Maize Growth Promotion by Inoculation with an Engineered Ammonium-Excreting Strain of Nitrogen-Fixing Pseudomonasstutzeri
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Media
2.2. In Vitro Screening for Plant Growth-Promoting Traits
2.3. Nitrogenase Activity Assays
2.4. Ammonium Excretion Determination
2.5. Pot Experiments and Inoculation Method
2.6. 15N Dilution Assay
2.7. Field Experiment for Crop Yield
2.8. Root Sampling and Bacterial Counts
2.9. Statistical Analysis
3. Results
3.1. Properties of the Wild-Type and Mutant Derivatives
3.2. Colonization of Wild-Type and Mutant Strains on the Maize Root Surface
3.3. Effects of Wild and Mutant Strains Inoculation on Maize Growth Promotion
3.4. Effects of Inoculation with P. stutzeri Strains on Maize N Concentrations
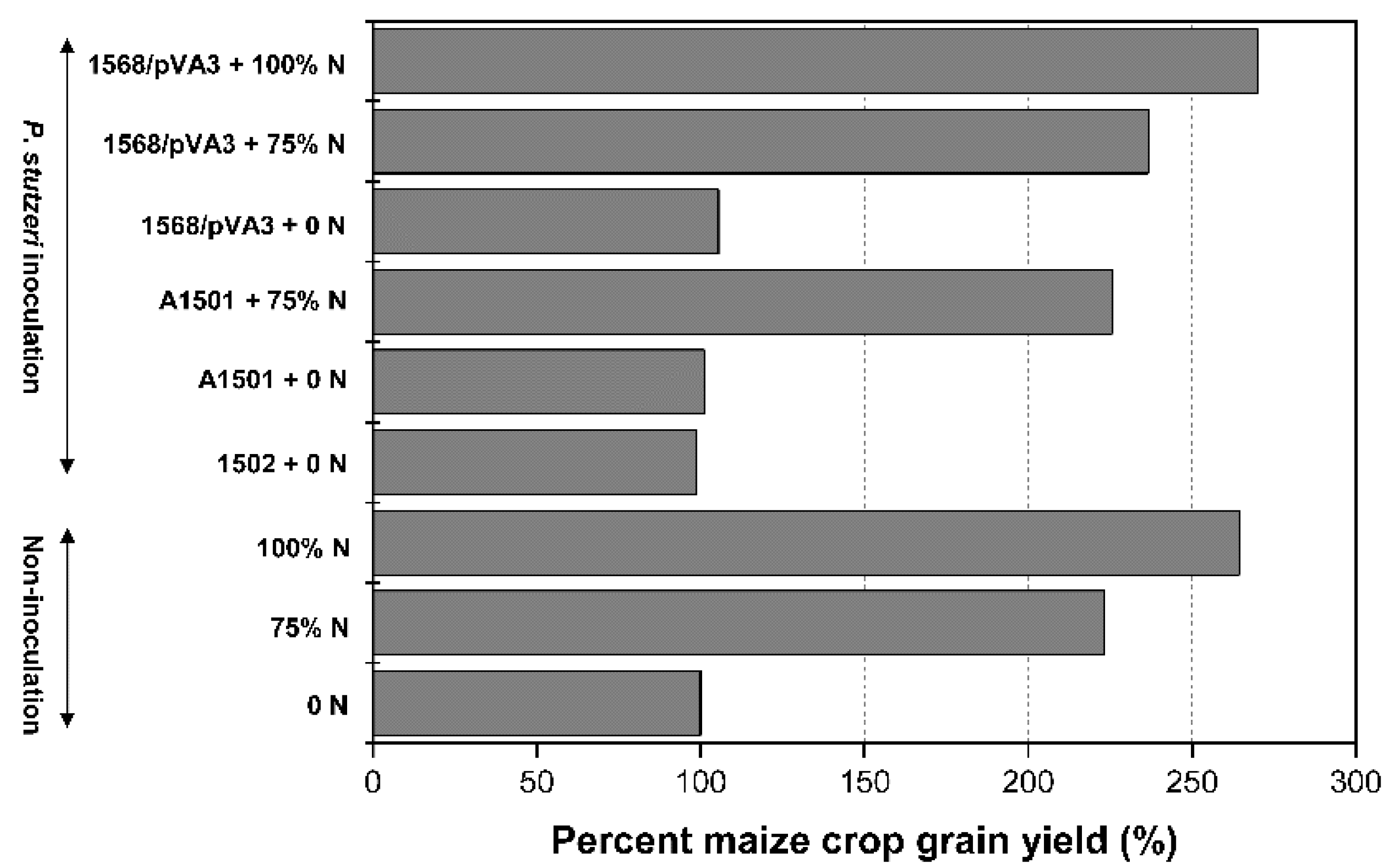
3.5. Crop Yield in Field Experiment
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Massa, F.; Defez, R.; Bianco, C. Exploitation of Plant Growth Promoting Bacteria for Sustainable Agriculture: Hierarchical Approach to Link Laboratory and Field Experiments. Microorganisms 2022, 10, 865. [Google Scholar] [CrossRef]
- Lugtenberg, B.; Kamilova, F. Plant-growth-promoting rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniel, A.I.; Fadaka, A.O.; Gokul, A.; Bakare, O.O.; Aina, O.; Fisher, S.; Burt, A.F.; Mavumengwana, V.; Keyster, M.; Klein, A. Biofertilizer: The Future of Food Security and Food Safety. Microorganisms 2022, 10, 1220. [Google Scholar] [CrossRef]
- Vejan, P.; Abdullah, R.; Khadiran, T.; Ismail, S.; Nasrulhaq Boyce, A. Role of plant growth promoting rhizobacteria in agricultural sustainability-a review. Molecules 2016, 21, 573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, J.H.; Kim, S.D. Induction of Drought Stress Resistance by Multi-Functional PGPR Bacillus licheniformis K11 in Pepper. Plant Pathol. J. 2013, 29, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Okon, Y.; Labandera-Gonzalez, C.A. Agronomic applications of Azospirillum-an evaluation of 20 years worldwide field inoculation. Soil Biol. Biochem. 1994, 26, 1591–1601. [Google Scholar] [CrossRef]
- Montañez, A.; Abreu, C.; Gill, P.; Hardarson, G.; Sicardi, M. Biological nitrogen fixation in maize (Zea mays L.) by 15N isotope-dilution and identification of associated culturable diazotrophs. Biol. Fert. Soils 2008, 45, 253–263. [Google Scholar] [CrossRef]
