Microencapsulation of Probiotics for Food Functionalization: An Update on Literature Reviews
Abstract
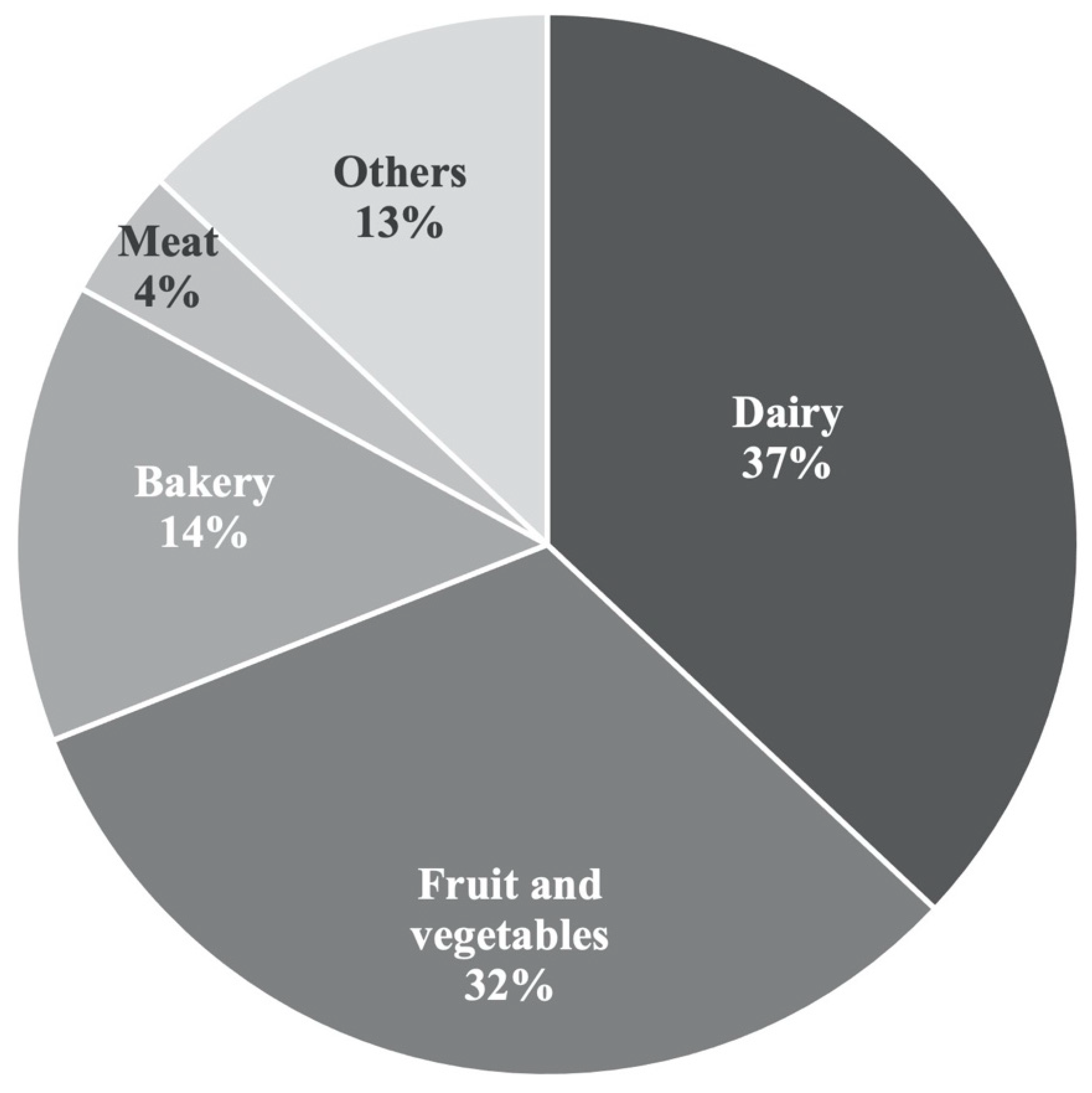
1. Introduction
2. Food Probiotication with Microencapsulated Cells
2.1. Baked Goods
2.2. Dairy Products
2.2.1. Milk
2.2.2. Fermented Milk and Yogurt
2.2.3. Ice Cream
2.2.4. Cheese
2.3. Fruits and Vegetable-Based Products
2.4. Other Products
3. Co-Microencapsulation of Probiotics
- Omega-3 and GABA;
- Soluble dietary fibres;
- Phytochemicals.
