Response of Carbon Emissions and the Bacterial Community to Freeze–Thaw Cycles in a Permafrost-Affected Forest–Wetland Ecotone in Northeast China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Soil Sampling and Experimental Design
2.3. Soil Microbial Analysis
2.4. Data Analysis
3. Results
3.1. Soil Carbon Emissions of Forest and Wetland Patches after FTCs
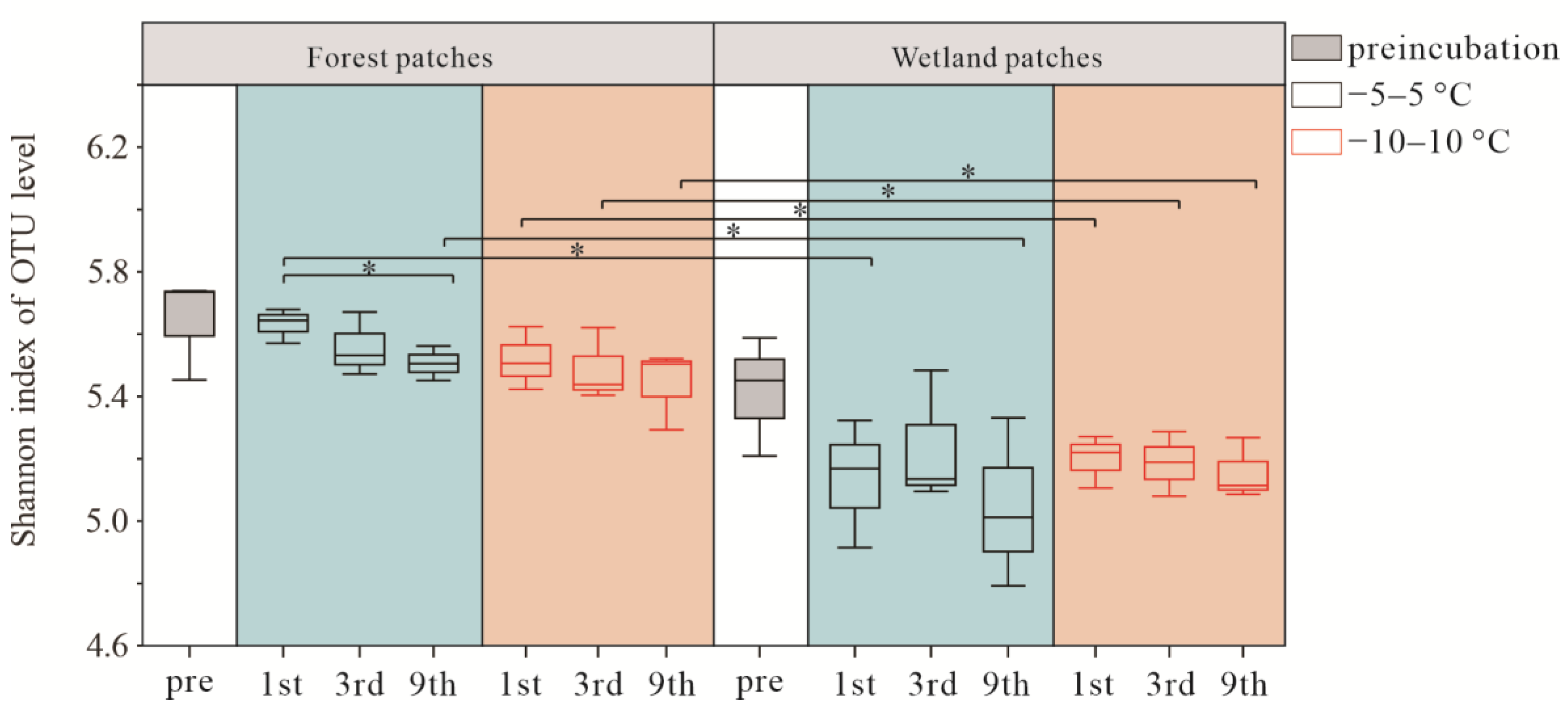
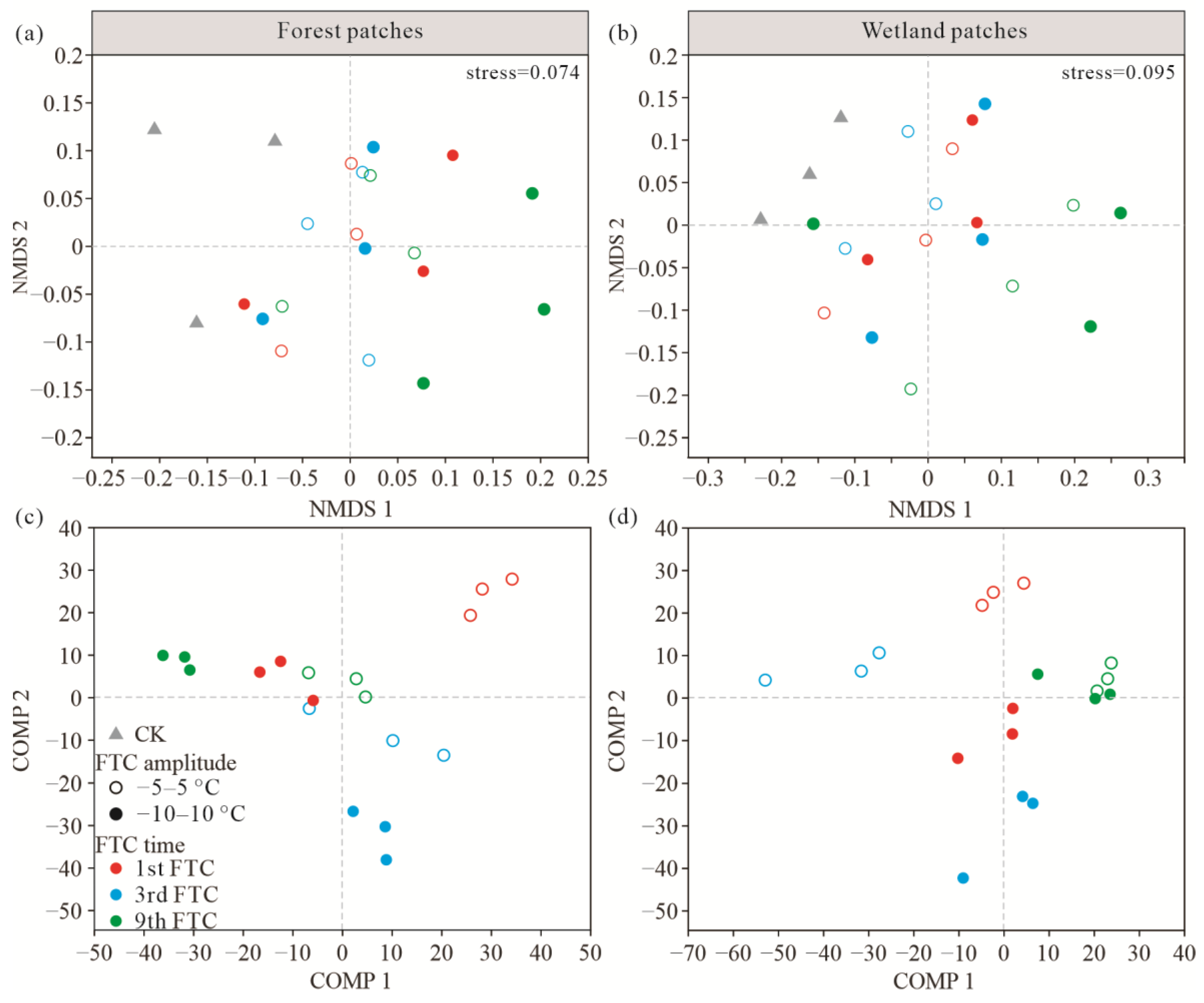
3.2. Soil Bacterial Community Composition and Diversity after FTCs
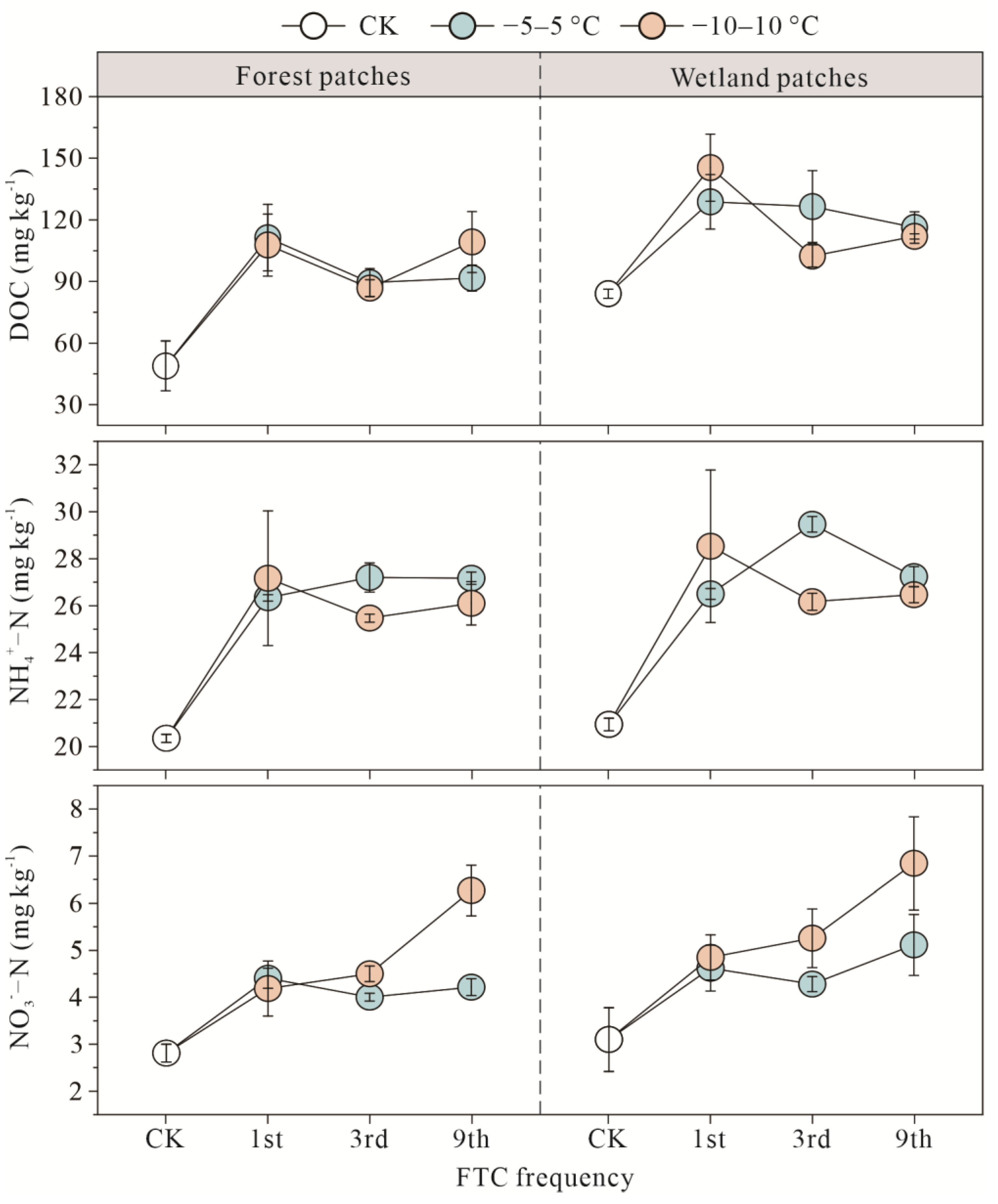
3.3. Soil Substrates of Forest and Wetland Patches after FTCs
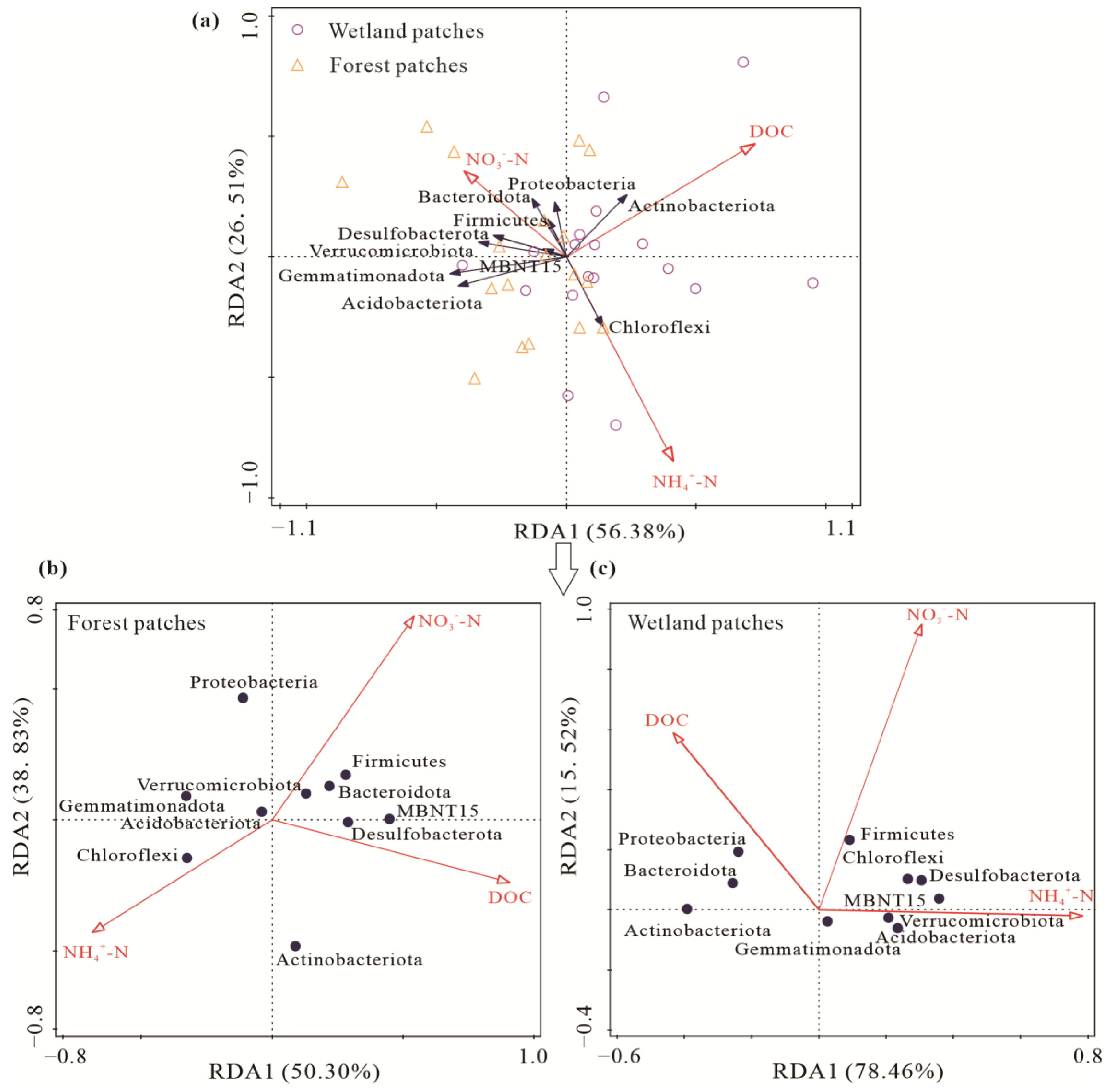
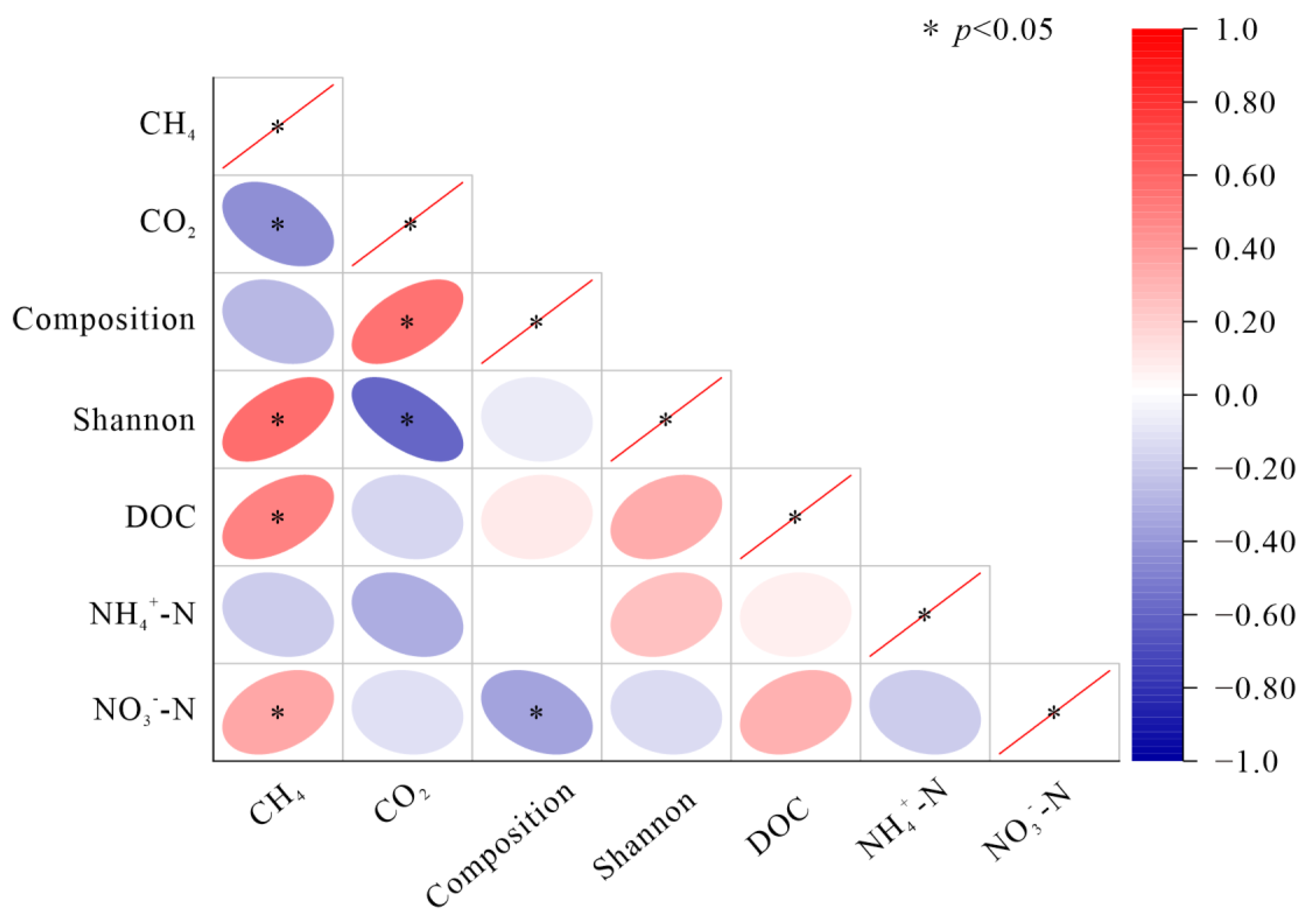
3.4. Relationship among Soil Carbon Emissions, the Soil Bacterial Community, and Soil Substrates
4. Discussion
4.1. Response of Carbon and Nitrogen to FTCs
4.2. Soil Bacterial Community Composition and Diversity Respond to FTCs
4.3. Response of Soil Carbon Emissions to FTCs and Its Relationship with the Soil Bacterial Community and Soil Substrates
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FTCs | freeze–thaw cycles |
| CO2 | carbon dioxide |
| CH4 | methane |
| DOC | dissolved organic carbon |
| NH4+-N | ammonium nitrogen |
| NO3−-N | nitrate nitrogen |
| RDA | redundancy analysis |
| NMDS | non-metric multidimensional scaling |
| PLS-DA | partial least squares discriminant analysis |
References
- Arias, P.; Bellouin, N.; Coppola, E.; Jones, R.; Krinner, G.; Marotzke, J.; Naik, V.; Palmer, M.; Plattner, G.K.; Rogelj, J.; et al. Climate Change 2021: The Physical Science Basis. Contribution of Working Group14 I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Technical Summary; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Mu, C.C.; Abbott, B.W.; Norris, A.J.; Mu, M.; Fan, C.Y.; Chen, X.; Jia, L.; Yang, R.M.; Zhang, T.J.; Wang, K. The status and stability of permafrost carbon on the Tibetan Plateau. Earth Sci. Rev. 2020, 211, 103433. [Google Scholar] [CrossRef]
- Schimel, D.S. Terrestrial ecosystem and carbon cycle. Glob. Chang. Biol. 2006, 1, 77–91. [Google Scholar] [CrossRef]
- Song, Y.Y.; Liu, C.; Song, C.C.; Wang, X.W.; Ma, X.Y.; Gao, J.L.; Gao, S.Q.; Wang, L.L. Linking soil organic carbon mineralization with soil microbial and substrate properties under warming in permafrost peatlands of Northeastern China. CATENA 2021, 203, 105348. [Google Scholar] [CrossRef]
- Hollesen, J.; Elberling, B.; Jansson, P.E. Future active layer dynamics and carbon dioxide production from thawing permafrost layers in Northeast Greenland. Glob. Chang. Biol. 2011, 17, 911–926. [Google Scholar] [CrossRef]
- Jin, H.J.; Yu, Q.H.; Lü, L.Z.; Guo, D.X.; He, R.X.; Yu, S.P.; Sun, G.Y.; Li, Y.W. Degradation of permafrost in the Xing’anling Mountains, Northeastern China. Permafr. Periglac. Process. 2007, 18, 245–258. [Google Scholar] [CrossRef]
- Li, X.Y.; Jin, H.J.; Wang, H.W.; Marchenko, S.S.; Shan, W.; Luo, D.L.; He, R.X.; Spektor, V.; Huang, Y.D.; Li, X.Y. Influences of forest fires on the permafrost environment: A review. Adv. Clim. Chang. Res. 2021, 12, 48–65. [Google Scholar] [CrossRef]
- Schuur, E.A.; McGuire, A.D.; Schädel, C.; Grosse, G.; Harden, J.; Hayes, D.J.; Hugelius, G.; Koven, C.D.; Kuhry, P.; Lawrence, D.M. Climate change and the permafrost carbon feedback. Nature 2015, 520, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.D.; Zhao, L.; Hu, G.J.; Liu, G.M.; Li, W.P.; Ding, Y.J. Permafrost and land cover as controlling factors for light fraction organic matter on the southern Qinghai-Tibetan Plateau. Sci. Total Environ. 2018, 613, 1165–1174. [Google Scholar] [CrossRef]