- Fox, A.R.; Soto, G.; Valverde, C.; Russo, D.; Lagares, A.; Zorreguieta, A.; Alleva, K.; Pascuan, C.; Frare, R.; Mercado-Blanco, J.; et al. Major cereal crops benefit from biological nitrogen fixation when inoculated with the nitrogen-fixing bacterium Pseudomonas protegens Pf-5 X940. Environ. Microbiol. 2016, 18, 3522–3534. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.T.; Rediers, H.; Ghequire, M.G.; Nguyen, H.H.; De Mot, R.; Vanderleyden, J.; Spaepen, S. The plant growth-promoting effect of the nitrogen-fixing endophyte Pseudomonas stutzeri A15. Arch. Microbiol. 2017, 199, 513–517. [Google Scholar] [CrossRef]
- Chalk, P.M. The strategic role of 15N in quantifying the contribution of endophytic N2 fixation to the N nutrition of non-legumes. Symbiosis 2016, 69, 63–80. [Google Scholar] [CrossRef]
- Sevilla, M.; Burris, R.H.; Gunapala, N.; Kennedy, C. Comparison of benefit to sugarcane plant growth and 15N2 incorporation following inoculation of sterile plants with Acetobacter diazotrophicus wild-type and nif mutant strains. Mol. Plant Microbe Interact. Interact. 2011, 14, 358–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iniguez, A.L.; Dong, Y.; Triplett, E.W. Nitrogen fixation in wheat provided by Klebsiella pneumoniae 342. Mol. Plant Microbe In. 2004, 17, 1078–1085. [Google Scholar] [CrossRef] [Green Version]
- Pankievicz, V.C.; do Amaral, F.P.; Santos, K.F.; Agtuca, B.; Xu, Y.; Schueller, M.J.; Arisi, A.C.M.; Steffen, M.B.R.; de Souza, E.M.; Pedrosa, F.O.; et al. Robust biological nitrogen fixation in a model grass-bacterial association. Plant J. 2015, 81, 907–919. [Google Scholar] [CrossRef] [PubMed]
- Christiansen-Weniger, C.; Van Veen, J.A. NH4+-excreting Azospirillum brasilense mutants enhance the nitrogen supply of a wheat host. Appl. Environ. Microbiol. 1991, 57, 3006–3012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roley, S.S.; Xue, C.; Hamilton, S.K.; Tiedje, J.M.; Roberson, G.P. Isotopic evidence for episodic nitrogen fixation in switchgrass (Panicum virgatum L.). Soil Biol. Biochem. 2019, 129, 90–98. [Google Scholar] [CrossRef]
- Turner, T.R.; James, E.K.; Poole, P.S. The plant microbiome. Genome Biol. 2013, 14, 209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alves, G.C.; Videira, S.S.; Urquiaga, S.; Reis, V.M. Differential plant growth promotion and nitrogen fixation in two genotypes of maize by several Herbaspirillum inoculants. Plant Soil. 2015, 387, 307–321. [Google Scholar] [CrossRef]
- Bueno Batista, M.; Dixon, R. Manipulating nitrogen regulation in diazotrophic bacteria for agronomic benefit. Biochem. Soc. Trans. 2019, 47, 603–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bali, A.; Blanco, G.; Hill, S.; Kennedy, C. Excretion of ammonium by a nifL mutant of Azotobacter vinelandii fixing nitrogen. Appl. Environ. Microbiol. 1992, 58, 1711–1718. [Google Scholar] [CrossRef] [Green Version]
