossRef] [PubMed]
- Seethaler, B.; Nguyen, N.K.; Basrai, M.; Kiechle, M.; Walter, J.; Delzenne, N.M.; Bischoff, S.C. Short-Chain Fatty Acids Are Key Mediators of the Favorable Effects of the Mediterranean Diet on Intestinal Barrier Integrity: Data from the Randomized Controlled LIBRE Trial. Am. J. Clin. Nutr. 2022, 116, 928–942. [Google Scholar] [CrossRef] [PubMed]
- Rizzolo-Brime, L.; Caro-Garcia, E.M.; Alegre-Miranda, C.A.; Felez-Nobrega, M.; Zamora-Ros, R. Lignan exposure: A worldwide perspective. Eur. J. Nutr. 2021, 61, 1143–1165. [Google Scholar] [CrossRef] [PubMed]
- Bess, E.N.; Bisanz, J.E.; Yarza, F.; Bustion, A.; Rich, B.E.; Li, X.; Kitamura, S.; Waligurski, E.; Ang, Q.Y.; Alba, D.L.; et al. Genetic Basis for the Cooperative Bioactivation of Plant Lignans by Eggerthella Lenta and Other Human Gut Bacteria. Nat. Microbiol. 2020, 5, 56–66. [Google Scholar] [CrossRef]
- Woting, A.; Clavel, T.; Loh, G.; Blaut, M. Bacterial Transformation of Dietary Lignans in Gnotobiotic Rats. FEMS Microbiol. Ecol. 2010, 72, 507–514. [Google Scholar] [CrossRef]
- Navarro, S.L.; Levy, L.; Curtis, K.R.; Elkon, I.; Kahsai, O.J.; Ammar, H.S.; Randolph, T.W.; Hong, N.N.; Neto, F.C.; Raftery, D.; et al. Effect of a Flaxseed Lignan Intervention on Circulating Bile Acids in a Placebo-Controlled Randomized, Crossover Trial. Nutrients 2020, 12, 1837. [Google Scholar] [CrossRef]
- Rietjens, I.M.C.M.; Louisse, J.; Beekmann, K. The Potential Health Effects of Dietary Phytoestrogens. Br. J. Pharmacol. 2017, 174, 1263–1280. [Google Scholar] [CrossRef]
- Ren, G.Y.; Chen, C.Y.; Chen, W.G.; Huang, Y.; Qin, L.Q.; Chen, L.H. The Treatment Effects of Flaxseed-Derived Secoisolariciresinol Diglycoside and Its Metabolite Enterolactone on Benign Prostatic Hyperplasia Involve the G Protein-Coupled Estrogen Receptor 1. Appl. Physiol. Nutr. Metab. 2016, 41, 1303–1310. [Google Scholar] [CrossRef]
- Eriksen, A.K.; Kyrø, C.; Nørskov, N.P.; Frederiksen, K.; Bach Knudsen, K.E.; Overvad, K.; Landberg, R.; Tjønneland, A.; Olsen, A. Pre-Diagnostic Plasma Enterolactone Concentrations Are Associated with Lower Mortality among Individuals with Type 2 Diabetes: A Case-Cohort Study in the Danish Diet, Cancer and Health Cohort. Diabetologia 2019, 62, 959–969. [Google Scholar] [CrossRef]
- Kyro, C.; Frederiksen, K.; Holm, M.; Norskov, N.P.; Knudsen, K.E.B.; Overvad, K.; Tjonneland, A.; Olsen, A. Prediagnosis Plasma Concentrations of Enterolactone and Survival after Colorectal Cancer: The Danish Diet, Cancer and Health Cohort. Br. J. Nutr. 2019, 122, 552–563. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, F.; Li, J.; Ivey, K.L.; Wilkinson, J.E.; Wang, D.D.; Li, R.; Liu, G.; Eliassen, H.A.; Chan, A.T.; et al. Dietary Lignans, Plasma Enterolactone Levels, and Metabolic Risk in Men: Exploring the Role of the Gut Microbiome. BMC Microbiol. 2022, 22, 82. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, X.; Liu, Y.; Tian, H.; Flickinger, B.; Empie, M.W.; Sun, S.Z. Dietary Flaxseed Lignan Extract Lowers Plasma Cholesterol and Glucose Concentrations in Hypercholesterolaemic Subjects. Br. J. Nutr. 2008, 99, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Hullar, M.A.J.; Lancaster, S.M.; Li, F.; Tseng, E.; Beer, K.; Atkinson, C.; Wähälä, K.; Copeland, W.K.; Randolph, T.W.; Newton, K.M.; et al. Enterolignan-Producing Phenotypes Are Associated with Increased Gut Microbial Diversity and Altered Composition in Premenopausal Women in the United States. Cancer Epidemiol. Biomark. Prev. 2015, 24, 546–554. [Google Scholar] [CrossRef]
- McCann, S.E.; Hullar, M.A.J.; Tritchler, D.L.; Cortes-Gomez, E.; Yao, S.; Davis, W.; O’connor, T.; Erwin, D.; Thompson, L.U.; Yan, L.; et al. Enterolignan Production in a Flaxseed Intervention Study in Postmenopausal Us Women of African Ancestry and European Ancestry. Nutrients 2021, 13, 919. [Google Scholar] [CrossRef]
- Corona, G.; Kreimes, A.; Barone, M.; Turroni, S.; Brigidi, P.; Keleszade, E.; Costabile, A. Impact of Lignans in Oilseed Mix on Gut Microbiome Composition and Enterolignan Production in Younger Healthy and Premenopausal Women: An in Vitro Pilot Study. Microb. Cell Fact. 2020, 19, 82. [Google Scholar] [CrossRef]
- Nishijima, S.; Suda, W.; Oshima, K.; Kim, S.W.; Hirose, Y.; Morita, H.; Hattori, M. The Gut Microbiome of Healthy Japanese and Its Microbial and Functional Uniqueness. DNA Res. 2016, 23, 125–133. [Google Scholar] [CrossRef]
- Peñalvo, J.L.; Adlercreutz, H.; Uehara, M.; Ristimaki, A.; Watanabe, S. Lignan Content of Selected Foods from Japan. J. Agric. Food Chem. 2008, 56, 401–409. [Google Scholar] [CrossRef]
- Asai, A.; Miyazawa, T. Occurrence of Orally Administered Curcuminoid as Glucuronide and Glucuronide/Sulfate Conjugates in Rat Plasma. Life Sci. 2000, 67, 2785–2793. [Google Scholar] [CrossRef]
- Shoji, M.; Nakagawa, K.; Watanabe, A.; Tsuduki, T.; Yamada, T.; Kuwahara, S.; Kimura, F.; Miyazawa, T. Comparison of the Effects of Curcumin and Curcumin Glucuronide in Human Hepatocellular Carcinoma HepG2 Cells. Food Chem. 2014, 151, 126–132. [Google Scholar] [CrossRef]
- Hosomi, K.; Ohno, H.; Murakami, H.; Natsume-Kitatani, Y.; Tanisawa, K.; Hirata, S.; Suzuki, H.; Nagatake, T.; Nishino, T.; Mizuguchi, K.; et al. Method for Preparing DNA from Feces in Guanidine Thiocyanate Solution Affects 16S RRNA-Based Profiling of Human Microbiota Diversity. Sci. Rep. 2017, 7, 4339. [Google Scholar] [CrossRef]
- Hosomi, K.; Saito, M.; Park, J.; Murakami, H.; Shibata, N.; Ando, M.; Nagatake, T.; Konishi, K.; Ohno, H.; Tanisawa, K.; et al. Oral Administration of Blautia Wexlerae Ameliorates Obesity and Type 2 Diabetes via Metabolic Remodeling of the Gut Microbiota. Nat. Commun. 2022, 13, 4477. [Google Scholar] [CrossRef]