- Koven, C.D.; Ringeval, B.; Friedlingstein, P.; Ciais, P.; Cadule, P.; Khvorostyanov, D.; Krinner, G.; Tarnocai, C. Permafrost carbon-climate feedbacks accelerate global warming. Proc. Natl. Acad. Sci. USA 2011, 108, 14769–14774. [Google Scholar] [CrossRef] [PubMed]
- Zimov, S.A.; Schuur, E.A.; Chapin, F.S., III. Permafrost and the global carbon budget. Science 2006, 312, 1612–1613. [Google Scholar] [CrossRef]
- Edwards, L.M. The effects of soil freeze–thaw on soil aggregate breakdown and concomitant sediment flow in Prince Edward Island: A review. Can. J. Soil Sci. 2013, 93, 459–472. [Google Scholar] [CrossRef]
- Congreves, K.; Wagner-Riddle, C.; Si, B.; Clough, T.J. Nitrous oxide emissions and biogeochemical responses to soil freezing-thawing and drying-wetting. Soil Biol. Biochem. 2018, 117, 5–15. [Google Scholar] [CrossRef]
- Groffman, P.M.; Hardy, J.P.; Fashu-Kanu, S.; Driscoll, C.T.; Cleavitt, N.L.; Fahey, T.J.; Fisk, M.C. Snow depth, soil freezing and nitrogen cycling in a northern hardwood forest landscape. Biogeochemistry 2011, 102, 223–238. [Google Scholar] [CrossRef]
- Margesin, R.; Neuner, G.; Storey, K.B. Cold-loving microbes, plants, and animals—Fundamental and applied aspects. Naturwissenschaften 2007, 94, 77–99. [Google Scholar] [CrossRef]
- Yang, J.S.; Zhou, W.M.; Liu, J.S.; Hu, X.J. Dynamics of greenhouse gas formation in relation to freeze/thaw soil depth in a flooded peat marsh of Northeast China. Soil Biol. Biochem. 2014, 75, 202–210. [Google Scholar] [CrossRef]
- Song, C.C.; Zhang, J.B.; Wang, Y.Y.; Wang, Y.S.; Zhao, Z.C. Emission of CO2, CH4 and N2O from freshwater marsh in northeast of China. J. Environ. Manag. 2008, 88, 428–436. [Google Scholar] [CrossRef]
- Song, C.C.; Xu, X.F.; Sun, X.X.; Tian, H.Q.; Sun, L.; Miao, Y.Q.; Wang, X.W.; Guo, Y.D. Large methane emission upon spring thaw from natural wetlands in the northern permafrost region. Environ. Res. Lett. 2012, 7, 034009. [Google Scholar] [CrossRef]
- Wisser, D.; Marchenko, S.; Talbot, J.; Treat, C.; Frolking, S. Soil temperature response to 21st century global warming: The role of and some implications for peat carbon in thawing permafrost soils in North America. Earth Syst. Dyn. 2011, 2, 121–138. [Google Scholar] [CrossRef]
- Jorgenson, M.T.; Racine, C.H.; Walters, J.C.; Osterkamp, T.E. Permafrost degradation and ecological changes associated with a warmingclimate in central Alaska. Clim. Chang. 2001, 48, 551–579. [Google Scholar] [CrossRef]
- Yang, Z.P.; Ou, Y.H.; Xu, X.L.; Zhao, L.; Song, M.H.; Zhou, C.P. Effects of permafrost degradation on ecosystems. Acta Ecol. Sin. 2010, 30, 33–39. [Google Scholar] [CrossRef]
- Gill, A.L.; Giasson, M.A.; Yu, R.; Finzi, A.C. Deep peat warming increases surface methane and carbon dioxide emissions in a black spruce-dominated ombrotrophic bog. Glob. Chang. Biol. 2017, 23, 5398–5411. [Google Scholar] [CrossRef]
- Ward, S.E.; Ostle, N.J.; Oakley, S.; Quirk, H.; Henrys, P.A.; Bardgett, R.D. Warming effects on greenhouse gas fluxes in peatlands are modulated by vegetation composition. Ecol. Lett. 2013, 16, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Chistyakov, K.; Amosov, M.; Kurochkin, Y.N.; Lessovaia, S.; Strelkov, I.; Tyusov, G. Tundra and Steppe Locations of Altai Highland Depressions: Features of Vegetation Community and Soil Cover and Their Thermal Regime. Contemp. Probl. Ecol. 2021, 14, 231–240. [Google Scholar] [CrossRef]