4. The Market of Microencapsulated Probiotics
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef] [PubMed]
- Kechagia, M.; Basoulis, D.; Konstantopoulou, S.; Dimitriadi, D.; Gyftopoulou, K.; Skarmoutsou, N.; Fakiri, E.M. Health benefits of probiotics: A review. ISRN Nutr. 2013, 2013. [Google Scholar] [CrossRef]
- Koirala, S.; Anal, A.K. Probiotics-based foods and beverages as future foods and overall safety and regulatory claims. Future Foods 2021, 3, 100013. [Google Scholar] [CrossRef]
- Thomas, M.B.; Vaidyanathan, M.; Radhakrishnan, K.; Raichur, A.M. Enhanced viability of probiotic Saccharomyces boulardii encapsulated by layer-by-layer approach in pH responsive chitosan–dextran sulfate polyelectrolytes. J. Food Eng. 2014, 136, 1–8. [Google Scholar] [CrossRef]
- Anselmo, A.C.; McHugh, K.J.; Webster, J.; Langer, R.; Jaklenec, A. Layer-by-layer encapsulation of probiotics for delivery to the microbiome. Adv. Mater. 2016, 28, 9486–9490. [Google Scholar] [CrossRef] [PubMed]
- Nguon, O.; Lagugné-Labarthet, F.; Brandys, F.A.; Li, J.; Gillies, E.R. Microencapsulation by in situ polymerization of amino resins. Polym. Rev. 2018, 58, 326–375. [Google Scholar] [CrossRef]
- Spigno, G.; Duserm Garrido, G.; Guidesi, M.; Elli, M. Spray-drying encapsulation of probiotics for ice-cream application. Chem. Eng. Trans. 2015, 43, 49–54. [Google Scholar] [CrossRef]
- Surnis, M.S.A.; Huparikar, M.K.B.; Kamble, M.P.A.; Mulla, M.M.I. Microencapsulation of Probiotics (Lactobacillus Casei and Bifidobacterium Longum) in Pineapple Jam by Spray Drying and its Comparitive Study. Int. J. Eng. Res. 2016, 5, 675–677. [Google Scholar]
- Naga Sivudu, S.; Ramesh, B.; Umamahesh, K.; Vijaya Sarathi Reddy, O. Probiotication of Tomato and Carrot Juices for Shelf-life Enhancement using Micro-encapsulation. J. Food Biosci. Technol. 2016, 6, 13–22. [Google Scholar]
- García-Ceja, A.; Mani-López, E.; Palou, E.; López-Malo, A. Viability during refrigerated storage in selected food products and during simulated gastrointestinal conditions of individual and combined lactobacilli encapsulated in alginate or alginate-chitosan. LWT Food Sci. Technol. 2015, 63, 482–489. [Google Scholar] [CrossRef]
- Alves, M.; Peres, C.M.; Hernandez-Mendonza, A.; Bronze, M.R.; Peres, C.; Malcata, F.X. Olive paste as vehicle for delivery of potential probiotic Lactobacillus plantarum 33. Food Res. Int. 2015, 75, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.M.B.; Shahidi, F.; Mortazavi, S.A.; Milani, E.; Eshaghi, Z. Synbiotic potential of Doogh supplemented with free and encapsulated Lactobacillus plantarum LS5 and Helianthus tuberosus inulin. J. Food Sci. Technol. 2015, 52, 4579–4585. [Google Scholar] [CrossRef] [PubMed]
- De Prisco, A.; Mauriello, G. Probiotication of foods: A focus on microencapsulation tool. Trends Food Sci. Technol. 2016, 48, 27–39. [Google Scholar] [CrossRef]
- Seyedain-Ardabili, M.; Sharifan, A.; Ghiassi Tarzi, B. The production of synbiotic bread by microencapsulation. Food Technol. Biotechnol. 2016, 54, 52–59. [Google Scholar] [CrossRef]