- Park, J.; Kato, K.; Murakami, H.; Hosomi, K.; Tanisawa, K.; Nakagata, T.; Ohno, H.; Konishi, K.; Kawashima, H.; Chen, Y.A.; et al. Comprehensive Analysis of Gut Microbiota of a Healthy Population and Covariates Affecting Microbial Variation in Two Large Japanese Cohorts. BMC Microbiol. 2021, 21, 151. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pẽa, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME Allows Analysis of High-Throughput Community Sequencing Data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME Improves Sensitivity and Speed of Chimera Detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Dray, S.; Dufour, A.B. The Ade4 Package: Implementing the Duality Diagram for Ecologists. J. Stat. Softw. 2007, 22, 1–20. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; Mcglinn, D.; Minchin, P.R.; O’hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Package “vegan” Title Community Ecology Package, Version 2.5-7; CRAN: Vienna, Austria, 2020. [Google Scholar]
- Team, R.C. R: A Language and Environment for Statistical Computing; R-Team Project: Copenhagen, Denmark, 2021. [Google Scholar]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic Biomarker Discovery and Explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Liaw, A.; Wiener, M. Classification and Regression by RandomForest. R News 2002, 2, 18–22. [Google Scholar]
- Kuhn, M. Package ‘caret’—Classification and Regression Training; CRAN: Vienna, Austria, 2019. [Google Scholar]
- Ginestet, C. Ggplot2: Elegant Graphics for Data Analysis. J. R. Stat. Soc. Ser. A (Stat. Soc.) 2011, 174, 245–246. [Google Scholar] [CrossRef]
- Nørskov, N.P.; Kyrø, C.; Olsen, A.; Tjønneland, A.; Bach Knudsen, K.E. High-Throughput LC-MS/MS Method for Direct Quantification of Glucuronidated, Sulfated, and Free Enterolactone in Human Plasma. J. Proteome Res. 2016, 15, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Knust, U.; Spiegelhalder, B.; Strowitzki, T.; Owen, R.W. Contribution of Linseed Intake to Urine and Serum Enterolignan Levels in German Females: A Randomised Controlled Intervention Trial. Food Chem. Toxicol. 2006, 44, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Mukker, J.K.; Michel, D.; Muir, A.D.; Krol, E.S.; Alcorn, J. Permeability and Conjugative Metabolism of Flaxseed Lignans by Caco-2 Human Intestinal Cells. J. Nat. Prod. 2014, 77, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Possemiers, S.; Bolca, S.; Eeckhaut, E.; Depypere, H.; Verstraete, W. Metabolism of Isoflavones, Lignans and Prenylflavonoids by Intestinal Bacteria: Producer Phenotyping and Relation with Intestinal Community. FEMS Microbiol. Ecol. 2007, 61, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Lagkouvardos, I.; Kläring, K.; Heinzmann, S.S.; Platz, S.; Scholz, B.; Engel, K.H.; Schmitt-Kopplin, P.; Haller, D.; Rohn, S.; Skurk, T.; et al. Gut Metabolites and Bacterial Community Networks during a Pilot Intervention Study with Flaxseeds in Healthy Adult Men. Mol. Nutr. Food Res. 2015, 59, 1614–1628. [Google Scholar] [CrossRef]
- Clavel, T.; Henderson, G.; Alpert, C.A.; Philippe, C.; Rigottier-Gois, L.; Doré, J.; Blaut, M. Intestinal Bacterial Communities That Produce Active Estrogen-like Compounds Enterodiol and Enterolactone in Humans. Appl. Environ. Microbiol. 2005, 71, 6077–6085. [Google Scholar] [CrossRef]
