Greek Remdesivir Cohort (GREC) Study: Effectiveness of Antiviral Drug Remdesivir in Hospitalized Patients with COVID-19 Pneumonia
Abstract
:1. Introduction
2. Materials and Methods
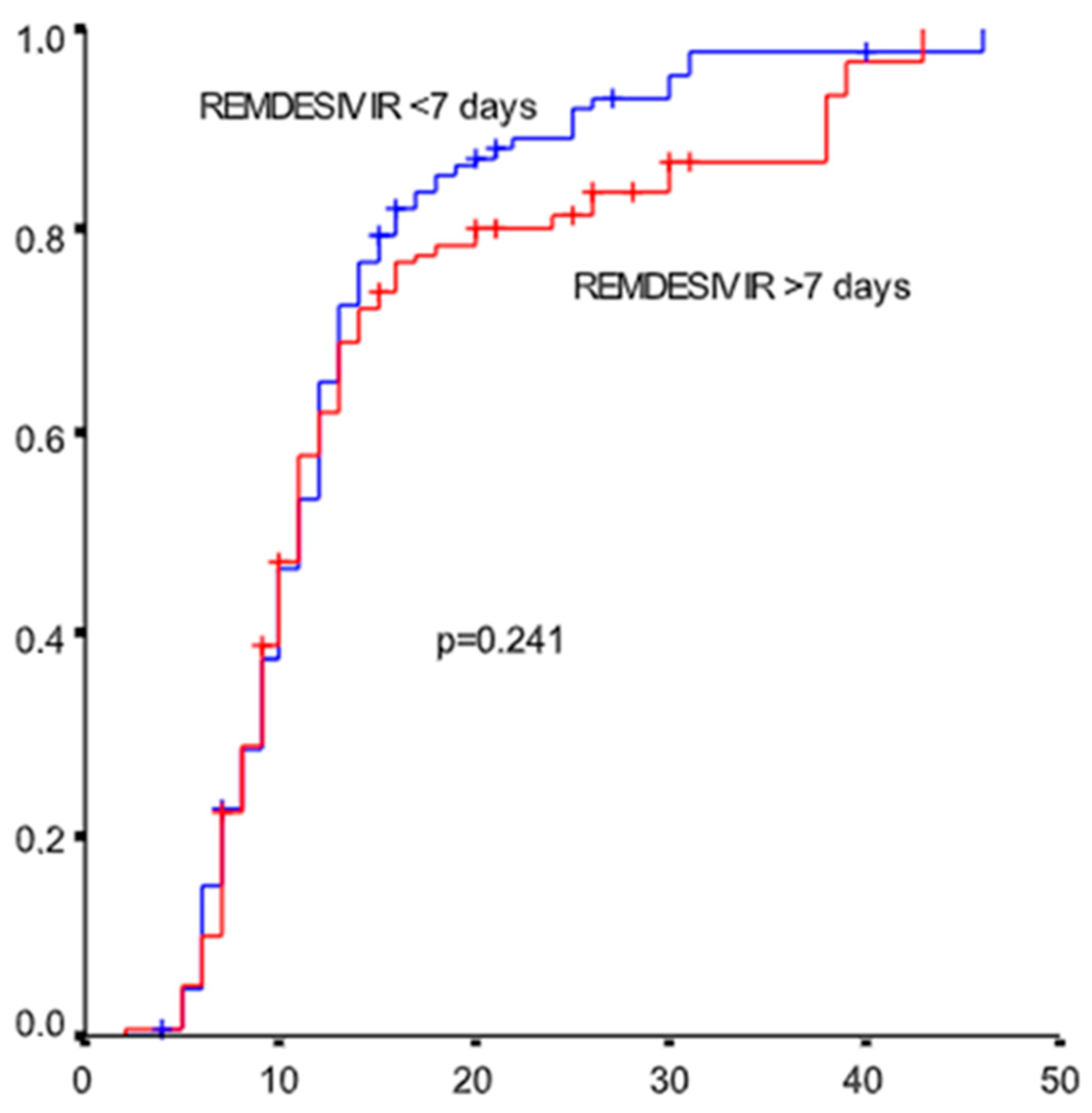
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef]
- World Health Organization. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (accessed on 26 August 2022).
