A Promising Use of Trimethyl Chitosan for Removing Microcystis aeruginosa in Water Treatment Processes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biological Material and Culture
2.2. Coagulation/Flocculation Materials
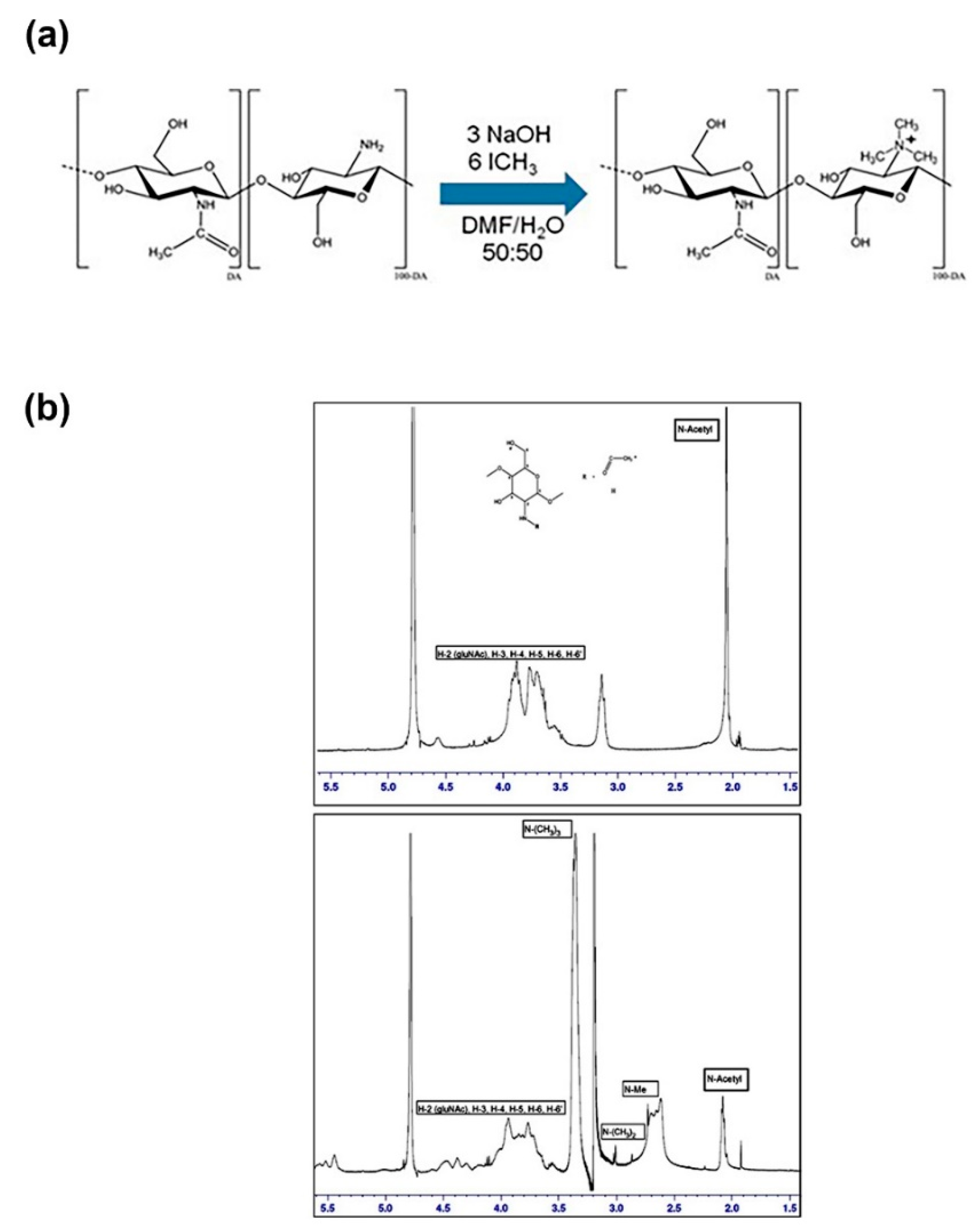
2.3. Synthesis and Characterization of N-Trimethyl Chitosan
2.4. C/F of M. aeruginosa Cells
2.4.1. Test Tube Assays
2.4.2. Jar-Test Assays
2.4.3. Determination of Cell Density, Turbidity, and Zeta Potential
2.5. M. aeruginosa Cell Integrity after TMC Treatment
2.5.1. Viability Determination
2.5.2. Electron Microscopy of M. aeruginosa Cells
2.6. Mathematical Model
2.7. Statistical Analysis
3. Results
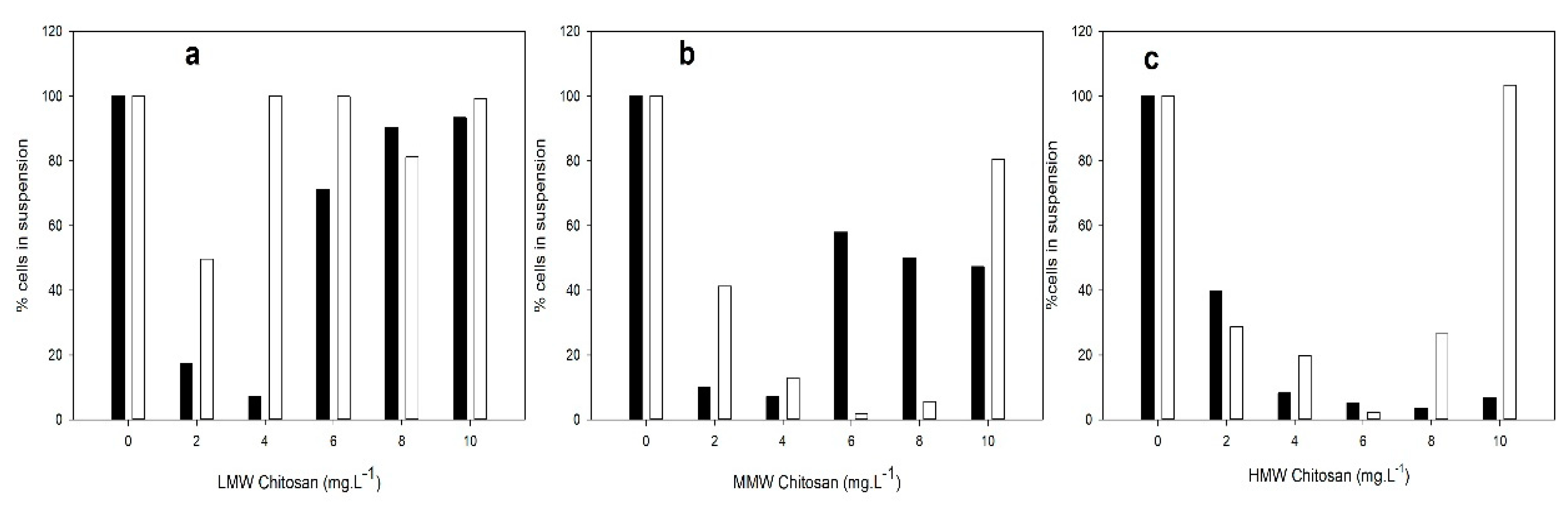
3.1. C/F Capacity of Chitosan with Different MW
3.2. Characterization of Trimethyl Chitosan (TMC)
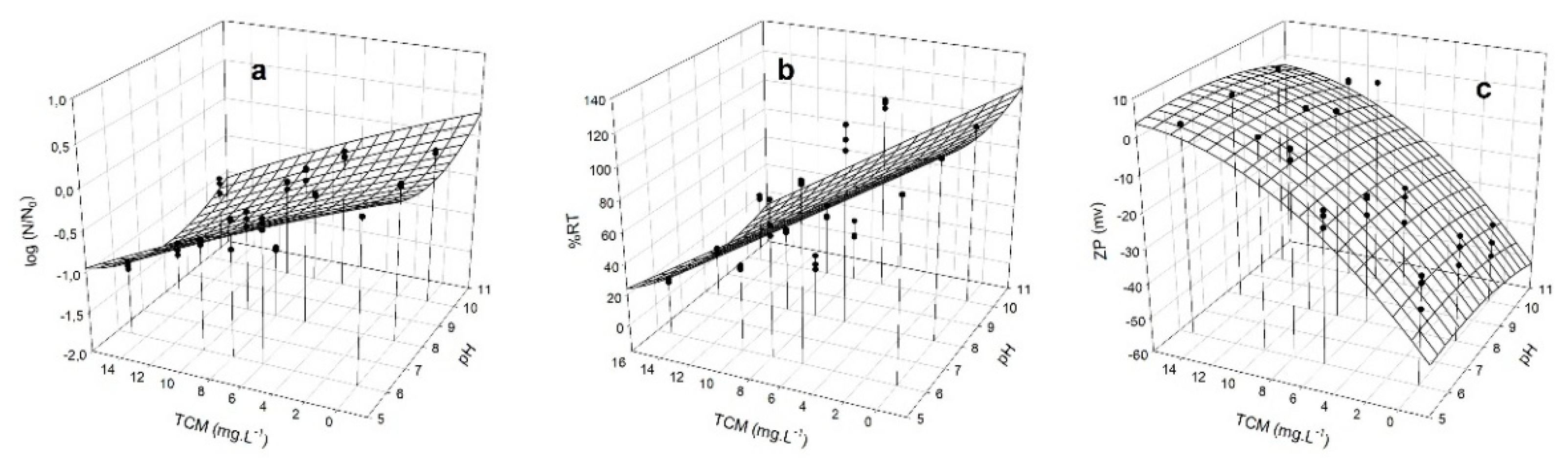
3.3. Determination of the C/F Capacity of TMC in Jar-Test Assays
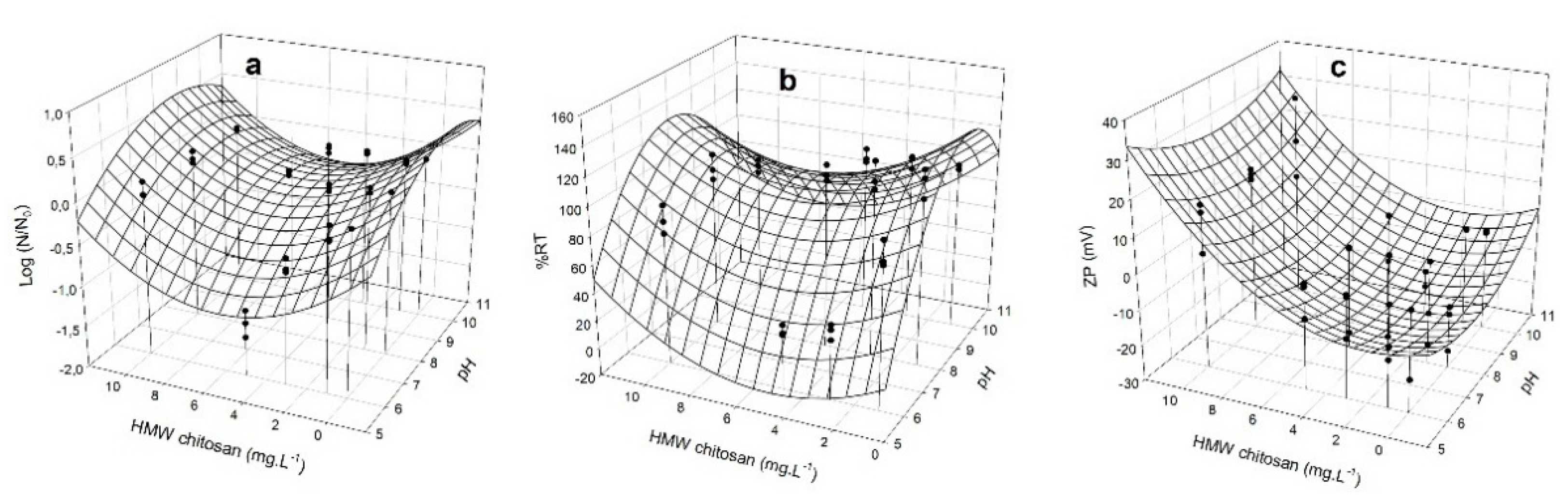
3.4. Removal Capacity of M. aeruginosa by HMW Chitosan and TMC
3.5. Effect of TMC on M. aeruginosa Cell Membrane Integrity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heisler, J.; Glibert, P.M.; Burkholder, J.M.; Anderson, D.M.; Cochlan, W.; Dennison, W.C.; Dortch, Q.; Gobler, C.J.; Heil, C.A.; Humphries, E.; et al. Eutrophication and harmful algal blooms: A scientific consensus. Harmful Algae 2008, 8, 3–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Neil, J.M.; Davis, T.W.; Burford, M.A.; Gobler, C.J. The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Reichwaldt, E.S.; Ghadouani, A. Effects of rainfall patterns on toxic cyanobacterial blooms in a changing climate: Between simplistic scenarios and complex dynamics. Water Res. 2012, 46, 1372–1393. [Google Scholar] [CrossRef]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial Blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef]