- Jin, H.J.; Jin, X.Y.; He, R.X.; Luo, D.L.; Chang, X.L.; Wang, S.L.; Marchenko, S.S.; Yang, S.Z.; Yi, C.L.; Li, S.J. Evolution of permafrost in China during the last 20 ka. Sci. China Earth Sci. 2019, 62, 1207–1223. [Google Scholar] [CrossRef]
- Dong, X.F.; Liu, C.; Li, M.; Ma, D.L.; Chen, Q.; Zang, S.Y. Variations in active layer soil hydrothermal dynamics of typical wetlands in permafrost region in the Great Hing’an Mountains, northeast China. Ecol. Indic. 2021, 129, 107880. [Google Scholar] [CrossRef]
- Song, Y.Y.; Song, C.C.; Shi, F.X.; Wang, M.Q.; Ren, J.S.; Wang, X.W.; Jiang, L. Linking plant community composition with the soil C pool, N availability and enzyme activity in boreal peatlands of Northeast China. Appl. Soil Ecol. 2019, 140, 144–154. [Google Scholar] [CrossRef]
- Schuman, G.; Reeder, J.; Manley, J.; Hart, R.; Manley, W. Impact of grazing management on the carbon and nitrogen balance of a mixed-grass rangeland. Ecol. Appl. 1999, 9, 65–71. [Google Scholar] [CrossRef]
- Teklay, T.; Shi, Z.; Attaeian, B.; Chang, S.X. Temperature and substrate effects on C & N mineralization and microbial community function of soils from a hybrid poplar chronosequence. Appl. Soil Ecol. 2010, 46, 413–421. [Google Scholar]
- Qin, S.Q.; Kou, D.; Mao, C.; Chen, Y.L.; Chen, L.Y.; Yang, Y.H. Temperature sensitivity of permafrost carbon release mediated by mineral and microbial properties. Sci. Adv. 2021, 7, eabe3596. [Google Scholar] [CrossRef]
- Schroeder, J.; Kammann, L.; Helfrich, M.; Tebbe, C.C.; Poeplau, C. Impact of common sample pre-treatments on key soil microbial properties. Soil Biol. Biochem. 2021, 160, 108321. [Google Scholar] [CrossRef]
- Wu, X.; Wang, F.F.; Li, T.; Fu, B.J.; Lv, Y.H.; Liu, G.H. Nitrogen additions increase N2O emissions but reduce soil respiration and CH4 uptake during freeze–thaw cycles in an alpine meadow. Geoderma 2020, 363, 114157. [Google Scholar] [CrossRef]
- Wang, J.Y.; Song, C.C.; Hou, A.X.; Wang, L.L. CO2 emissions from soils of different depths of a permafrost peatland, Northeast China: Response to simulated freezing–thawing cycles. J. Plant Nutr. Soil Sci. 2014, 177, 524–531. [Google Scholar] [CrossRef]
- Lang, M.; Cai, Z.C.; Chang, S.X. Effects of land use type and incubation temperature on greenhouse gas emissions from Chinese and Canadian soils. J. Soils Sediments 2011, 11, 15–24. [Google Scholar] [CrossRef]
- Ghani, A.; Dexter, M.; Perrott, K. Hot-water extractable carbon in soils: A sensitive measurement for determining impacts of fertilisation, grazing and cultivation. Soil Biol. Biochem. 2003, 35, 1231–1243. [Google Scholar] [CrossRef]
- Lu, R.K. Soil Agricultural Chemical Analysis Method; China Agricultural Science and Technology Press: Beijing, China, 2000; pp. 1–315. [Google Scholar]
- Wood, T.E.; Lawrence, D. No short-term change in soil properties following four-fold litter addition in a Costa Rican rain forest. Plant Soil 2008, 307, 113–122. [Google Scholar]
- De Jonge, L.; Jacobsen, O.; Moldrup, P. Soil water repellency: Effects of water content, temperature, and particle size. Soil Sci. Soc. Am. J. 1999, 63, 437–442. [Google Scholar] [CrossRef]
- Chen, S.F.; Zhou, Y.Q.; Chen, Y.R.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Mago, T.; Salzberg, S.L. FLASH: Fast Length Adjustment of Short Reads to Improve Genome Assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996. [Google Scholar] [CrossRef]