- Muzzafar, A.; Sharma, V. Microencapsulation of probiotics for incorporation in cream biscuits. J. Food Meas. Charact. 2018, 12, 2193–2201. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, X.D.; Boom, R.M.; Schutyser, M.A. Survival of encapsulated Lactobacillus plantarum during isothermal heating and bread baking. LWT 2018, 93, 396–404. [Google Scholar] [CrossRef]
- Zhang, L.; Taal, M.A.; Boom, R.M.; Chen, X.D.; Schutyser, M.A. Effect of baking conditions and storage on the viability of Lactobacillus plantarum supplemented to bread. LWT 2018, 87, 318–325. [Google Scholar] [CrossRef]
- Thang, T.D.; Hang, H.T.T.; Luan, N.T.; KimThuy, D.T.; Lieu, D.M. Survival Survey of Lactobacillus acidophilus In Additional Probiotic Bread. Turk. J. Agric. Food Sci. Technol. 2019, 7, 588–592. [Google Scholar]
- Arslan-Tontul, S.; Erbas, M.; Gorgulu, A. The Use of probiotic-loaded single-and double-layered microcapsules in cake production. Probiotics Antimicrob. Proteins 2019, 11, 840–849. [Google Scholar] [CrossRef]
- Dimitrellou, D.; Kandylis, P.; Petrović, T.; Dimitrijević-Branković, S.; Lević, S.; Nedović, V.; Kourkoutas, Y. Survival of spray dried microencapsulated Lactobacillus casei ATCC 393 in simulated gastrointestinal conditions and fermented milk. LWT Food Sci. Technol. 2016, 71, 169–174. [Google Scholar] [CrossRef]
- Chaikham, P. Stability of probiotics encapsulated with Thai herbal extracts in fruit juices and yoghurt during refrigerated storage. Food Biosci. 2015, 12, 61–66. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, D. Development of antioxidant rich fruit supplemented probiotic yogurts using free and microencapsulated Lactobacillus rhamnosus culture. J. Food Sci. Technol. 2016, 53, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Amakiri, A.C.; Thantsha, M.S. Survival of Bifidobacterium longum LMG 13197 microencapsulated in Vegetal or Vegetal-inulin matrix in simulated gastrointestinal fluids and yoghurt. SpringerPlus 2016, 5, 1343. [Google Scholar] [CrossRef] [PubMed]
- Bosnea, L.A.; Moschakis, T.; Biliaderis, C.G. Microencapsulated cells of Lactobacillus paracasei subsp. paracasei in biopolymer complex coacervates and their function in a yogurt matrix. Food Funct. 2017, 8, 554–562. [Google Scholar] [CrossRef]
- Jiang, Y.; Zheng, Z.; Zhang, T.; Hendricks, G.; Guo, M. Microencapsulation of Lactobacillus acidophilus NCFM using polymerized whey proteins as wall material. Int. J. Food Sci. Nutr. 2016, 67, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, C.L.; Sun, Y.; Li, A.L.; Liu, F.; Meng, X.C. Microencapsulation of Lactobacillus rhamnosus GG by Transglutaminase Cross-Linked Soy Protein Isolate to Improve Survival in Simulated Gastrointestinal Conditions and Yoghurt. J. Food Sci. 2016, 81, M1726–M1734. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, S.; Zheng, J.; Gao, F.; Ahmad, S.; Guo, M. Microencapsulation of Lactobacillus acidophilus (La-5), its evaluation and application in the yoghurt. Pak. J. Agric. Sci. 2016, 53, 933–939. [Google Scholar]
- Patrignani, F.; Siroli, L.; Serrazanetti, D.I.; Braschi, G.; Betoret, E.; Reinheimer, J.A.; Lanciotti, R. Microencapsulation of functional strains by high pressure homogenization for a potential use in fermented milk. Food Res. Int. 2017, 97, 250–257. [Google Scholar] [CrossRef]