- World Health Organization. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management, 2nd ed.; Chorus, I., Welker, M., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 401–432. [Google Scholar]
- Alam, Z.B.; Otaki, M.; Furumai, H.; Ohgaki, S. Direct and indirect inactivation of Microcystis aeruginosa by UV-radiation. Water Res. 2001, 35, 1008–1014. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, B.; Zhang, P.; Wang, L.; Wang, H. Removal of algae by sonication-coagulation. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2006, 41, 1379–1390. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Hobson, P.; Ho, L.; Daly, R.; Brookes, J. The effects of various control and water treatment processes on the membrane integrity and toxin fate of cyanobacteria. J. Hazard. Mater. 2014, 264, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Harke, M.J.; Steffen, M.M.; Gobler, C.J.; Otten, T.G.; Wilhelm, S.W.; Wood, S.A.; Paerl, H.W. A review of the global ecology, genomics, and biogeography of the toxic cyanobacterium, Microcystis spp. Harmful Algae 2016, 54, 4–20. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Liu, Y.; Conklin, A.; Westrick, J.; Weavers, L.; Dionysiou, D.D.; Lenhart, J.J.; Mouser, P.J.; Szlag, D.; Walker, H.W. Toxic cyanobacteria and drinking water: Impacts, detection, and treatment. Harmful Algae 2016, 54, 174–193. [Google Scholar] [CrossRef]
- Yang, R.; Li, H.; Huang, M.; Yang, H.; Li, A. A review on chitosan-based flocculants and their applications in water treatment. Water Res. 2016, 95, 59–89. [Google Scholar] [CrossRef]
- Jančula, D.; Maršálek, B. Critical review of actually available chemical compounds for prevention and management of cyanobacterial blooms. Chemosphere 2011, 85, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.J.; Ho, L.; Hobson, P.; Brookes, J. Evaluating the effectiveness of copper sulphate chlorine, potassium permanganate, hydrogen peroxide and ozone on cyanobacterial cell integrity. Water Res. 2013, 47, 5153–5164. [Google Scholar] [CrossRef] [PubMed]