- Stackebrandt, E.; Goebel, B.M. Taxonomic note: A place for DNA-DNA reassociation and 16S rRNA sequence analysis in the present species definition in bacteriology. Int. J. Syst. Evol. Microbiol. 1994, 44, 846–849. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Gerald, B. A Brief Review of Independent, Dependent and One Sample t-test. Int. J. Appl. Math. Theor. Phys. 2018, 4, 50. [Google Scholar] [CrossRef] [Green Version]
- van Eeuwijk, F.A.; Kroonenberg, P.M. Multiplicative models for interaction in three-way ANOVA, with applications to plant breeding. Biometrics 1998, 54, 1315–1333. [Google Scholar] [CrossRef]
- Bubier, J.L.; Moore, T.R.; Bellisario, L.; Comer, N.T.; Crill, P.M. Ecological controls on methane emissions from a northern peatland complex in the zone of discontinuous permafrost, Manitoba, Canada. Glob. Biogeochem. Cycles 1995, 9, 455–470. [Google Scholar] [CrossRef]
- Van Den Wollenberg, A.L. Redundancy analysis an alternative for canonical correlation analysis. Psychometrika 1977, 42, 207–219. [Google Scholar] [CrossRef]
- Messan, K.S.; Jones, R.M.; Doherty, S.J.; Foley, K.; Douglas, T.A.; Barbato, R.A. The role of changing temperature in microbial metabolic processes during permafrost thaw. PLoS ONE 2020, 15, e0232169. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, Y.-H.; Oono, Y. Relational patterns of gene expression via non-metric multidimensional scaling analysis. Bioinformatics 2005, 21, 730–740. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.D.; Zhao, L.; Liu, G.M.; Xu, H.Y.; Zhang, X.L.; Ding, Y.J. Effects of permafrost thaw-subsidence on soil bacterial communities in the southern Qinghai-Tibetan Plateau. Appl. Soil Ecol. 2018, 128, 81–88. [Google Scholar] [CrossRef]
- Gibbons, J.D.; Chakraborti, S. Comparisons of the Mann-Whitney, Student’st, and alternate t tests for means of normal distributions. J. Exp. Educ. 1991, 59, 258–267. [Google Scholar] [CrossRef]
- Wang, J.Y.; Song, C.C.; Wang, X.W.; Song, Y.Y. Changes in labile soil organic carbon fractions in wetland ecosystems along a latitudinal gradient in Northeast China. CATENA 2012, 96, 83–89. [Google Scholar] [CrossRef]
- Feng, X.J.; Nielsen, L.L.; Simpson, M.J. Responses of soil organic matter and microorganisms to freeze–thaw cycles. Soil Biol. Biochem. 2007, 39, 2027–2037. [Google Scholar] [CrossRef]
- Grogan, P.; Michelsen, A.; Ambus, P.; Jonasson, S. Freeze–thaw regime effects on carbon and nitrogen dynamics in sub-arctic heath tundra mesocosms. Soil Biol. Biochem. 2004, 36, 641–654. [Google Scholar] [CrossRef]
- Yu, X.F.; Zou, Y.C.; Jiang, M.; Lu, X.G.; Wang, G.P. Response of soil constituents to freeze–thaw cycles in wetland soil solution. Soil Biol. Biochem. 2011, 43, 1308–1320. [Google Scholar] [CrossRef]
- Song, Y.; Zou, Y.C.; Wang, G.P.; Yu, X.F. Altered soil carbon and nitrogen cycles due to the freeze-thaw effect: A meta-analysis. Soil Biol. Biochem. 2017, 109, 35–49. [Google Scholar] [CrossRef]
- Vestgarden, L.S.; Austnes, K. Effects of freeze–thaw on C and N release from soils below different vegetation in a montane system: A laboratory experiment. Glob. Chang. Biol. 2009, 15, 876–887. [Google Scholar] [CrossRef]
- Herrmann, A.; Witter, E. Sources of C and N contributing to the flush in mineralization upon freeze–thaw cycles in soils. Soil Biol. Biochem. 2002, 34, 1495–1505. [Google Scholar] [CrossRef]
- Henry, H.A. Soil freeze–thaw cycle experiments: Trends, methodological weaknesses and suggested improvements. Soil Biol. Biochem. 2007, 39, 977–986. [Google Scholar] [CrossRef]
- Gao, D.C.; Bai, E.; Yang, Y.; Zong, S.W.; Hagedorn, F. A global meta-analysis on freeze-thaw effects on soil carbon and phosphorus cycling. Soil Biol. Biochem. 2021, 159, 108283. [Google Scholar] [CrossRef]