- Agostini, M.L.; Andres, E.L.; Sims, A.C.; Graham, R.L.; Sheahan, T.P.; Lu, X.; Smith, E.C.; Case, J.B.; Feng, J.Y.; Jordan, R.; et al. Coronavirus Susceptibility to the Antiviral Remdesivir (GS-5734) Is Mediated by the Viral Polymerase and the Proofreading Exoribonuclease. mBio 2018, 9, e00221-e18. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.J.; Tchesnokov, E.P.; Woolner, E.; Perry, J.K.; Feng, J.Y.; Porter, D.P.; Götte, M. Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. J. Biol. Chem. 2020, 295, 6785–6797. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, B.; Thakur, S.S. Remdesivir and Its Combination with Repurposed Drugs as COVID-19 Therapeutics. Front. Immunol. 2022, 13, 830990. [Google Scholar] [CrossRef]
- Kokic, G.; Hillen, H.S.; Tegunov, D.; Dienemann, C.; Seitz, F.; Schmitzova, J.; Farnung, L.; Siewert, A.; Höbartner, C.; Cramer, P. Mechanism of SARS-CoV-2 polymerase stalling by remdesivir. Nat. Commun. 2021, 12, 279. [Google Scholar] [CrossRef]
- Malin, J.J.; Suárez, I.; Priesner, V.; Fätkenheuer, G.; Rybniker, J. Remdesivir against COVID-19 and Other Viral Diseases. Clin. Microbiol. Rev. 2020, 34, e00162-20. [Google Scholar] [CrossRef]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the Treatment of COVID-19—Final Report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef]
- WHO Solidarity Trial Consortium. Remdesivir and three other drugs for hospitalised patients with COVID-19: Final results of the WHO Solidarity randomised trial and updated meta-analyses. Lancet 2022, 399, 1941–1953. [Google Scholar] [CrossRef]
- Ali, K.; Azher, T.; Baqi, M.; Binnie, A.; Borgia, S.; Carrier, F.M.; Cavayas, Y.A.; Chagnon, N.; Cheng, M.P.; Conly, J.; et al. Remdesivir for the treatment of patients in hospital with COVID-19 in Canada: A randomized controlled trial. Can. Med. Assoc. J. 2022, 194, E242–E251. [Google Scholar] [CrossRef]
- European Respiratory Journal. Available online: https://erj.ersjournals.com/content/57/4/2100048 (accessed on 26 August 2022).
- Infectious Diseases Society of America. Available online: https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management (accessed on 26 August 2022).
- National Institutes of Health. Available online: https://www.covid19treatmentguidelines.nih.gov/management/clinical-management-of-adults/hospitalized-adults--therapeutic-management (accessed on 26 August 2022).
- Hagman, K.; Hedenstierna, M.; Gille-Johnson, P.; Hammas, B.; Grabbe, M.; Dillner, J.; Ursing, J. Severe Acute Respiratory Syndrome Coronavirus 2 RNA in Serum as Predictor of Severe Outcome in Coronavirus Disease 2019: A Retrospective Cohort Study. Clin. Infect. Dis. 2021, 73, e2995–e3001. [Google Scholar] [CrossRef]
- Hogan, C.A.; Stevens, B.A.; Sahoo, M.K.; Huang, C.; Garamani, N.; Gombar, S.; Yamamoto, F.; Murugesan, K.; Kurzer, J.; Zehnder, J.; et al. High Frequency of SARS-CoV-2 RNAemia and Association with Severe Disease. Clin. Infect. Dis. 2021, 72, e291–e295. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.K.H.; Lau, K.T.K.; Au, I.C.H.; Xiong, X.; Chung, M.S.H.; Lau, E.H.Y.; Cowling, B.J. Optimal Timing of Remdesivir Initiation in Hospitalized Patients with Coronavirus Disease 2019 (COVID-19) Administered with Dexamethasone. Clin. Infect. Dis. 2022, 75, e499–e508. [Google Scholar] [CrossRef] [PubMed]
- Spinner, C.D.; Gottlieb, R.L.; Criner, G.J.; Arribas López, J.R.; Cattelan, A.M.; Soriano Viladomiu, A.; Ogbuagu, O.; Malhotra, P.; Mullane, K.M.; Castagna, A.; et al. Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients with Moderate COVID-19: A Randomized Clinical Trial. JAMA 2020, 324, 1048–1057. [Google Scholar] [CrossRef]
- Gottlieb, R.L.; Vaca, C.E.; Paredes, R.; Mera, J.; Webb, B.J.; Perez, G.; Oguchi, G.; Ryan, P.; Nielsen, B.U.; Brown, M.; et al. Early Remdesivir to Prevent Progression to Severe COVID-19 in Outpatients. N. Engl. J. Med. 2022, 386, 305–315. [Google Scholar] [CrossRef]
- European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/veklury (accessed on 26 August 2022).