- Fast, S.A.; Kokabian, B.; Gude, V.G. Chitosan enhanced coagulation of algal turbid waters-Comparison between rapid mix and ultrasound coagulation methods. Chem. Eng. J. 2014, 244, 403–410. [Google Scholar] [CrossRef]
- Dearfield, K.L.; Douglas, G.R.; Ehling, U.H.; Moore, M.M.; Sega, G.A.; Brusick, D.J. Acrylamide: A review of its genotoxicity and an assessment of heritable genetic risk. Mutat. Res. 1995, 330, 71–99. [Google Scholar] [CrossRef]
- Xiong, B.; Loss, R.D.; Shields, D.; Pawlik, R.; Hochreiter, R.; Zydney, A.; Kumar, A. Polyacrylamide degradation and its implications in environmental systems. NPJ Clean Water 2018, 1, 17. [Google Scholar] [CrossRef]
- Renault, F.; Sancey, B.; Badot, P.M.; Crini, G. Chitosan for coagulation/flocculation processes—An eco-friendly approach. Eur. Polym. J. 2009, 45, 1337–1348. [Google Scholar] [CrossRef]
- Pei, H.Y.; Ma, C.X.; Hu, W.R.; Sun, F. The behaviors of Microcystis aeruginosa cells and extracellular microcystins during chitosan flocculation and flocs storage processes. Bioresour. Technol. 2014, 151, 314–322. [Google Scholar] [CrossRef]
- Elieh-Ali-Komi, D.; Hamblin, M.R. Chitin and Chitosan: Production and application of versatile biomedical nanomaterials. Int. J. Adv. Res. 2016, 4, 411–427. [Google Scholar]
- Kasaai, M.R. Determination of the degree of N-acetylation for chitin and chitosan by various NMR spectroscopy techniques: A review. Carbohydr. Polym. 2010, 79, 801–810. [Google Scholar] [CrossRef]
- Riske, F.; Schroeder, J.; Belliveau, J.; Kang, X.; Kutzko, J.; Menon, M.K. The use of chitosan as a flocculant in mammalian cell culture dramatically improves clarification throughput without adversely impacting monoclonal antibody recovery. J. Biotechnol. 2007, 128, 813–823. [Google Scholar] [CrossRef]