- Setten, L.; Soto, G.; Mozzicafreddo, M.; Fox, A.R.; Lisi, C.; Cuccioloni, M.; Angeletti, M.; Pagano, E.; Diaz-Paleo, A.; Ayub, N.D. Engineering Pseudomonas protegens Pf-5 for nitrogen fixation and its application to improve plant growth under nitrogen-deficient conditions. PLoS ONE 2013, 8, e63666. [Google Scholar] [CrossRef]
- Santos, K.F.D.N.; Moure, V.R.; Hauer, V.; Santos, A.R.S.; Donatti, L.; Galvao, C.W.; Pedrosa, F.O.; Souza, E.M.; Wassem, R.; Steffen, M.B.R. Wheat colonization by an Azospirillum brasilense ammonium excreting strain reveals upregulation of nitrogenase and superior plant growth promotion. Plant Soil 2017, 367, 639–650. [Google Scholar] [CrossRef]
- Colnaghi, R.; Green, A.; He, L.; Rudnick, P.; Kennedy, C. Strategies for increased ammonium production in free-living or plant associated nitrogen fixing bacteria. Plant Soil 1997, 194, 145–154. [Google Scholar] [CrossRef]
- You, C.B.; Zhou, F. Non-nodular endorhizosperic nitrogen fixation in wetland rice. Can. J. Microbiol. 1988, 198835, 403–408. [Google Scholar]
- Vermeiren, H.; Willems, A.; Schoofs, G.; de Mot, R.; Keijers, V.; Hai, W.; Vanderleyden, J. The rice inoculant strain Alcaligenes faecalis A15 is a nitrogen-fixing Pseudomonas stutzeri. Syst. Appl. Microbiol. 1999, 22, 215–224. [Google Scholar] [CrossRef]
- Yan, Y.; Yang, J.; Dou, Y.; Chen, M.; Ping, S.; Peng, J.; Lu, W.; Zhang, W.; Yao, Z.; Li, H.; et al. Nitrogen fixation island and rhizosphere competence traits in the genome of root-associated Pseudomonas stutzeri A1501. Proc. Natl. Acad. Sci. USA 2008, 21, 7564–7569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- You, C.B.; Lin, M.; Fang, X.J.; Song, W. Attachment of Alcaligenes to rice roots. Soil Biol. Biochem. 1995, 27, 463–466. [Google Scholar] [CrossRef]
- Rediers, H.; Bonnecarrère, V.; Rainey, P.B.; Hamonts, K.; Vanderleyden, J.; De Mot, R. Development and application of a dapB-based in vivo expression technology system to study colonization of rice by the endophytic nitrogen-fixing bacterium Pseudomonas stutzeri A15. Appl. Environ. Microbiol. 2003, 69, 6864–6874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.; Wang, R.; Yang, Z.; Zhan, Y.; Ma, Y.; Ping, S.; Zhang, L.; Lin, M.; Yan, Y. 1-Aminocyclopropane-1-Carboxylate Deaminase from Pseudomonas stutzeri A1501 Facilitates the Growth of Rice in the Presence of Salt or Heavy Metals. J. Microbiol. Biotechnol. 2015, 25, 1119–1128. [Google Scholar] [CrossRef]