- Ader, F.; Bouscambert-Duchamp, M.; Hites, M.; Peiffer-Smadja, N.; Poissy, J.; Belhadi, D.; Diallo, A.; Lê, M.P.; Peytavin, G.; Staub, T.; et al. Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): A phase 3, randomised, controlled, open-label trial. Lancet Infect. Dis. 2022, 22, 209–221. [Google Scholar] [CrossRef]
- WHO Solidarity Trial Consortium; Pan, H.; Peto, R.; Henao-Restrepo, A.M.; Preziosi, M.P.; Sathiyamoorthy, V.; Abdool Karim, Q.; Alejandria, M.M.; Hernández García, C.; Kieny, M.P.; et al. Repurposed Antiviral Drugs for COVID-19—Interim WHO Solidarity Trial Results. N. Engl. J. Med. 2021, 384, 497–511. [Google Scholar] [PubMed]
- Garibaldi, B.T.; Wang, K.; Robinson, M.L.; Betz, J.; Caleb Alexander, G.; Andersen, K.M.; Joseph, C.S.; Mehta, H.B.; Korwek, K.; Sands, K.E.; et al. Real-World Effectiveness of Remdesivir in Adults Hospitalized with Coronavirus Disease 2019 (COVID-19): A Retrospective, Multicenter Comparative Effectiveness Study. Clin. Infect. Dis. 2022, 75, e516–e524. [Google Scholar] [CrossRef] [PubMed]
- Olender, S.A.; Walunas, T.L.; Martinez, E.; Perez, K.K.; Castagna, A.; Wang, S.; Kurbegov, D.; Goyal, P.; Ripamonti, D.; Balani, B.; et al. Remdesivir Versus Standard-of-Care for Severe Coronavirus Disease 2019 Infection: An Analysis of 28-Day Mortality. Open Forum Infect. Dis. 2021, 8, ofab278. [Google Scholar] [CrossRef]
- Benfield, T.; Bodilsen, J.; Brieghel, C.; Harboe, Z.B.; Helleberg, M.; Holm, C.; Israelsen, S.B.; Jensen, J.; Jensen, T.Ø.; Johansen, I.S.; et al. Improved Survival Among Hospitalized Patients with Coronavirus Disease 2019 (COVID-19) Treated with Remdesivir and Dexamethasone. A Nationwide Population-Based Cohort Study. Clin. Infect. Dis. 2021, 73, 2031–2036. [Google Scholar] [CrossRef]
- Joo, E.J.; Ko, J.H.; Kim, S.E.; Kang, S.J.; Baek, J.H.; Heo, E.Y.; Shi, H.J.; Eom, J.S.; Choe, P.G.; Bae, S.; et al. Clinical and Virologic Effectiveness of Remdesivir Treatment for Severe Coronavirus Disease 2019 (COVID-19) in Korea: A Nationwide Multicenter Retrospective Cohort Study. J. Korean Med. Sci. 2021, 36, e83. [Google Scholar] [CrossRef]
- Arch, B.; Kovacs, D.; Scott, J.T.; Jones, A.P.; Harrison, E.M.; Rosala-Hallas, A.; Gamble, C.G.; Openshaw, P.J.M.; Baillie, J.K.; Semple, M.G.; on behalf of ISARIC4C Investigators. Evaluation of the effectiveness of remdesivir in treating severe COVID-19 using data from the ISARIC WHO Clinical Characterisation Protocol UK: A prospective, national cohort study. medRxiv 2021. [Google Scholar] [CrossRef]
- Mozaffari, E.; Chandak, A.; Zhang, Z.; Liang, S.; Thrun, M.; Gottlieb, R.L.; Kuritzkes, D.R.; Sax, P.E.; Wohl, D.A.; Casciano, R.; et al. Remdesivir Treatment in Hospitalized Patients with Coronavirus Disease 2019 (COVID-19): A Comparative Analysis of In-hospital All-cause Mortality in a Large Multicenter Observational Cohort. Clin. Infect. Dis. 2022, 75, e450–e458. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vidal, C.; Meira, F.; Cózar-Llistó, A.; Dueñas, G.; Puerta-Alcalde, P.; Garcia-Pouton, N.; Chumbita, M.; Cardozo, C.; Hernandez-Meneses, M.; Alonso-Navarro, R.; et al. Real-life use of remdesivir in hospitalized patients with COVID-19. Rev. Esp. Quimioter. 2021, 34, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vidal, C.; Alonso, R.; Camon, A.M.; Cardozo, C.; Albiach, L.; Agüero, D.; Marcos, M.A.; Ambrosioni, J.; Bodro, M.; Chumbita, M.; et al. Impact of remdesivir according to the pre-admission symptom duration in patients with COVID-19. J. Antimicrob. Chemother. 2021, 76, 3296–3302. [Google Scholar] [CrossRef] [PubMed]
- Paranjape, N.; Husain, M.; Priestley, J.; Koonjah, Y.; Watts, C.; Havlik, J. Early Use of Remdesivir in Patients Hospitalized With COVID-19 Improves Clinical Outcomes: A Retrospective Observational Study. Infect. Dis. Clin. Pract. 2021, 29, e282–e286. [Google Scholar] [CrossRef]
- French, G.; Hulse, M.; Nguyen, D.; Sobotka, K.; Webster, K.; Corman, J.; Aboagye-Nyame, B.; Dion, M.; Johnson, M.; Zalinger, B.; et al. Impact of hospital strain on excess deaths during the COVID-19 pandemic-United States, July 2020–July 2021. Am. J. Transplant. 2022, 22, 654–657. [Google Scholar] [CrossRef]
- Wikipedia. Available online: https://en.wikipedia.org/wiki/COVID-19_pandemic_in_Greece (accessed on 26 August 2022).