- Zeng, D.; Wu, J.; Kennedy, J.F. Application of a chitosan flocculant to water treatment. Carbohydr. Polym. 2008, 71, 135–139. [Google Scholar] [CrossRef]
- Sun, F.; Pei, H.-Y.; Hu, W.-R.; Ma, C.-X. The lysis of Microcystis aeruginosa in AlCl3 coagulation and sedimentation processes. Chem. Eng. J. 2012, 193, 196–202. [Google Scholar] [CrossRef]
- Li, L.; Zhang, H.; Pan, G. Influence of zeta potential on the flocculation of cyanobacteria cells using chitosan-modified soil. J. Environ. Sci. 2015, 28, 47–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dao, V.H.; Cameron, N.R.; Saito, K. Synthesis, properties and performance of organic polymers employed in flocculation applications. Polym. Chem. 2016, 7, 11–25. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Pei, H.; Hu, W.; Zhu, Y.; Xu, H.; Ma, C.; Sun, J.; Li, H. A promising application of chitosan quaternary ammonium salt to removal of Microcystis aeruginosa cells from drinking water. Sci. Total Environ. 2017, 583, 496–504. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C.; Wang, P.; Qian, J.; Hou, J.; Ao, Y.; Wu, B. The performance of chitosan/montmorillonite nanocomposite during the flocculation and floc storage processes of Microcystis aeruginosa cells. Environ. Sci. Pollut. Res. 2015, 22, 11148–11161. [Google Scholar] [CrossRef]
- Ma, C.; Hu, W.; Pei, H.; Xu, H.; Pei, R. Enhancing integrated removal of Microcystis aeruginosa and adsorption of microcystins using chitosan-aluminum chloride combined coagulants: Effect of chemical dosing orders and coagulation mechanisms. Colloids Surf. A Physicochem. Eng. Asp. 2016, 490, 258–267. [Google Scholar] [CrossRef]
- Zou, X.; Xu, K.; Xue, Y.; Qu, Y.; Li, Y. Removal of harmful algal blooms in freshwater by buoyant-bead flotation using chitosan-coated fly ash cenospheres. Environ. Sci. Pollut. Res. 2020, 27, 29239–29247. [Google Scholar] [CrossRef]