- Ke, X.; Feng, S.; Wang, J.; Lu, W.; Zhang, W.; Chen, M.; Lin, M. Effect of inoculation with nitrogen-fixing bacterium Pseudomonas stutzeri A1501 on maize plant growth and the microbiome indigenous to the rhizosphere. Syst. Appl. Microbiol. 2019, 42, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yan, Y.; He, S.; Ping, S.; Alam, K.M.; Han, Y.; Liu, X.; Lu, W.; Zhang, W.; Chen, M.; et al. Involvement of the ammonium transporter AmtB in nitrogenase regulation and ammonium excretion in Pseudomonas stutzeri A1501. Res. Microbiol. 2012, 163, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Zhou, S.; Mo, X.; You, C.; Wang, D. Investigation of dinitrogen fixation bacteria isolated from rice rhizosphere. Chin. Sci. Bull. 1981, 26, 383–384. [Google Scholar]
- Desnoues, N.; Lin, M.; Guo, X.; Ma, L.; Carrenolopez, R.; Elmerich, C. Nitrogen fixation genetics and regulation in a Pseudomonas stutzeri strain associated with rice. Microbiology 2003, 149, 2251–2262. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Sitepu, I.R.; Tang, S.Y.; Hashidoko, Y. Salkowski’s reagent test as a primary screening index for functionalities of rhizobacteria isolated from wild dipterocarp saplings growing naturally on medium-strongly acidic tropical peat soil. Biosci. Biotechnol. Biochem. 2010, 74, 202–208. [Google Scholar] [CrossRef] [Green Version]
- Fiske, C.H.; Subbarow, Y.J. The colorimetric determination of phosphorus. Biol. Chem. 1925, 66, 375–400. [Google Scholar] [CrossRef]
- Pande, A.; Pandey, P.; Mehra, S.; Singh, M.; Kaushik, S. Phenotypic and genotypic characterization of phosphate solubilizing bacteria and their efficiency on the growth of maize. J. Genet. Eng. Biotechnol. 2017, 15, 379–391. [Google Scholar] [CrossRef]
- Honma, S.; and Shimomura, T. Metabolism of 1-aminocyclopropane-1-carboxylic acid. Agric. Biol. Chem. 1978, 42, 1825–1831. [Google Scholar] [CrossRef]
- Zhan, Y.; Yan, Y.; Deng, Z.; Chen, M.; Lu, W.; Lu, C.; Shang, L.; Yang, Z.; Zhang, W.; Wang, W.; et al. The novel regulatory ncRNA, NfiS, optimizes nitrogen fixation via base pairing with the nitrogenase gene nifK mRNA in Pseudomonas stutzeri A1501. Proc. Natl. Acad. Sci. USA 2016, 113, E4348–E4356. [Google Scholar] [CrossRef] [Green Version]
- Murase, J.; Noll, M.; Frenzel, P. Impact of protists on the activity and structure of the bacterial community in a rice field soil. Appl. Environ. Microbiol. 2006, 72, 5436–5444. [Google Scholar] [CrossRef] [Green Version]