- Risk, N.; Snider, D.; Wagner-Riddle, C. Mechanisms leading to enhanced soil nitrous oxide fluxes induced by freeze–thaw cycles. Can. J. Soil Sci. 2013, 93, 401–414. [Google Scholar] [CrossRef]
- Xiao, L.; Zhang, Y.; Li, P.; Xu, G.; Shi, P.; Zhang, Y. Effects of freeze-thaw cycles on aggregate-associated organic carbon and glomalin-related soil protein in natural-succession grassland and Chinese pine forest on the Loess Plateau. Geoderma 2019, 334, 1–8. [Google Scholar] [CrossRef]
- Zhang, B.L.; Wu, X.K.; Zhang, W.; Chen, X.M.; Zhang, G.S.; Ai, X.; Sun, L.K.; Zhang, B.G.; Liu, G.X.; Chen, T. Diversity and succession of Actinobacteria in the forelands of the Tianshan Glacier, China. Geomicrobiol. J. 2016, 33, 716–723. [Google Scholar] [CrossRef]
- Larsen, K.S.; Jonasson, S.; Michelsen, A. Repeated freeze–thaw cycles and their effects on biological processes in two arctic ecosystem types. Appl. Soil Ecol. 2002, 21, 187–195. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, Q.; Wen, J.H.; Ding, X.L.; Agathokleous, E. Straw addition decreased the resistance of bacterial community composition to freeze–thaw disturbances in a clay loam soil due to changes in physiological and functional traits. Geoderma 2022, 424, 116007. [Google Scholar] [CrossRef]
- Han, C.L.; Gu, Y.J.; Kong, M.; Hu, L.W.; Jia, Y.; Li, F.M.; Sun, G.J.; Siddique, K.H. Responses of soil microorganisms, carbon and nitrogen to freeze–thaw cycles in diverse land-use types. Appl. Soil Ecol. 2018, 124, 211–217. [Google Scholar] [CrossRef]
- Kielak, A.M.; Barreto, C.C.; Kowalchuk, G.A.; Van Veen, J.A.; Kuramae, E.E. The ecology of Acidobacteria: Moving beyond genes and genomes. Front. Microbiol. 2016, 7, 744. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Grogan, P.; Chu, H.; Christiansen, C.T.; Walker, V.K. The effect of freeze-thaw conditions on arctic soil bacterial communities. Biology 2013, 2, 356–377. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.E.; Ho, L.; Rees, C.A.; Hill, J.E.; Nodwell, J.R.; Elliot, M.A. Streptomyces exploration is triggered by fungal interactions and volatile signals. eLife 2017, 6, e21738. [Google Scholar] [CrossRef] [PubMed]
- Männistö, M.K.; Tiirola, M.; Häggblom, M.M. Effect of freeze-thaw cycles on bacterial communities of Arctic tundra soil. Microb. Ecol. 2009, 58, 621–631. [Google Scholar] [CrossRef]
- Traxler, M.F.; Kolter, R. Natural products in soil microbe interactions and evolution. Nat. Prod. Rep. 2015, 32, 956–970. [Google Scholar] [CrossRef]
- Paredes-Sabja, D.; Setlow, P.; Sarker, M.R. Germination of spores of Bacillales and Clostridiales species: Mechanisms and proteins involved. Trends microbiol. 2011, 19, 85–94. [Google Scholar] [CrossRef]
- DeBruyn, J.M.; Nixon, L.T.; Fawaz, M.N.; Johnson, A.M.; Radosevich, M. Global biogeography and quantitative seasonal dynamics of Gemmatimonadetes in soil. Appl. Environ. Microbiol. 2011, 77, 6295–6300. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.L.; Walker, V.K. Selection of low-temperature resistance in bacteria and potential applications. Environ. Technol. 2010, 31, 943–956. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef]
- Schostag, M.; Priemé, A.; Jacquiod, S.; Russel, J.; Ekelund, F.; Jacobsen, C.S. Bacterial and protozoan dynamics upon thawing and freezing of an active layer permafrost soil. ISME J. 2019, 13, 1345–1359. [Google Scholar] [CrossRef]
- Ho, A.; Di Lonardo, D.P.; Bodelier, P.L. Revisiting life strategy concepts in environmental microbial ecology. FEMS Microbiol. Ecol. 2017, 93, fix006. [Google Scholar] [CrossRef] [PubMed]