- Li, H.; Pan, G. Simultaneous removal of harmful algal bloom sand microcystins using microorganism-and chitosan-modified local soil. Environ. Sci. Technol. 2015, 49, 6249–6256. [Google Scholar] [CrossRef] [Green Version]
- Aguilera, A.; Haakonsson, S.; Martin, M.V.; Salerno, G.; Echenique, R. Bloom-forming cyanobacteria and cyanotoxins in Argentina: A growing health and environmental concern. Limnologica 2018, 69, 103–114. [Google Scholar] [CrossRef]
- Rosso, L.; Sedan, D.; Kolman, M.; Caixach, J.; Flores, C.; Oteiza, J.M.; Salerno, G.L.; Echenique, R.; Giannuzzi, L.; Andrinolo, D. Microcystis aeruginos strain [D-Leu1] Mcyst-LR producer, from Buenos Aires province, Argentina. J. Coast. Life Med. 2014, 2, 287–296. [Google Scholar] [CrossRef]
- Rippka, R.; Deruelles, J.; Waterburry, J.B.; Herdman, M.; Stanier, R.Y. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 1979, 111, 1–61. [Google Scholar] [CrossRef] [Green Version]
- Rúnarsson, Ö.V.; Holappa, J.; Jónsdóttir, S.; Steinsson, H.; Másson, M. N-selective ‘one pot’ synthesis of highly N-substituted trimethyl chitosan (TMC). Carbohydr. Polym. 2008, 74, 740–744. [Google Scholar] [CrossRef]
- Szymańska, E.; Winnicka, K. Stability of chitosan-a challenge for pharmaceutical and biomedical applications. Mar. Drugs 2015, 13, 1819–1846. [Google Scholar] [CrossRef]
- Schulze, K.; López, D.A.; Tillich, U.M.; Frohme, M. A simple viability analysis for unicellular cyanobacteria using a new autofluorescence assay, automated microscopy, and Image. BMC Biotechnol. 2011, 11, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venable, J.H.; Coggeshall, R. A simplified lead citrate stain for use in electron microscopy. J Cell Biol. 1965, 25, 407–408. [Google Scholar] [CrossRef] [Green Version]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 6th ed.; Iowa State Univ. Press: Ames, IA, USA, 1969. [Google Scholar]