- Afzaal, M.; Khan, A.U.; Saeed, F.; Ahmed, A.; Ahmad, M.H.; Maan, A.A.; Tufail, T.; Anjum, F.M.; Hussain, S. Functional exploration of free and encapsulated probiotic bacteria in yogurt and simulated gastrointestinal conditions. Food Sci. Nutr. 2019, 7, 3931–3940. [Google Scholar] [CrossRef]
- Pinto, S.S.; Cavalcante, B.D.; Verruck, S.; Alves, L.F.; Prudêncio, E.S.; Amboni, R.D. Effect of the incorporation of Bifidobacterium BB-12 microencapsulated with sweet whey and inulin on the properties of Greek-style yogurt. J. Food Sci. Technol. 2017, 54, 2804–2813. [Google Scholar] [CrossRef] [PubMed]
- Seth, D.; Mishra, H.N.; Deka, S.C. Effect of microencapsulation using extrusion technique on viability of bacterial cells during spray drying of sweetened yoghurt. Int. J. Biol. Macromol. 2017, 103, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Kalkan, S.; Öztürk, D.; Selimoğlu, B.S. Determining some of the quality characteristics of probiotic yogurts manufactured by using microencapsulated Saccharomyces cerevisiae var. boulardii. Turk. J. Vet. Anim. Sci. 2018, 42, 617–623. [Google Scholar] [CrossRef]
- Wang, M.; Wang, C.; Gao, F.; Guo, M. Effects of polymerised whey protein-based microencapsulation on survivability of Lactobacillus acidophilus LA-5 and physiochemical properties of yoghurt. J. Microencapsul. 2018, 35, 504–512. [Google Scholar] [CrossRef]
- Fazilah, N.F.; Hamidon, N.H.; Ariff, A.B.; Khayat, M.E.; Wasoh, H.; Halim, M. Microencapsulation of Lactococcus lactis Gh1 with Gum Arabic and Synsepalum dulcificum via Spray Drying for Potential Inclusion in Functional Yogurt. Molecules 2019, 24, 1422. [Google Scholar] [CrossRef]
- Verruck, S.; Barretta, C.; Miotto, M.; Canella, M.H.M.; de Liz, G.R.; Maran, B.M.; Garcia, S.G.; da Silveira, S.M.; Vieira, C.R.W.; da Cruz, A.G.; et al. Evaluation of the interaction between microencapsulated Bifidobacterium BB-12 added in goat’s milk Frozen Yogurt and Escherichia coli in the large intestine. Food Res. Int. 2020, 127, 108690. [Google Scholar] [CrossRef]
- Obradović, N.; Volić, M.; Nedović, V.; Rakin, M.; Bugarski, B. Microencapsulation of probiotic starter culture in protein–carbohydrate carriers using spray and freeze-drying processes: Implementation in whey-based beverages. J. Food Eng. 2022, 321, 110948. [Google Scholar] [CrossRef]
- Silva, R.; Pimentel, T.C.; Junior, F.E.D.M.; Esmerino, E.A.; Freitas, M.Q.; Fávaro-Trindade, C.S.; Silva, M.C.; Cruz, A.G. Microencapsulation with spray-chilling as an innovative strategy for probiotic low sodium requeijão cremoso processed cheese processing. Food Biosci. 2022, 46, 101517. [Google Scholar] [CrossRef]
- Afzaal, M.; Saeed, F.; Arshad, M.U.; Nadeem, M.T.; Saeed, M.; Tufail, T. The effect of encapsulation on the stability of probiotic bacteria in ice cream and simulated gastrointestinal conditions. Probiotics Antimicrob. Proteins 2019, 11, 1348–1354. [Google Scholar] [CrossRef]
- Kataria, A.; Achi, S.C.; Halami, P.M. Effect of encapsulation on viability of Bifidobacterium longum CFR815j and physiochemical properties of ice cream. Indian J. Microbiol. 2018, 58, 248–251. [Google Scholar] [CrossRef]