- Nikrad, M.P.; Kerkhof, L.J.; Häggblom, M.M. The subzero microbiome: Microbial activity in frozen and thawing soils. FEMS Microbiol. Ecol. 2016, 92, fiw081. [Google Scholar] [CrossRef] [PubMed]
- Urakawa, R.; Shibata, H.; Kuroiwa, M.; Inagaki, Y.; Tateno, R.; Hishi, T.; Fukuzawa, K.; Hirai, K.; Toda, H.; Oyanagi, N. Effects of freeze–thaw cycles resulting from winter climate change on soil nitrogen cycling in ten temperate forest ecosystems throughout the Japanese archipelago. Soil Biol. Biochem. 2014, 74, 82–94. [Google Scholar] [CrossRef]
- Jiang, N.; Juan, Y.H.; Tian, L.L.; Chen, X.D.; Sun, W.T.; Chen, L.J. Soil water contents control the responses of dissolved nitrogen pools and bacterial communities to freeze-thaw in temperate soils. BioMed Res. Int. 2020, 2020, 6867081. [Google Scholar] [CrossRef] [PubMed]
- Isobe, K.; Oka, H.; Watanabe, T.; Tateno, R.; Urakawa, R.; Liang, C.; Senoo, K.; Shibata, H. High soil microbial activity in the winter season enhances nitrogen cycling in a cool-temperate deciduous forest. Soil Biol. Biochem. 2018, 124, 90–100. [Google Scholar] [CrossRef]
- Ren, J.S.; Song, C.C.; Hou, A.X.; Song, Y.Y.; Zhu, X.Y.; Cagle, G.A. Shifts in soil bacterial and archaeal communities during freeze-thaw cycles in a seasonal frozen marsh, Northeast China. Sci. Total Environ. 2018, 625, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.M.; Liu, M.H.; Yang, J.L.; Feng, F.J. Meta-analysis of the impact of freeze–thaw cycles on soil microbial diversity and C and N dynamics. Soil Biol. Biochem. 2022, 168, 108608. [Google Scholar] [CrossRef]
- Henry, H.A. Climate change and soil freezing dynamics: Historical trends and projected changes. Clim. Chang. 2008, 87, 421–434. [Google Scholar] [CrossRef]
- Dong, Z.H.; Li, J.F.; Chen, L.; Wang, S.; Shao, T. Effects of freeze–thaw event on microbial community dynamics during red clover ensiling. Front. Microbiol. 2019, 10, 1559. [Google Scholar] [CrossRef] [PubMed]
- Juan, Y.H.; Jiang, N.; Tian, L.L.; Chen, X.D.; Sun, W.T.; Chen, L.J. Effect of freeze-thaw on a midtemperate soil bacterial community and the correlation network of its members. BioMed Res. Int. 2018, 2018, 8412429. [Google Scholar] [CrossRef]
- Li, Y.X.; Wang, L.X.; Tian, L.; Zheng, H.F.; Ou, Y.; Yan, B.X.; Cui, H.; Bao, M.W.; Zhang, S.Q.; Guan, F.C. Dissolved organic carbon, an indicator of soil bacterial succession in restored wetland under freeze-thaw cycle. Ecol. Eng. 2022, 177, 106569. [Google Scholar] [CrossRef]
- Liu, X.; Qi, Z.M.; Wang, Q.; Ma, Z.W.; Li, L.H. Effects of biochar addition on CO2 and CH4 emissions from a cultivated sandy loam soil during freeze-thaw cycles. Plant Soil Environ. 2017, 63, 243–249. [Google Scholar]
- Ludwig, B.; Teepe, R.; de Gerenyu, V.L.; Flessa, H. CO2 and N2O emissions from gleyic soils in the Russian tundra and a German forest during freeze–thaw periods—a microcosm study. Soil Biol. Biochem. 2006, 38, 3516–3519. [Google Scholar] [CrossRef]
- Walz, J.; Knoblauch, C.; Böhme, L.; Pfeiffer, E.-M. Regulation of soil organic matter decomposition in permafrost-affected Siberian tundra soils-Impact of oxygen availability, freezing and thawing, temperature, and labile organic matter. Soil Biol. Biochem. 2017, 110, 34–43. [Google Scholar] [CrossRef]
- Kim, D.; Vargas, R.; Bond-Lamberty, B.; Turetsky, M. Effects of soil rewetting and thawing on soil gas fluxes: A review of current literature and suggestions for future research. Biogeosciences 2012, 9, 2459–2483. [Google Scholar] [CrossRef]