- National Public Health Organization. Available online: https://eody.gov.gr/neos-koronaios-covid-19/ (accessed on 26 August 2022).
- Hellenic Society for Infectious Diseases. Available online: https://www.loimoxeis.gr/covid-19-info-banner/ (accessed on 26 August 2022).
| Total | Initiation ≤7 Days | Initiation >7 Days | p Value | |
|---|---|---|---|---|
| Male gender, n (%) | 335 (60.8) | 210 (59.8) | 125 (62.5) | 0.537 |
| Age | 59.97 ± 14.38 | 60.40 ± 14.93 | 59.23 ± 13.34 | 0.356 |
| Immunomodulators | 157 (28.5) | 83 (23.6) | 74 (37.0) | 0.001 |
| Comorbidities | 0.145 | |||
| Hypertension | 221 (40.1) | 150 (42.7) | 71 (35.5) | 0.096 |
| Diabetes | 112 (20.3) | 76 (21.7) | 36 (18.0) | 0.306 |
| Dyslipidemia | 152 (27.6) | 103 (29.3) | 49 (24.5) | 0.221 |
| CVD | 62 (11.3) | 44 (12.5) | 18 (9.0) | 0.207 |
| None | 234 (42.5) | 141 (40.2) | 93 (46.5) | |
| One | 152 (27.6) | 95 (27.1) | 57 (28.5) | |
| Two or more | 165 (29.9) | 115 (32.8) | 50 (25.0) | |
| Recovery | 496 (90.0) | 320 (91.2) | 176 (88.0) | 0.233 |
| Time to recovery | 9 (6–13) | 9 (6–12) | 9 (6–12.75) | 0.370 |
| Intubation | 60 (10.9) | 30 (8.5) | 30 (15.0) | 0.019 |
| Time to intubation | 10.5 (3.5–14.75) | 8.5 (3–12.5) | 12 (8.25–17.25) | 0.067 |
| Mortality at day 14th | 16 (3.7) | 10 (3.6) | 6 (3.8) | 0.884 |
| Mortality (overall) | 55 (10.0) | 31 (8.8) | 24 (12.0) | 0.233 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrakis, V.; Rapti, V.; Akinosoglou, K.; Bonelis, C.; Athanasiou, K.; Dimakopoulou, V.; Syrigos, N.K.; Spernovasilis, N.; Trypsianis, G.; Marangos, M.; et al. Greek Remdesivir Cohort (GREC) Study: Effectiveness of Antiviral Drug Remdesivir in Hospitalized Patients with COVID-19 Pneumonia. Microorganisms 2022, 10, 1949. https://doi.org/10.3390/microorganisms10101949
Petrakis V, Rapti V, Akinosoglou K, Bonelis C, Athanasiou K, Dimakopoulou V, Syrigos NK, Spernovasilis N, Trypsianis G, Marangos M, et al. Greek Remdesivir Cohort (GREC) Study: Effectiveness of Antiviral Drug Remdesivir in Hospitalized Patients with COVID-19 Pneumonia. Microorganisms. 2022; 10(10):1949. https://doi.org/10.3390/microorganisms10101949
Chicago/Turabian StylePetrakis, Vasilis, Vasiliki Rapti, Karolina Akinosoglou, Constantinos Bonelis, Kalomoira Athanasiou, Vasiliki Dimakopoulou, Nikolaos K. Syrigos, Nikolaos Spernovasilis, Grigoris Trypsianis, Markos Marangos, and et al. 2022. "Greek Remdesivir Cohort (GREC) Study: Effectiveness of Antiviral Drug Remdesivir in Hospitalized Patients with COVID-19 Pneumonia" Microorganisms 10, no. 10: 1949. https://doi.org/10.3390/microorganisms10101949
APA StylePetrakis, V., Rapti, V., Akinosoglou, K., Bonelis, C., Athanasiou, K., Dimakopoulou, V., Syrigos, N. K., Spernovasilis, N., Trypsianis, G., Marangos, M., Gogos, C., Papazoglou, D., Panagopoulos, P., & Poulakou, G. (2022). Greek Remdesivir Cohort (GREC) Study: Effectiveness of Antiviral Drug Remdesivir in Hospitalized Patients with COVID-19 Pneumonia. Microorganisms, 10(10), 1949. https://doi.org/10.3390/microorganisms10101949