- Song, W.C.; Li, X.; Sun, S.H.; Yang, Y.L.; Jia, R.B. Test study of enhanced coagulation for conventional treatment with low temperature and low turbidity. J. Residuals Sci. Technol. 2015, 12, 1544–8053. [Google Scholar] [CrossRef] [Green Version]
- Zheng, B.G.; Peng, W.G.; Zhang, J.B.; Zheng, Z. Flocculation removal of Microcystis aeruginosa by chitosan-bentonite compound material. Adv. Mat. Res. 2011, 335–336, 1381–1384. [Google Scholar] [CrossRef]
- Bangde, P.S.; Jain, R.D.; Dandekar, P. Alternative approach to synthesize methylated chitosan using deep eutectic solvents, biocatalyst and “green” methylating agents. Acs Sustain. Chem. Eng. 2016, 4, 3552–3557. [Google Scholar] [CrossRef]
- Hemming, E.B.; Masters, A.F.; Perosa, A.; Selva, M.; Maschmeyer, T. Single-step methylation of chitosan using dimethyl carbonate as a green methylating agent. Molecules 2019, 24, 3986. [Google Scholar] [CrossRef] [Green Version]
- Verspagen, J.M.; Van De Waal, D.B.; Finke, J.F.; Visser, P.M.; Van Donk, E.; Huisman, J. Rising CO2 levels will intensify phytoplankton blooms in eutrophic and hypertrophic lakes. PLoS ONE 2014, 9, e104325. [Google Scholar] [CrossRef] [PubMed]
- Bullerjahn, G.S.; Mckay, R.M.; Davis, T.W.; Baker, D.B.; Boyer, G.L.; D’anglada, L.V.; Doucette, G.J.; Ho, J.C.; Irwin, E.G.; Kling, C.L.; et al. Global solutions to regional problems: Collecting global expertise to address the problem of harmful cyanobacterial blooms. A Lake Erie case study. Harmful Algae 2016, 54, 223–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krausfeldt, L.E.; Farmer, A.T.; Castro Gonzalez, H.; Zepernick, B.N.; Campagna, S.R.; Wilhelm, S.W. Urea is both a carbon and nitrogen source for Microcystis aeruginosa: Tracking 13C incorporation at bloom pH conditions. Front. Microbiol. 2019, 10, 1064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, C.Y.; Lo, S.L.; Chang, C.L.; Chen, F.L.; Wu, Y.D.; Ma, J.L. Treatment of highly turbid water using chitosan and aluminum salts. Sep. Purif. Technol. 2013, 104, 322–326. [Google Scholar] [CrossRef]