- Bageshwar, U.K.; Srivastava, M.; Pardha-Saradhi, P.; Paul, S.; Gothandapani, S.; Jaat, R.S.; Shankar, P.; Yadav, R.; Biswas, D.R.; Kumar, P.A.; et al. An environmentally friendly engineered Azotobacter strain that replaces a substantial amount of urea fertilizer while sustaining the same wheat yield. Appl. Environ. Microbiol. 2017, 83, e00590-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christiansen-Weniger, C.; Vanderleyden, J. Ammonium-excreting Azospirillum sp. become intracellularly established in maize (Zea mays) para-nodules. Biol. Fertil. Soils 1994, 17, 1–8. [Google Scholar] [CrossRef]
- Van Dommelen, A.; Croonenborghs, A.; Spaepen, S.; Vanderleyden, J. Wheat growth promotion through inoculation with an ammonium-excreting mutant of Azospirillum brasilense. Biol. Fert. Soils 2009, 45, 549–553. [Google Scholar] [CrossRef]
- Fukami, J.; Nogueira, M.A.; Araujo, R.S.; Hungria, M. Accessing inoculation methods of maize and wheat with Azospirillum brasilense. AMB Express 2016, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, F.O.; Oliveira, A.L.M.; Guimarães, V.F.; Etto, R.M.; Souza, E.M.; Furmam, F.G.; Gonçalves, D.R.P.; Santos, O.J.A.P.; Gonçalves, L.S.A.; Battistus, A.G.; et al. The ammonium excreting Azospirillum brasilense strain HM053: A new alternative inoculant for maize. Plant Soil 2020, 451, 45–56. [Google Scholar] [CrossRef]
- Cassán, F.; Diaz-Zorita, M. Azospirillum sp. in current agriculture: From the laboratory to the field. Soil Biol. Biochem. 2016, 103, 117–130. [Google Scholar] [CrossRef]
- Naqqash, T.; Hameed, S.; Imran, A.; Hanif, M.K.; Majeed, A.; van Elsas, J.D. Differential Response of Potato Toward Inoculation with Taxonomically Diverse Plant Growth Promoting Rhizobacteria. Front. Plant. Sci. 2016, 7, 144. [Google Scholar] [CrossRef] [Green Version]
- Souza, R.D.; Ambrosini, A.; Passaglia, L.M. Plant growth-promoting bacteria as inoculants in agricultural soils. Genet. Mol. Biol. 2015, 38, 401–419. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Marquez, J.C.; Do Nascimento, M.; Dublan Mde, L.; Curatti, L. Association with an ammonium-excreting bacterium allows diazotrophic culture of oil-rich eukaryotic microalgae. Appl. Environ. Microbiol. 2012, 78, 2345–2352. [Google Scholar] [CrossRef] [Green Version]
- Ramos, L.D.L.; Rodrigues, E.P.; Silva, M.B.; Oliveira, J.E.; Zuluaga, M.Y.A.; Milani, K.M.L.; Oliveira, A.L.M.D. Ammonium excretion, auxin production and effects of maize inoculation with ethylenediamine-resistant mutants of Pseudomonas sp. Bragantia 2018, 77, 415–428. [Google Scholar] [CrossRef] [Green Version]