- Afzaal, M.; Khan, A.U.; Saeed, F.; Arshad, M.S.; Khan, M.A.; Saeed, M.; Maan, A.A.; Khan, M.K.; Ismail, Z.; Ahmed, A.; et al. Survival and stability of free and encapsulated probiotic bacteria under simulated gastrointestinal conditions and in ice cream. Food Sci. Nutr. 2020, 8, 1649–1656. [Google Scholar] [CrossRef] [PubMed]
- Gruskiene, R.; Bockuviene, A.; Sereikaite, J. Microencapsulation of bioactive ingredients for their delivery into fermented milk products: A review. Molecules 2021, 26, 4601. [Google Scholar] [CrossRef] [PubMed]
- Picciotti, U.; Massaro, A.; Galiano, A.; Garganese, F. Cheese fortification: Review and possible improvements. Food Rev. Int. 2021, 1–27. [Google Scholar] [CrossRef]
- Sharifi, S.; Rezazad-Bari, M.; Alizadeh, M.; Almasi, H.; Amiri, S. Use of whey protein isolate and gum Arabic for the co-encapsulation of probiotic Lactobacillus plantarum and phytosterols by complex coacervation: Enhanced viability of probiotic in Iranian white cheese. Food Hydrocoll. 2021, 113, 106496. [Google Scholar] [CrossRef]
- Mudgil, P.; Aldhaheri, F.; Hamdi, M.; Punia, S.; Maqsood, S. Fortification of Chami (traditional soft cheese) with probiotic-loaded protein and starch microparticles: Characterization, bioactive properties, and storage stability. LWT 2022, 158, 113036. [Google Scholar] [CrossRef]
- Kavas, N.; Kavas, G.; Kınık, Ö.; Ateş, M.; Şatır, G.; Kaplan, M. The effect of using microencapsulated pro and prebiotics on the aromatic compounds and sensorial properties of synbiotic goat cheese. Food Biosci. 2021, 43, 101233. [Google Scholar] [CrossRef]
- Kavas, N.; Kavas, G.; Kınık, Ö.; Ateş, M.; Kaplan, M.; Şatır, G. Symbiotic microencapsulation to enhance Bifidobacterium longum and Lactobacillus paracasei survival in goat cheese. Food Sci. Technol. 2021, 42. [Google Scholar] [CrossRef]
- Mukhtar, H.; Yaqub, S. Production of probiotic Mozzarella cheese by incorporating locally isolated Lactobacillus acidophilus. Ann. Microbiol. 2020, 70, 56. [Google Scholar] [CrossRef]
- Phoem, A.N.; Chanthachum, S.; Voravuthikunchai, S.P. Applications of microencapsulated Bifidobacterium longum with Eleutherine americana in fresh milk tofu and pineapple juice. Nutrients 2015, 7, 2469–2484. [Google Scholar] [CrossRef]
- Gandomi, H.; Abbaszadeh, S.; Misaghi, A.; Bokaie, S.; Noori, N. Effect of chitosan-alginate encapsulation with inulin on survival of Lactobacillus rhamnosus GG during apple juice storage and under simulated gastrointestinal conditions. LWT Food Sci. Technol. 2016, 69, 365–371. [Google Scholar] [CrossRef]
- Roy, D.; Savard, P.; Guertin, N.; Martoni, C.J.; Jones, M.L.; Champagne, C.P. Viability of Lactobacillus reuteri NCIMB 30242 during storage in fruit juice and soy beverage. J. Microbiol. Biotechnol. Food Sci. 2020, 9, 320–325. [Google Scholar] [CrossRef]
- Dias, C.O.; de Almeida, J.D.S.O.; Pinto, S.S.; de Oliveira Santana, F.C.; Verruck, S.; Müller, C.M.O.; Prudêncio, E.S.; de Mello Castanho Amboni, R.D. Development and physico-chemical characterization of microencapsulated bifidobacteria in passion fruit juice: A functional non-dairy product for probiotic delivery. Food Biosci. 2018, 24, 26–36. [Google Scholar] [CrossRef]