- Peterson, H.G.; Hrudey, S.E.; Cantin, I.A.; Perley, T.R.; Kenefick, S.K. Physiological toxicity, cell membrane damage and the release of dissolved organic carbon and geosmin by Aphanizomenon flos-aquae after exposure to water treatment chemicals. Water Res. 1995, 29, 1515–1523. [Google Scholar] [CrossRef]
- Mourya, V.K.; Inamdar, N.N. Trimethyl chitosan and its applications in drug delivery. J. Mater. Sci. Mater. Med. 2009, 20, 1057–1079. [Google Scholar] [CrossRef]
- Benediktsdóttir, B.E.; Gaware, V.S.; Rúnarsson, Ö.V.; Jónsdóttir, S.; Jensen, K.J.; Másson, M. Synthesis of N,N,N-trimethyl chitosan homopolymer and highly substituted N-alkyl-N, N-dimethyl chitosan derivatives with the aid of di-tert-butyldimethylsilyl chitosan. Carbohydr. Polym. 2011, 86, 1451–1460. [Google Scholar] [CrossRef]
- Jintapattanakit, A.; Mao, S.; Kissel, T.; Junyaprasert, V.B. Physicochemical properties and biocompatibility of N-trimethyl chitosan: Effect of quaternization and dimethylation. J. Pharm. Biopharm. 2008, 70, 563–571. [Google Scholar] [CrossRef]

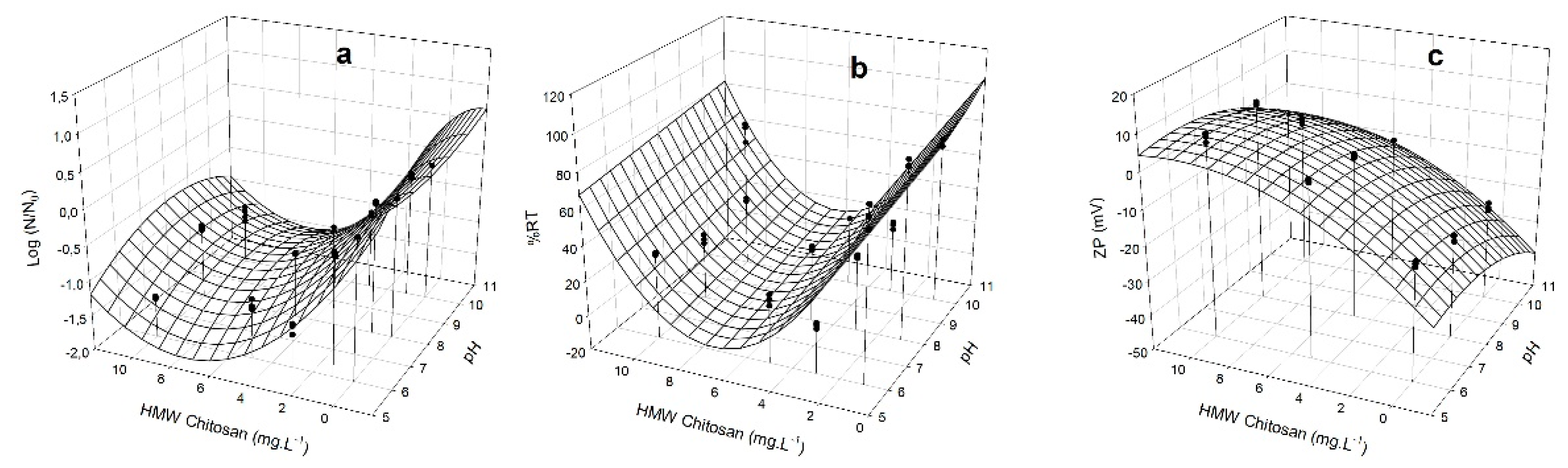

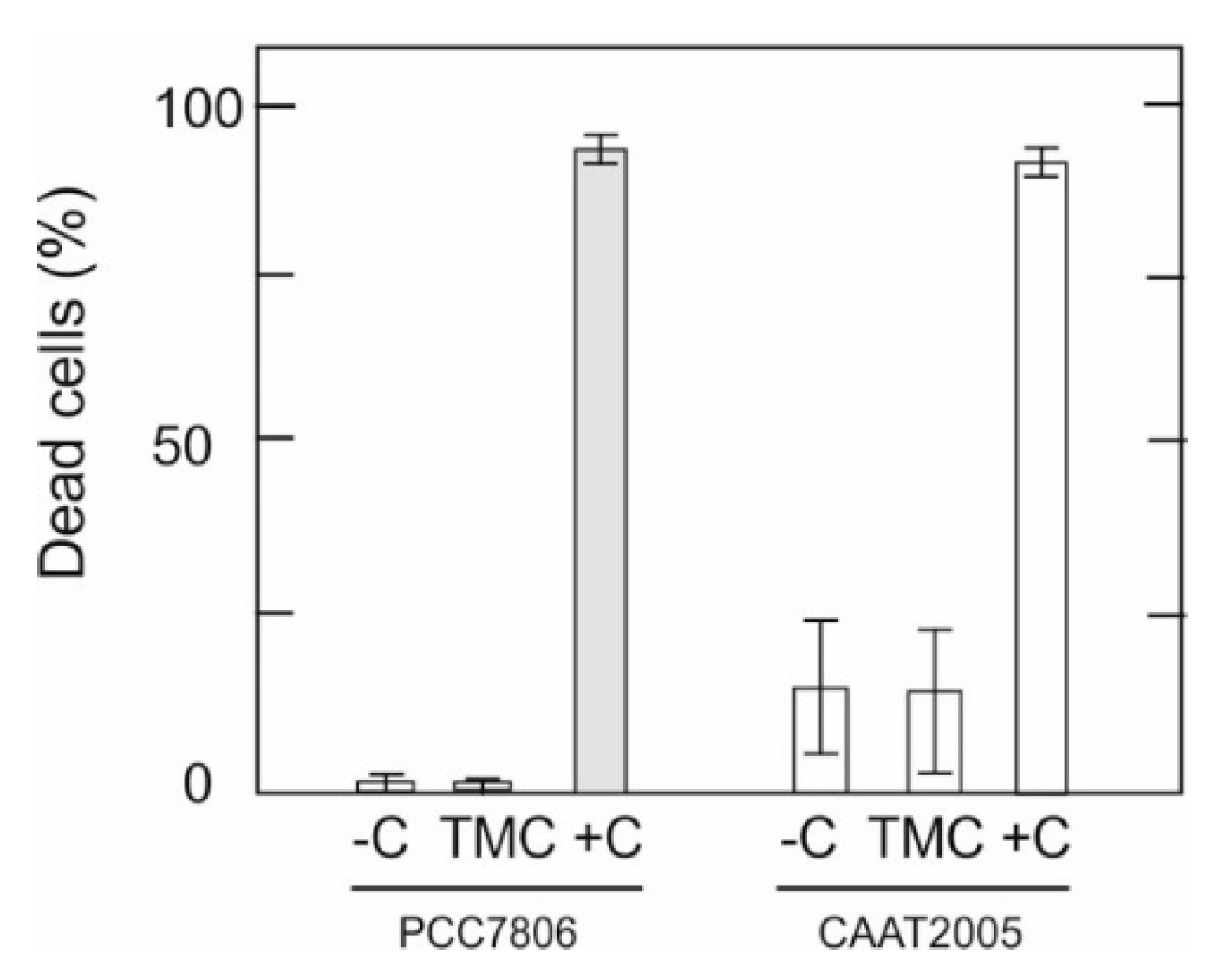
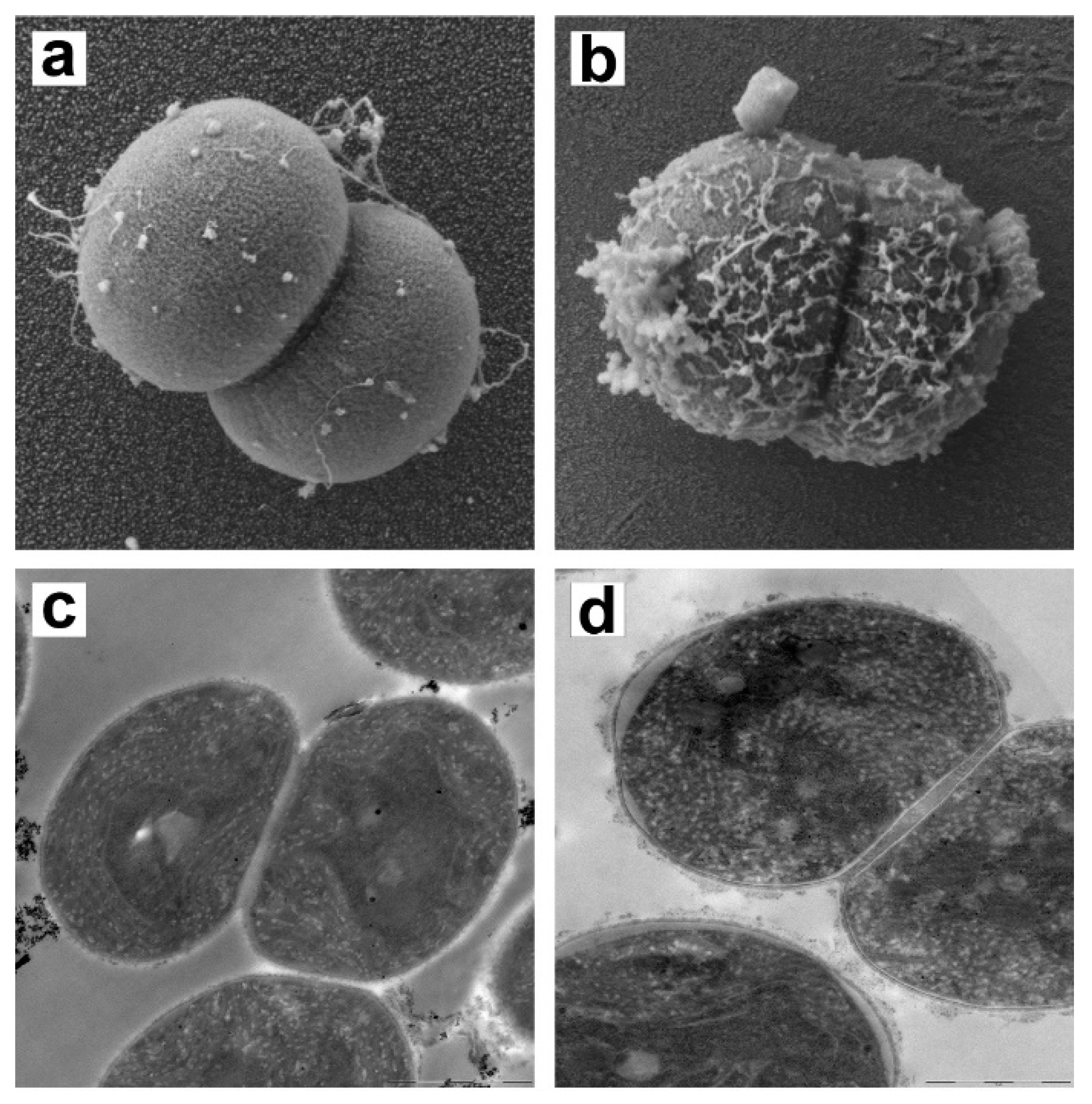
| Strain | Flocculant | Coefficients | |||||||
|---|---|---|---|---|---|---|---|---|---|
| y0 | a | b | c | d | R2 | RMSE | |||
| PCC 7806 | HMW chitosan | Log (N/N0) | −3.84 ± 1.33 | 0.911 ± 0.3444 | −0.165 ± 0.041 | −0.051 ± 0.002 | 0.0162 ± 0.003 | 0.487 | 0.271 |
| % RT | −133.8 ± 10.11 | −9.08 ± 0.25 | −33.73 ± 6.10 | 56.23 ± 1.67 | 2.28 ± 0.37 | 0.775 | 0.426 | ||
| ZP | 37.19 ± 44.33 | −14.90 11.50 | 2.34 ± 1.40 | 1.02 ± 0.72 | 0.06 ± 0.13 | 0.755 | 7.281 | ||
| TMC | Log (N/N0) | 1.36 ± 0.14 | −0.08 ± 0.03 | −0.01 ± 0.001 | 0.005 ± 0.003 | −1.10−4 ± 2.10−5 | 0.791 | 0.030 | |