- Zhu, R.B.; Liu, Y.S.; Ma, E.D.; Sun, J.J.; Xu, H.; Sun, L.G. Greenhouse gas emissions from penguin guanos and ornithogenic soils in coastal Antarctica: Effects of freezing–thawing cycles. Atmos. Environ. 2009, 43, 2336–2347. [Google Scholar] [CrossRef]
- Kreyling, J.; Schumann, R.; Weigel, R. Soils from cold and snowy temperate deciduous forests release more nitrogen and phosphorus after soil freeze–thaw cycles than soils from warmer, snow-poor conditions. Biogeosciences 2020, 17, 4103–4117. [Google Scholar] [CrossRef]
- Pelster, D.E.; Chantigny, M.H.; Rochette, P.; Bertrand, N.; Angers, D.A.; Zebarth, B.J.; Goyer, C. Rates and intensity of freeze–thaw cycles affect nitrous oxide and carbon dioxide emissions from agricultural soils. Can. J. Soil Sci. 2019, 99, 472–484. [Google Scholar] [CrossRef]
- Dong, X.F.; Liu, C.; Ma, D.L.; Wu, Y.F.; Man, H.R.; Wu, X.W.; Li, M.; Zang, S.Y. Organic carbon mineralization and bacterial community of active layer soils response to short-term warming in the Great Hing’an Mountains of Northeast China. Front. Microbiol. 2021, 12, 802213. [Google Scholar] [CrossRef] [PubMed]
- Ernakovich, J.G.; Lynch, L.M.; Brewer, P.E.; Calderon, F.J.; Wallenstein, M.D. Redox and temperature-sensitive changes in microbial communities and soil chemistry dictate greenhouse gas loss from thawed permafrost. Biogeochemistry 2017, 134, 183–200. [Google Scholar] [CrossRef]
- Barret, M.; Morrissey, J.P.; O’gara, F. Functional genomics analysis of plant growth-promoting rhizobacterial traits involved in rhizosphere competence. Biol. Fertil. Soils 2011, 47, 729–743. [Google Scholar] [CrossRef]
- Pold, G.; Billings, A.F.; Blanchard, J.L.; Burkhardt, D.B.; Frey, S.D.; Melillo, J.M.; Schnabel, J.; van Diepen, L.T.; DeAngelis, K.M. Long-term warming alters carbohydrate degradation potential in temperate forest soils. Appl. Environ. Microbiol. 2016, 82, 6518–6530. [Google Scholar] [CrossRef]
- Samad, T.; Billings, N.; Birjiniuk, A.; Crouzier, T.; Doyle, P.S.; Ribbeck, K. Swimming bacteria promote dispersal of non-motile staphylococcal species. ISME J. 2017, 11, 1933–1937. [Google Scholar] [CrossRef]
- Guo, Z.; Han, J.; Li, J.; Xu, Y.; Wang, X. Effects of long-term fertilization on soil organic carbon mineralization and microbial community structure. PLoS ONE 2019, 14, e0211163. [Google Scholar]
- Yang, Y.J.; Liu, H.X.; Dai, Y.C.; Tian, H.X.; Zhou, W.; Lv, J.L. Soil organic carbon transformation and dynamics of microorganisms under different organic amendments. Sci. Total Environ. 2021, 750, 141719. [Google Scholar] [CrossRef]
- Yamada, T.; Sekiguchi, Y. Cultivation of uncultured chloroflexi subphyla: Significance and ecophysiology of formerly uncultured chloroflexi’subphylum i’with natural and biotechnological relevance. Microbes Environ. 2009, 24, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.F.; Chen, J.H.; Pan, G.X.; Wang, G.M.; Liu, X.Y.; Zhang, X.H.; Li, L.Q.; Bian, R.J.; Cheng, K.; Zheng, J.W. A long-term hybrid poplar plantation on cropland reduces soil organic carbon mineralization and shifts microbial community abundance and composition. Appl. Soil Ecol. 2017, 111, 94–104. [Google Scholar] [CrossRef]
- Jiang, L.; Song, Y.Y.; Sun, L.; Song, C.C.; Wang, X.W.; Ma, X.Y.; Liu, C.; Gao, J.L. Effects of warming on carbon emission and microbial abundances across different soil depths of a peatland in the permafrost region under anaerobic condition. Appl. Soil Ecol. 2020, 156, 103712. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Song, C.C.; Wang, X.W.; Chen, N.; Zhang, H.; Du, Y.; Zhang, Z.G.; Zhu, X.H. Warming effects on the flux of CH4 from peatland mesocosms are regulated by plant species composition: Richness and functional types. Sci. Total Environ. 2022, 806, 150831. [Google Scholar] [CrossRef]