- Takagi, T.; Inoue, R.; Oshima, A.; Sakazume, H.; Ogawa, K.; Tominaga, T.; Mihara, Y.; Sugaya, T.; Mizushima, K.; Uchiyama, K.; et al. Typing of the Gut Microbiota Community in Japanese Subjects. Microorganisms 2022, 10, 664. [Google Scholar] [CrossRef]
- Oki, K.; Toyama, M.; Banno, T.; Chonan, O.; Benno, Y.; Watanabe, K. Comprehensive Analysis of the Fecal Microbiota of Healthy Japanese Adults Reveals a New Bacterial Lineage Associated with a Phenotype Characterized by a High Frequency of Bowel Movements and a Lean Body Type. BMC Microbiol. 2016, 16, 284. [Google Scholar] [CrossRef]
- Odamaki, T.; Kato, K.; Sugahara, H.; Hashikura, N.; Takahashi, S.; Xiao, J.Z.; Abe, F.; Osawa, R. Age-Related Changes in Gut Microbiota Composition from Newborn to Centenarian: A Cross-Sectional Study. BMC Microbiol. 2016, 16, 90. [Google Scholar] [CrossRef]
- Lampe, J.; Kim, E.; Levy, L.; Davidson, L.; Goldsby, J.; Miles, F.; Navarro, S.; Randolph, T.; Zhao, N.; Ivan Ivanov, I.; et al. Colonic mucosal and exfoliome transcriptomic profiling and fecal microbiome response to a flaxseed lignan extract intervention in humans. Am. J. Clin. Nutr. 2019, 110, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, S.; Matsuoka, T.; Hosomi, K.; Park, J.; Nishimura, M.; Murakami, H.; Konishi, K.; Miyachi, M.; Kawashima, H.; Mizuguchi, K.; et al. Classification of the Occurrence of Dyslipidemia Based on Gut Bacteria Related to Barley Intake. Front. Nutr. 2022, 9, 812469. [Google Scholar] [CrossRef] [PubMed]

| Accuracy | AUC | |
|---|---|---|
| Model 1: training data set | 0.741 ± 0.025 | 0.712 ± 0.018 |
| Model 1: test data set | 0.758 ± 0.054 | 0.747 ± 0.061 |
| Model 2: training data set | 0.780 ± 0.027 | 0.760 ± 0.027 |
| Model 2: test data set | 0.762 ± 0.051 | 0.760 ± 0.048 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawane, K.; Hosomi, K.; Park, J.; Ookoshi, K.; Nanri, H.; Nakagata, T.; Chen, Y.-A.; Mohsen, A.; Kawashima, H.; Mizuguchi, K.; et al. Identification of Human Gut Microbiome Associated with Enterolignan Production. Microorganisms 2022, 10, 2169. https://doi.org/10.3390/microorganisms10112169
Sawane K, Hosomi K, Park J, Ookoshi K, Nanri H, Nakagata T, Chen Y-A, Mohsen A, Kawashima H, Mizuguchi K, et al. Identification of Human Gut Microbiome Associated with Enterolignan Production. Microorganisms. 2022; 10(11):2169. https://doi.org/10.3390/microorganisms10112169
Chicago/Turabian StyleSawane, Kento, Koji Hosomi, Jonguk Park, Kouta Ookoshi, Hinako Nanri, Takashi Nakagata, Yi-An Chen, Attayeb Mohsen, Hitoshi Kawashima, Kenji Mizuguchi, and et al. 2022. "Identification of Human Gut Microbiome Associated with Enterolignan Production" Microorganisms 10, no. 11: 2169. https://doi.org/10.3390/microorganisms10112169
APA StyleSawane, K., Hosomi, K., Park, J., Ookoshi, K., Nanri, H., Nakagata, T., Chen, Y.-A., Mohsen, A., Kawashima, H., Mizuguchi, K., Miyachi, M., & Kunisawa, J. (2022). Identification of Human Gut Microbiome Associated with Enterolignan Production. Microorganisms, 10(11), 2169. https://doi.org/10.3390/microorganisms10112169