- Beneduzi, A.; Ambrosini, A.; Passaglia, L.M. Plant growth-promoting rhizobacteria (PGPR): Their potential as antagonists and biocontrol agents. Genet. Mol. Biol. 2012, 35, 1044–1051. [Google Scholar] [CrossRef] [Green Version]
- Mathimaran, N.; Jegan, S.; Thimmegowda, M.N.; Prabavathy, V.R.; Yuvaraj, P.; Kathiravan, R.; Sivakumar, M.N.; Manjunatha, B.N.; Bhavitha, N.C.; Sathish, A.; et al. Intercropping transplanted pigeon pea with finger millet: Arbuscular mycorrhizal fungi and plant growth promoting rhizobacteria boost yield while reducing fertilizer input. Front. Sustain. Food Syst. 2020, 4, 88. [Google Scholar] [CrossRef]
- Lalucat, J.; Bennasar, A.; Bosch, R.; García-Valdés, E.; Palleroni, N.J. Biology of Pseudomonas stutzeri. Microbiol. Mol. Biol. Rev. 2006, 70, 510–547. [Google Scholar] [CrossRef] [PubMed]
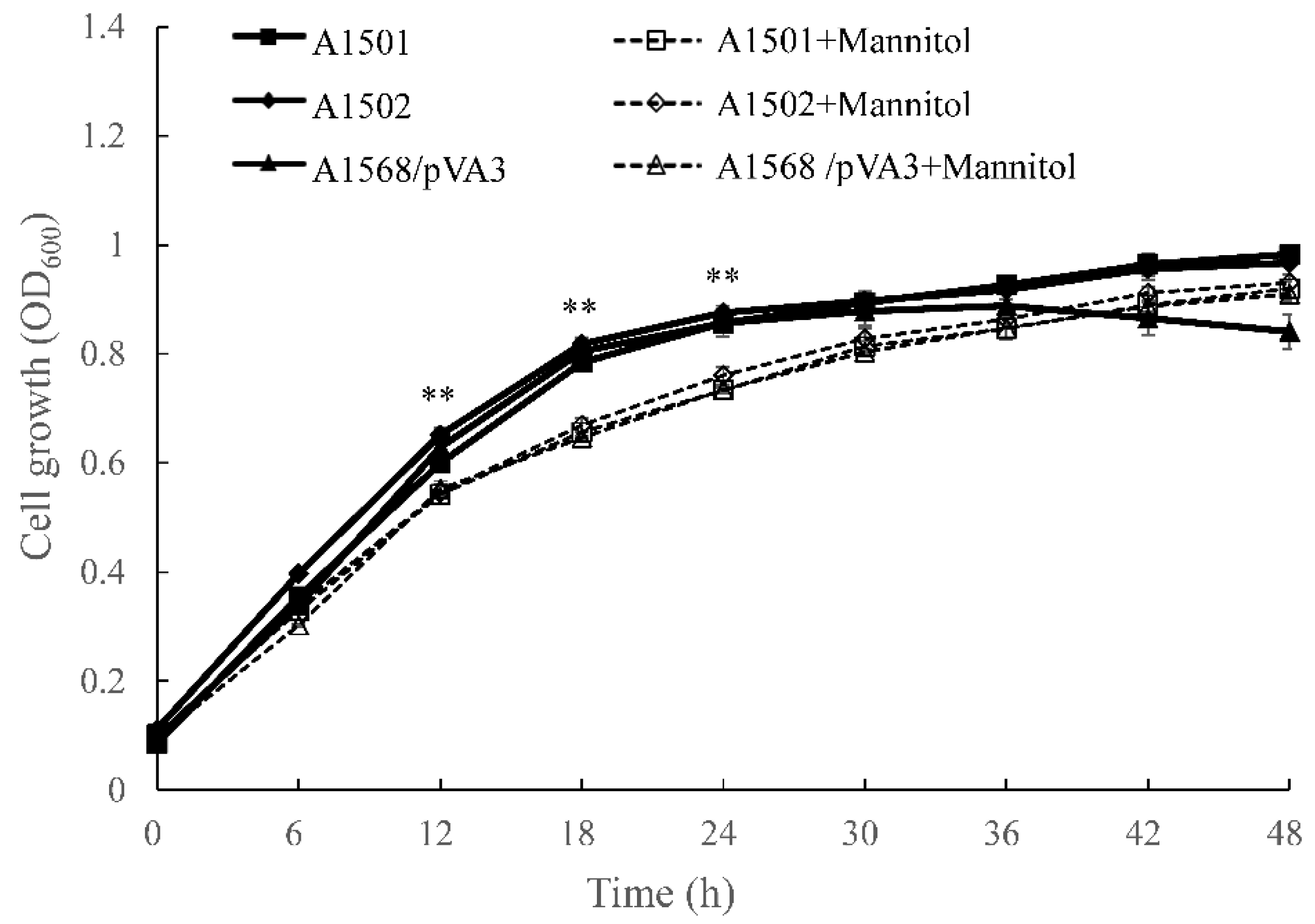

| Strains | Mannitol-Treated Condition | Phosphate Solubilization Ability | IAA Production (mg/L) | ACC Deaminase Activity (μmol a-Ketobutyrate mg Protein−1 h−1) | Nitrogenase Activity (nmol of Ethylene mg Protein−1 h−1) | Extracellular Ammonium Concentration (μM) | ||
|---|---|---|---|---|---|---|---|---|
| Diameter of Halo (cm) | Diameter of Colony (cm) | PSI | ||||||
| 1568/pVA3 | 0 | 1.70 ± 0.01 a | 1.11 ± 0.02 a | 1.53 ± 0.04 a | 28.61 ± 1.11 b | 3.88 ± 0.10 a | 1792.3 ± 98.7 b | 20.3 ± 0.4 b |
| 200 mM | 1.74 ± 0.02 a | 1.13 ± 0.02 a | 1.54 ± 0.06 a | 32.67 ± 0.99 c | 3.74 ± 0.06 a | 1675.1 ± 112.9 ab | 18.2 ± 0.2 a | |
| A1501 | 0 | 1.68 ± 0.04 a | 1.06 ± 0.03 a | 1.59 ± 0.12 a | 22.88 ± 1.17 a | 3.80 ± 0.09 a | 1618.2 ± 101.4 ab | n.d. |
| 200 mM | 1.72 ± 0.02 a | 1.10 ± 0.02 a | 1.57 ± 0.07 a | 29.26 ± 2.47 b | 3.85 ± 0.11 a | 1564.8 ± 90.6 a | n.d. | |
| nifH-mutant | 0 | 1.69 ± 0.03 a | 1.12 ± 0.01 a | 1.51 ± 0.05 a | 24.79 ± 2.30 a | 3.76 ± 0.05 a | n.d. | n.d. |
| 200 mM | 1.73 ± 0.03 a | 1.12 ± 0.04 a | 1.55 ± 0.10 a | 28.14 ± 1.03 b | 3.81 ± 0.07 a | n.d. | n.d. | |
| Inoculant Treatments | SW (g) | RW (g) | SL (cm) | RL (cm) | Bacteria Root Colonization (CFU g−1) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| WS | WW | WS | WW | WS | WW | WS | WW | WS | WW | |
| 1568/pVA3 | 22.7 ± 0.9 b | 53.1 ± 2.8 c | 5.1 ± 0.2 c | 10.4 ± 0.9 c | 61.1 ± 4.2 b | 74.3 ± 2.8 b | 21.2 ± 3.8 a | 30.7 ± 3.5 a | 8.15 × 105 b | 1.54 × 107 b |
| A1501 | 21.2 ± 0.8 b | 47.6 ± 3.9 b | 4.9 ± 0.3 bc | 9.8 ± 0.6 c | 59.7 ± 3.6 b | 67.6 ± 4.0 b | 20.9 ± 2.4 a | 29.8 ± 2.6 a | 9.09 × 105 b | 1.46 × 107 b |