- Hütsch, B.W.; Webster, C.P.; Powlson, D.S. Methane oxidation in soil as affected by land use, soil pH and N fertilization. Soil Biol. Biochem. 1994, 26, 1613–1622. [Google Scholar] [CrossRef]
- Wang, Z.P.; Delaune, R.D.; Lindau, C.W.; Patrick, W.H. Methane production from anaerobic soil amended with rice straw and nitrogen fertilizers. Fertil. Res. 1992, 33, 115–121. [Google Scholar] [CrossRef]
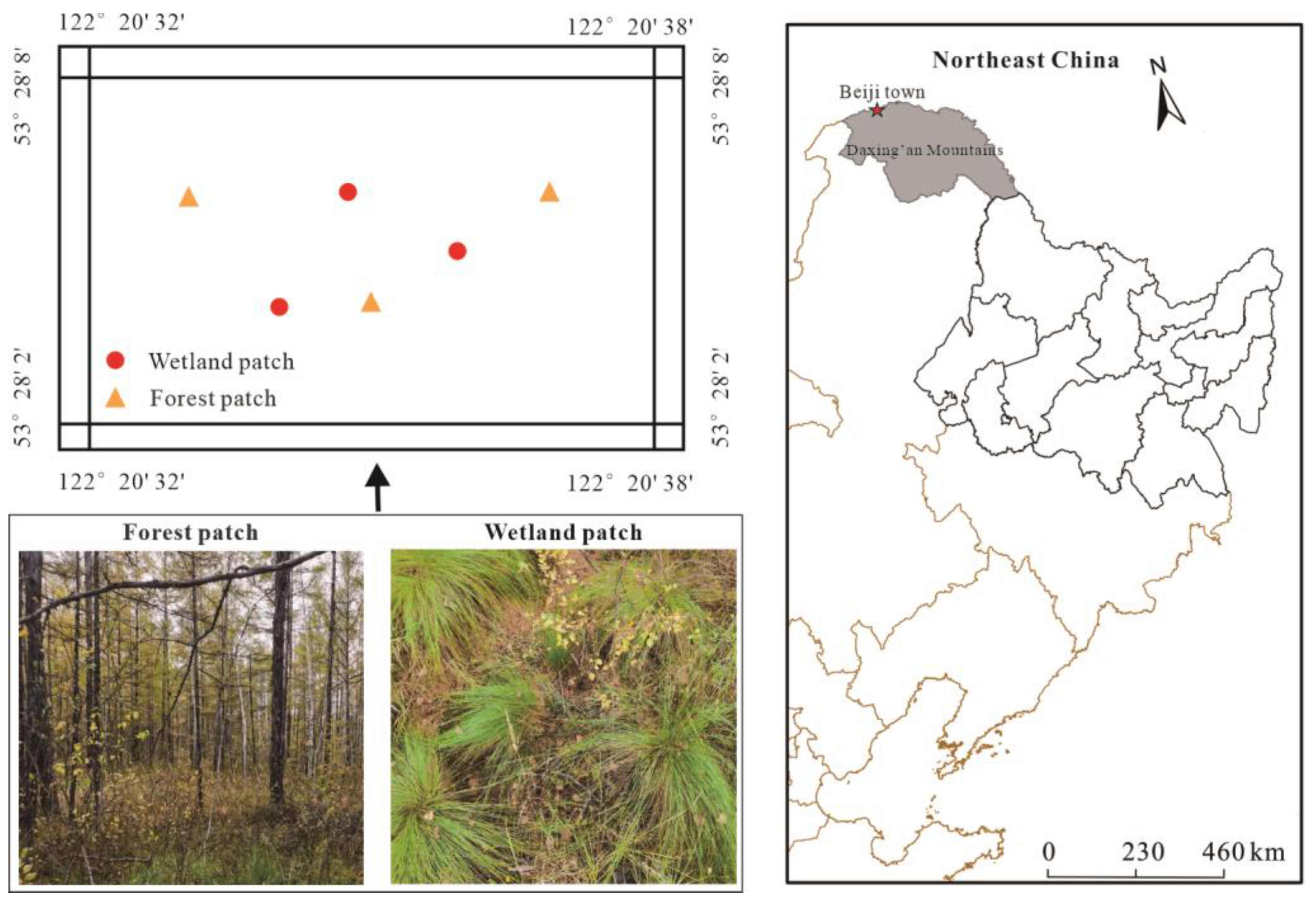
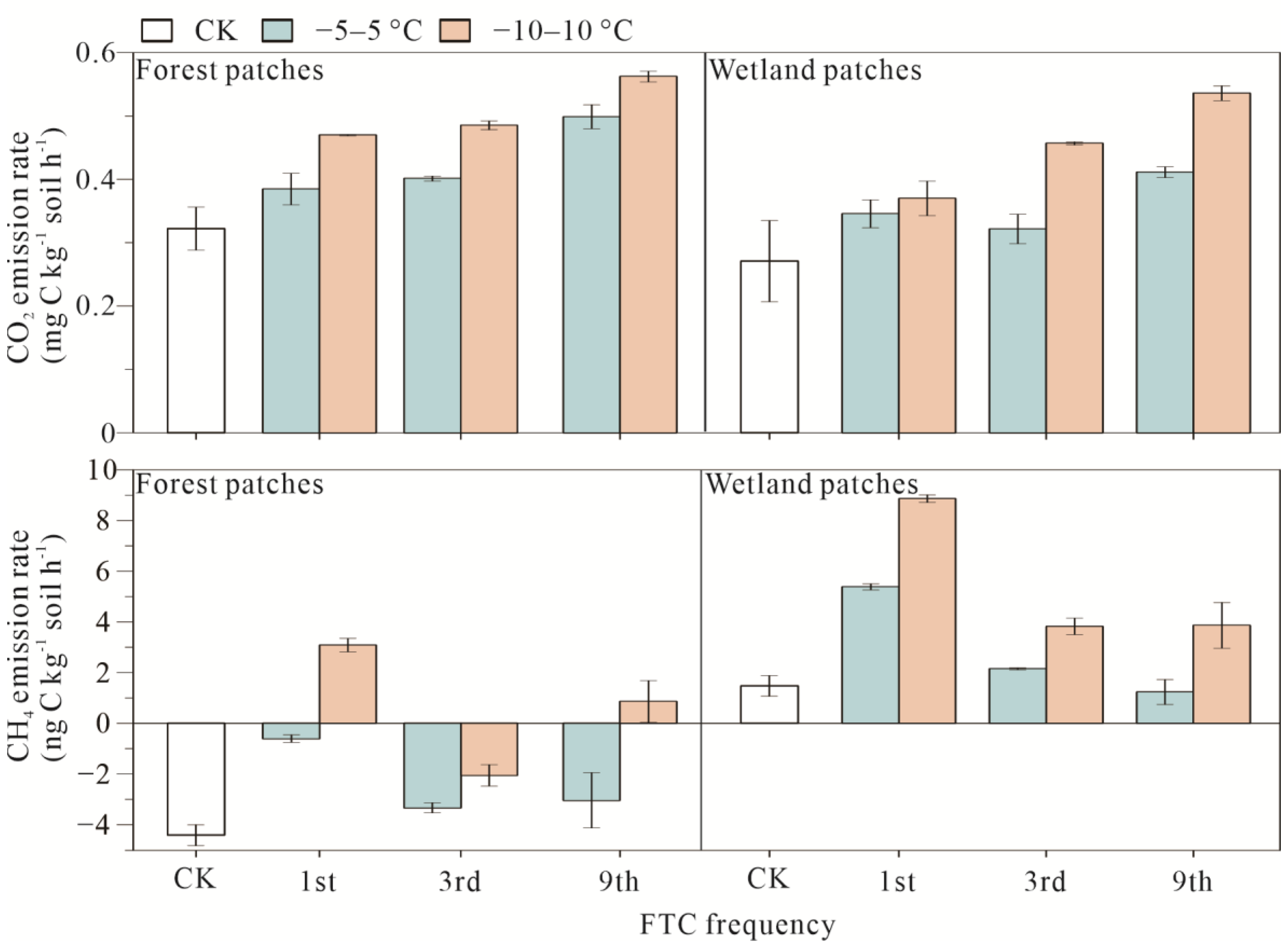
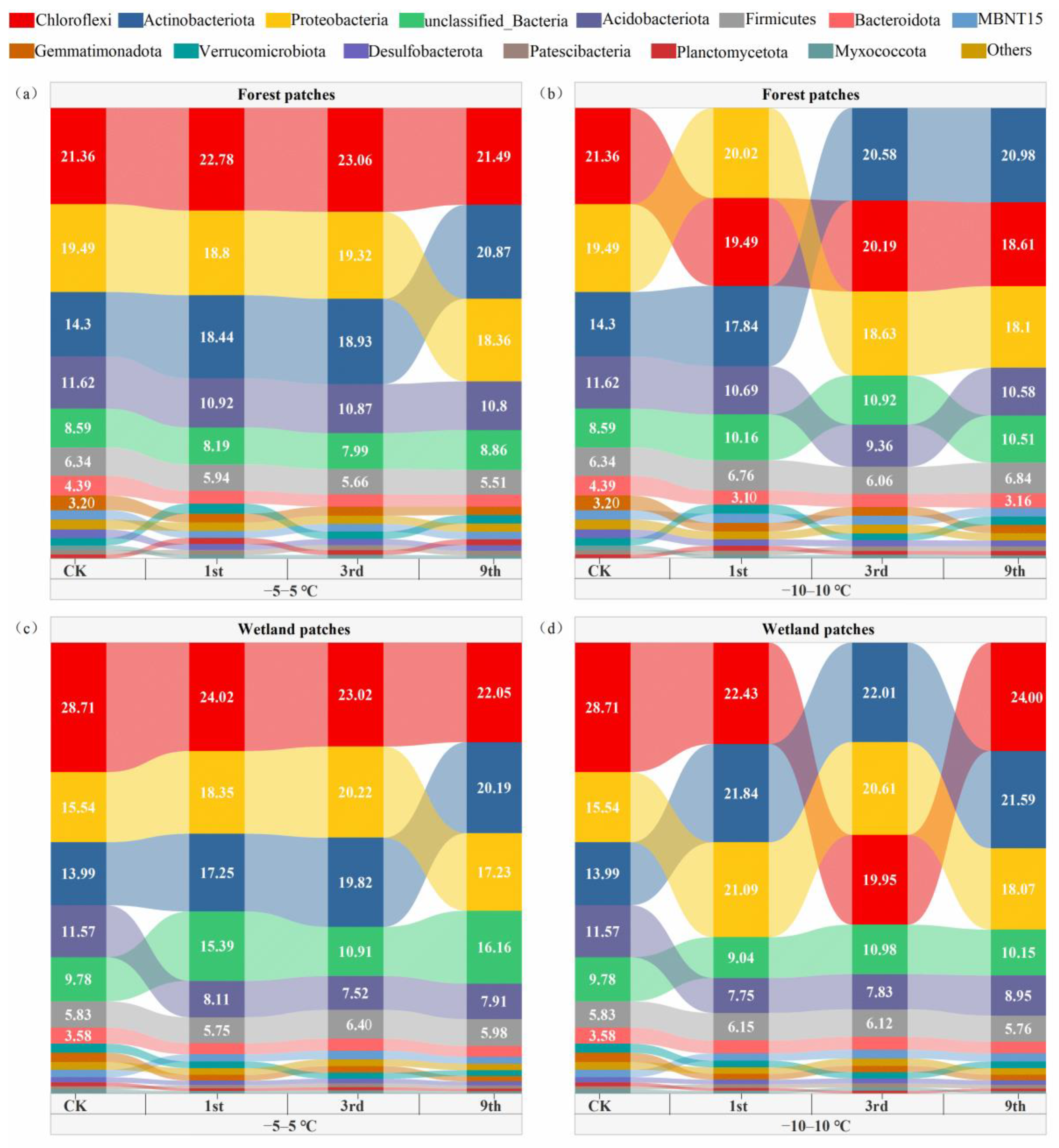
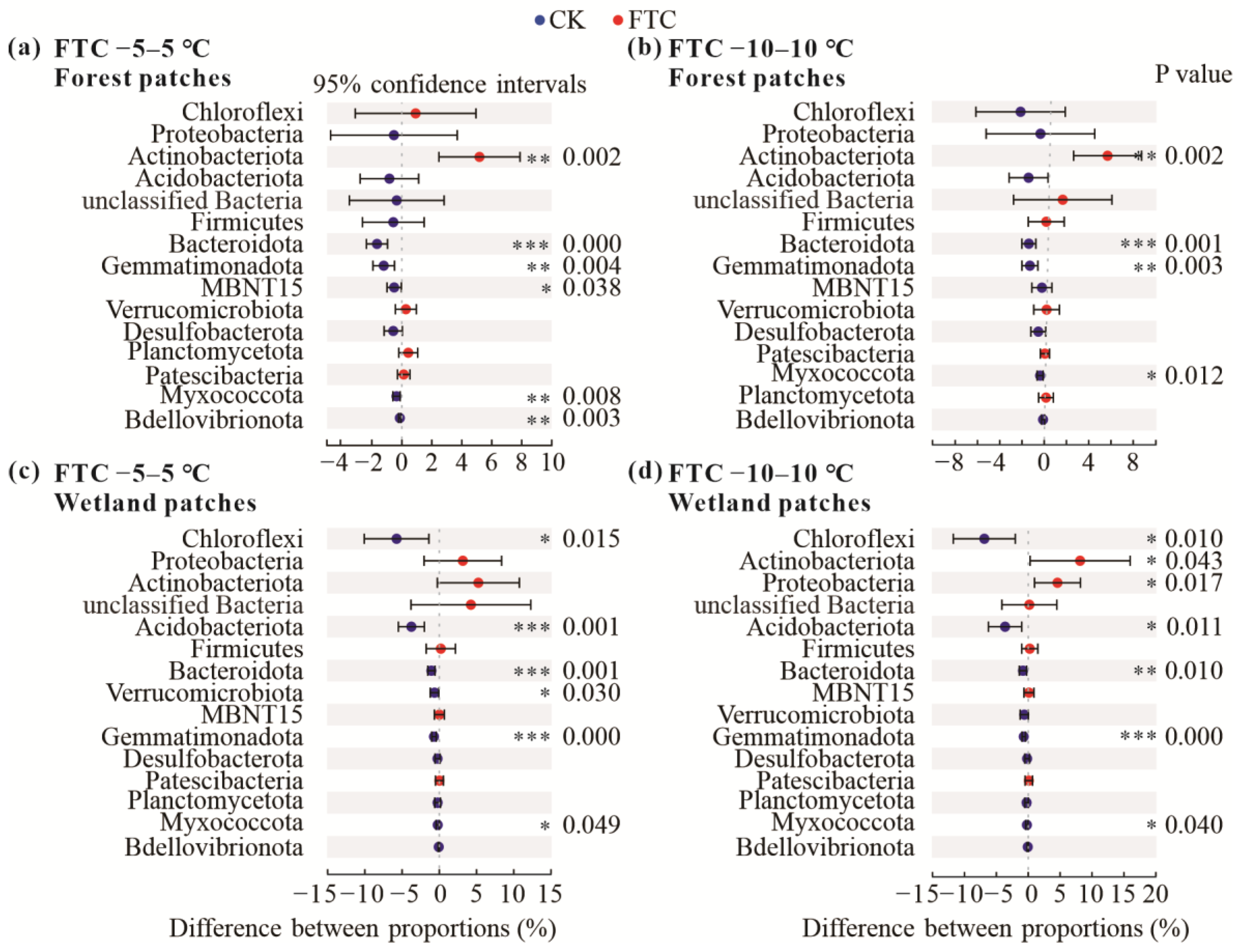
| Ecotone | Soil Water Content | FTC Amplitude | FTC Period | Sampling Time |
|---|---|---|---|---|
| Forest patches | Field water content (23.38 ± 0.81%) | −5–5 °C and −10–10 °C | 2 days (the treated sample was frozen at −5 °C or −10 °C for 24 h and thawed at 5 °C or 10 °C for 24 h) | After preincubation (CK) and the 1st, 3rd, and 9th FTCs |
| Wetland patches | Field water content (84.41 ± 5.36%) |
| Patch type | Adjusted R2 | F-Statistic | p | Significant Variables (p < 0.05) | Beta Coefficient | ||
|---|---|---|---|---|---|---|---|
| Forest patches | Biological factors | CH4 | 0.894 | 5.751 | 0.035 | Proteobacteria | 0.575 |
| Bacteroidota | 1.145 | ||||||
| CO2 | 0.878 | 18.487 | <0.001 | Chloroflexi | −0.547 | ||
| Actinobacteriota | 0.295 | ||||||
| Proteobacteria | −0.423 | ||||||
| MBNT15 | 0.646 | ||||||
| Gemmatimonadota | −0.608 | ||||||
| Non-biological factors | CH4 | 0.456 | 11.254 | 0.029 | NO3−-N | 0.536 | |
| CO2 | 0.312 | 8.727 | 0.009 | DOC | 0.594 | ||
| Wetland patches | Biological factors | CH4 | 0.316 | 13.617 | 0.04 | Proteobacteria | 1.689 |
| Firmicutes | −1.295 | ||||||
| Patescibacteria | −1.287 | ||||||
| CO2 | 0.729 | 8.643 | 0.01 | Actinobacteriota | 2.181 | ||
| Proteobacteria | −1.370 | ||||||
| Acidobacteriota | 0.646 | ||||||
| Gemmatimonadota | 0.672 | ||||||
| Desulfobacterota | 0.518 | ||||||
| Non-biological factors | CH4 | 0.689 | 19.842 | 0.009 | NH4+-N | −0.712 | |
| NO3−-N | 0.615 | ||||||
| CO2 | - | - | - | None | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Dong, X.; Wu, X.; Ma, D.; Wu, Y.; Man, H.; Li, M.; Zang, S. Response of Carbon Emissions and the Bacterial Community to Freeze–Thaw Cycles in a Permafrost-Affected Forest–Wetland Ecotone in Northeast China. Microorganisms 2022, 10, 1950. https://doi.org/10.3390/microorganisms10101950
Liu C, Dong X, Wu X, Ma D, Wu Y, Man H, Li M, Zang S. Response of Carbon Emissions and the Bacterial Community to Freeze–Thaw Cycles in a Permafrost-Affected Forest–Wetland Ecotone in Northeast China. Microorganisms. 2022; 10(10):1950. https://doi.org/10.3390/microorganisms10101950
Chicago/Turabian StyleLiu, Chao, Xingfeng Dong, Xiaodong Wu, Dalong Ma, Yufei Wu, Haoran Man, Miao Li, and Shuying Zang. 2022. "Response of Carbon Emissions and the Bacterial Community to Freeze–Thaw Cycles in a Permafrost-Affected Forest–Wetland Ecotone in Northeast China" Microorganisms 10, no. 10: 1950. https://doi.org/10.3390/microorganisms10101950
APA StyleLiu, C., Dong, X., Wu, X., Ma, D., Wu, Y., Man, H., Li, M., & Zang, S. (2022). Response of Carbon Emissions and the Bacterial Community to Freeze–Thaw Cycles in a Permafrost-Affected Forest–Wetland Ecotone in Northeast China. Microorganisms, 10(10), 1950. https://doi.org/10.3390/microorganisms10101950