| % RT | 209.78 ± 114.8 | −24.57 ± 29.64 | −6.15 ± 2.09 | 1.39 ± 1.84 | 0.002 ± 0.001 | 0.656 | 0.615 | ||
| ZP | −50.23 ± 38.79 | 4.50 ± 10.01 | 4.71 ± 0.70 | −0.35 ± 0.62 | −0.13 ± 0.04 | 0.773 | 7.892 | ||
| CAAT 2005-3 | HMW chitosan | Log (N/N0) | −3.18 ± 0.80 | 0.79 ± 0.20 | −0.39 ± 0.02 | −4.75 ± 0.01 | 0.02 ± 0.002 | 0.912 | 0.164 |
| % RT | 79.20 ± 8.14 | 2.14 ± 0.22 | −28.71 ± 3.57 | 0.008 ± 0.001 | 2.235 ± 0.314 | 0.711 | 14.842 | ||
| ZP | −42.60 ± 16.51 | 8.32 ± 4.27 | 4.27 ± 0.48 | −0.66 ± 0.27 | −0.204 ± 0.047 | 0.955 | 2.490 | ||
| TMC | Log (N/N0) | −0.50 ± 0.21 | 0.11 ± 0.08 | −0.02 ± 0.001 | −0.006 ± 0.00 | 8.9.10−4 ± 3.10−5 | 0.535 | 0.063 | |
| % RT | −20.34 ± 6.24 | 42.49 ± 15.12 | −16.67 ± 4.41 | −2.48 ± 0.94 | 0.70 ± 0.20 | 0.544 | 10.660 | ||
| ZP | 14.01 ± 18.30 | −8.25 ± 4.72 | 1.60 ± 0.33 | 0.45 ± 0.29 | −1.10−3 ± 1.10−4 | 0.837 | 3.721 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannuzzi, L.; Bacciadone, J.; Salerno, G.L. A Promising Use of Trimethyl Chitosan for Removing Microcystis aeruginosa in Water Treatment Processes. Microorganisms 2022, 10, 2052. https://doi.org/10.3390/microorganisms10102052
Giannuzzi L, Bacciadone J, Salerno GL. A Promising Use of Trimethyl Chitosan for Removing Microcystis aeruginosa in Water Treatment Processes. Microorganisms. 2022; 10(10):2052. https://doi.org/10.3390/microorganisms10102052
Chicago/Turabian StyleGiannuzzi, Leda, Julián Bacciadone, and Graciela L. Salerno. 2022. "A Promising Use of Trimethyl Chitosan for Removing Microcystis aeruginosa in Water Treatment Processes" Microorganisms 10, no. 10: 2052. https://doi.org/10.3390/microorganisms10102052
APA StyleGiannuzzi, L., Bacciadone, J., & Salerno, G. L. (2022). A Promising Use of Trimethyl Chitosan for Removing Microcystis aeruginosa in Water Treatment Processes. Microorganisms, 10(10), 2052. https://doi.org/10.3390/microorganisms10102052