| nifH-mutant | 18.1 ± 1.0 a | 39.4 ± 2.7 a | 4.7 ± 0.1 b | 8.1 ± 0.3 b | 49.3 ± 3.0 a | 58.9 ± 1.8 a | 20.5 ± 3.9 a | 29.4 ± 3.3 a | 1.04 × 106 b | 1.38 × 107 b |
| No inoculation | 17.3 ± 0.5 a | 37.4 ± 3.4 a | 4.3 ± 0.1 a | 6.8 ± 1.1 a | 48.2 ± 1.4 a | 57.4 ± 3.3 a | 20.1 ± 4.2 a | 29.1 ± 4.2 a | 7.02 × 102 a | 2.26 × 102 a |
| Inoculant Treatments | Shoot N Concentrations (%) | Shoot Atom% 15N Excess (%) | % Ndfa | Total N Fixed (g/Plant) | ||||
|---|---|---|---|---|---|---|---|---|
| WS | WW | WS | WW | WS | WW | WS | WW | |
| 1568/pVA3 | 2.23 ± 0.06 b | 2.44 ± 0.07 b | 3.98 ± 0.12 b | 3.73 ± 0.16 b | 18.9 ± 1.2 b | 28.4 ± 0.9 b | 0.13 ± 0.01 b | 0.39 ± 0.04 b |
| A1501 | 2.17 ± 0.03 ab | 2.38 ± 0.05 ab | 4.17 ± 0.20 b | 3.99 ± 0.22 b | 15.1 ± 0.7 a | 23.3 ± 1.0 a | 0.09 ± 0.01 a | 0.29 ± 0.02 a |
| nifH-mutant | 2.18 ± 0.02 ab | 2.30 ± 0.06 a | 4.91 ± 0.13 a | 5.21 ± 0.10 a | - | - | - | - |
| No inoculation | 2.12 ± 0.08 a | 2.31 ± 0.02 a | 5.10 ± 0.09 a | 5.34 ± 0.17 a | - | - | - | - |
| Water Treatment | Inoculant Treatment | NH4+-N (mg/kg) | NO3−N (mg/kg) | Organic C (mg/kg) |
|---|---|---|---|---|
| WS | 1568/pVA3 | 10.7 ± 1.03 b | 5.42 ± 0.40 b | 10.43 ± 0.05 a |
| A1501 | 2.47 ± 0.11 a | 3.91 ± 0.23 a | 11.10 ± 0.03 a | |
| nifH-mutant | 2.61 ± 0.06 a | 4.04 ± 0.13 a | 10.56 ± 0.07 a | |
| Non-inoculation | 2.50 ± 0.09 a | 3.90 ± 0.14 a | 11.02 ± 0.02 a | |
| WW | 1568/pVA3 | 13.3 ± 1.26 b | 8.91 ± 0.51 b | 11.21 ± 0.10 a |
| A1501 | 2.83 ± 0.07 a | 6.23 ± 0.62 a | 10.49 ± 0.06 a | |
| nifH-mutant | 2.87 ± 0.10 a | 6.42 ± 0.98 a | 10.84 ± 1.02 a | |
| Non-inoculation | 2.90 ± 0.08 a | 6.73 ± 0.80 a | 10.88 ± 0.09 a |
| Inoculant Treatments | Nitrogen Application | SH (cm) | SDW (g/Plant) | NC (mg/g) | LL (cm) | WL (cm) | DS (cm) | RN (no./Plant) | GN (no./Plant) | TSW (g) | GY (t/ha) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 N | 245.1 ± 2.0 ab | 232.1 ± 3.8 b | 18.1 ± 0.2 b | 97.9 ± 1.1 a | 9.7 ± 0.1 b | 2.76 ± 0.05 bc | 16.6 ± 0.2 ab | 37.1 ± 1.2 ab | 369.4 ± 10.0 b | 4.75 ± 0.04 b | |
| 1568/pVA3 | 75% N | 250.2 ± 1.4 c | 248.2 ± 1.3 c | 19.3 ± 0.2 c | 101.4 ± 1.5 b | 10.3 ± 0.2 b | 2.89 ± 0.07 c | 16.8 ± 0.3 b | 38.2 ± 1.0 b | 416.3 ± 7.5 cd | 10.65 ± 0.04 d |
| 100% N | 253.2 ± 1.8 d | 276.1 ± 4.2 f | 21.3 ± 0.3 d | 105.0 ± 1.0 c | 11.0 ± 0.1 c | 3.01 ± 0.08 d | 16.9 ± 0.1 b | 39.7 ± 1.3 bc | 443.7 ± 8.8 e | 12.15 ± 0.07 e | |
| 0 N | 242.8 ± 1.1 a | 230.0 ± 1.7 b | 17.4 ± 0.1 ab | 97.0 ± 1.5 a | 9.4 ± 0.1 a | 2.69 ± 0.04 b | 16.3 ± 0.2 a | 35.7 ± 0.9 a | 352.9 ± 9.1 a | 4.55 ± 0.03 a | |
| A1501 | 75% N | 247.5 ± 2.9 bc | 251.6 ± 2.3 c | 18.9 ± 0.3 c | 99.5 ± 1.7 b | 9.9 ± 0.2 b | 2.80 ± 0.06 c | 16.4 ± 0.1 a | 37.8 ± 1.8 b | 402.5 ± 11.3 c | 10.15 ± 0.08 c |
| 100% N | 251.4 ± 1.0 cd | 278.3 ± 4.5 f | 19.9 ± 0.2 d | 104.6 ± 1.4 c | 10.9 ± 0.1 c | 2.93 ± 0.04 d | 16.8 ± 0.2 b | 38.9 ± 1.6 bc | 431.7 ± 9.2 e | 11.60 ± 0.09 e | |
| 0 N | 241.2 ± 1.2 a | 224.5 ± 1.6 a | 17.2 ± 0.1 a | 95.7 ± 1.2 a | 9.2 ± 0.1 a | 2.60 ± 0.07 a | 16.2 ± 0.1 a | 35.9 ± 1.5 a | 350.6 ± 12.0 a | 4.45 ± 0.05 a | |
| nifH-mutant | 75% N | 249.9 ± 1.1 c | 259.1 ± 1.9 d | 18.8 ± 0.3 c | 98.3 ± 1.4 ab | 9.8 ± 0.1 b | 2.86 ± 0.02 c | 16.5 ± 0.1 a | 38.2 ± 2.0 bc | 402.5 ± 10.7 c | 9.95 ± 0.08 c |
| 100% N | 255.6 ± 2.7 d | 270.4 ± 3.2 e | 20.5 ± 0.2 d | 104.2 ± 1.0 c | 10.8 ± 0.2 c | 2.98 ± 0.04 d | 16.8 ± 0.3 b | 39.9 ± 1.1 bc | 434.7 ± 8.7 e | 12.00 ± 0.04 e | |
| 0 N | 242.6 ± 2.8 a | 228.5 ± 2.4 ab | 17.5 ± 0.1 a | 96.8 ± 1.0 a | 9.3 ± 0.1 a | 2.62 ± 0.03 a | 16.3 ± 0.1 a | 36.6 ± 1.3 a | 355.3 ± 11.2 a | 4.50 ± 0.05 a | |
| No inoculation | 75% N | 249.1 ± 2.5 c | 258.1 ± 2.9 d | 19.0 ± 0.2 c | 99.3 ± 1.3 ab | 10.1 ± 0.1 b | 2.83 ± 0.05 c | 16.8 ± 0.3 b | 38.9 ± 1.8 bc | 410.1 ± 9.8 cd | 10.05 ± 0.06 c |
| 100% N | 254.4 ± 3.1 d | 279.8 ± 3.1 f | 20.7 ± 0.4 d | 103.1 ± 2.2 bc | 11.2 ± 0.1 c | 2.99 ± 0.02 d | 16.9 ± 0.2 b | 40.2 ± 2.1 c | 440.1 ± 10.5 e | 11.90 ± 0.08 e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, S.; Li, J.; Wang, Q.; Yin, C.; Zhan, Y.; Yan, Y.; Lin, M.; Ke, X. Maize Growth Promotion by Inoculation with an Engineered Ammonium-Excreting Strain of Nitrogen-Fixing Pseudomonasstutzeri. Microorganisms 2022, 10, 1986. https://doi.org/10.3390/microorganisms10101986
Jiang S, Li J, Wang Q, Yin C, Zhan Y, Yan Y, Lin M, Ke X. Maize Growth Promotion by Inoculation with an Engineered Ammonium-Excreting Strain of Nitrogen-Fixing Pseudomonasstutzeri. Microorganisms. 2022; 10(10):1986. https://doi.org/10.3390/microorganisms10101986
Chicago/Turabian StyleJiang, Shanshan, Jiang Li, Qingyu Wang, Changyan Yin, Yuhua Zhan, Yongliang Yan, Min Lin, and Xiubin Ke. 2022. "Maize Growth Promotion by Inoculation with an Engineered Ammonium-Excreting Strain of Nitrogen-Fixing Pseudomonasstutzeri" Microorganisms 10, no. 10: 1986. https://doi.org/10.3390/microorganisms10101986
APA StyleJiang, S., Li, J., Wang, Q., Yin, C., Zhan, Y., Yan, Y., Lin, M., & Ke, X. (2022). Maize Growth Promotion by Inoculation with an Engineered Ammonium-Excreting Strain of Nitrogen-Fixing Pseudomonasstutzeri. Microorganisms, 10(10), 1986. https://doi.org/10.3390/microorganisms10101986