- Azarkhavarani, P.R.; Ziaee, E.; Hosseini, S.M.H. Effect of encapsulation on the stability and survivability of Enterococcus faecium in a non-dairy probiotic beverage. Food Sci. Technol. Int. 2019, 25, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Rovinaru, C.; Pasarin, D. Application of Microencapsulated Synbiotics in Fruit-Based Beverages. Probiotics Antimicrob. Proteins 2019, 12, 764–773. [Google Scholar] [CrossRef] [PubMed]
- Santos Monteiro, S.; Albertina Silva Beserra, Y.; Miguel Lisboa Oliveira, H.; Pasquali, M.A.D.B. Production of probiotic passion fruit (Passiflora edulis Sims f. flavicarpa Deg.) drink using Lactobacillus reuteri and microencapsulation via spray drying. Foods 2020, 9, 335. [Google Scholar]
- Nami, Y.; Lornezhad, G.; Kiani, A.; Abdullah, N.; Haghshenas, B. Alginate-Persian Gum-Prebiotics microencapsulation impacts on the survival rate of Lactococcus lactis ABRIINW-N19 in orange juice. LWT 2020, 124, 109190. [Google Scholar] [CrossRef]
- González-Ferrero, C.; Irache, J.M.; Marín-Calvo, B.; Ortiz-Romero, L.; Virto-Resano, R.; González-Navarro, C.J. Encapsulation of probiotics in soybean protein-based microparticles preserves viable cell concentration in foods all along the production and storage processes. J. Microencapsul. 2020, 37, 242–253. [Google Scholar] [CrossRef]
- González-Cuello, R.E.; Pájaro, K.; Acevedo, W.; Ortega-Toro, R. Study of the Shelf Life of a Low-Calorie Jam Added with Microencapsulated Probiotics. Contemp. Eng. Sci. 2018, 11, 1235–1244. [Google Scholar] [CrossRef][Green Version]
- Talebzadeh, S.; Sharifan, A. Developing probiotic jelly desserts with Lactobacillus acidophilus. J. Food Process. Preserv. 2017, 41, e13026. [Google Scholar] [CrossRef]
- Bora, A.F.M.; Li, X.; Zhu, Y.; & Du, L. Improved viability of microencapsulated probiotics in a freeze-dried banana powder during storage and under simulated gastrointestinal tract. Probiotics Antimicrob. Proteins 2019, 11, 1330–1339. [Google Scholar] [CrossRef]
- Kiani, A.; Nami, Y.; Hedayati, S.; Jaymand, M.; Samadian, H.; Haghshenas, B. Tarkhineh as a new microencapsulation matrix improves the quality and sensory characteristics of probiotic Lactococcus lactis KUMS-T18 enriched potato chips. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lipan, L.; Rusu, B.; Sendra, E.; Hernández, F.; Vázquez-Araújo, L.; Vodnar, D.C.; Carbonell-Barrachina, Á.A. Spray drying and storage of probiotic-enriched almond milk: Probiotic survival and physicochemical properties. J. Sci. Food Agric. 2020, 100, 3697–3708. [Google Scholar] [CrossRef] [PubMed]
- Song, C.E.; Shim, H.H.; Kuppusamy, P.; Jeong, Y.I.; Lee, K.D. Potential Sustainable Properties of Microencapsulated Endophytic Lactic Acid Bacteria (KCC-42) in In-Vitro Simulated Gastrointestinal Juices and Their Fermentation Quality of Radish Kimchi. BioMed Res. Int. 2018, 2018. [Google Scholar] [CrossRef]
- Song, C.E.; Kuppusamy, P.; Jeong, Y.I.; Shim, H.H.; Lee, K.D. Microencapsulation of endophytic LAB (KCC-41) and its probiotic and fermentative potential for cabbage kimchi. Int. Microbiol. 2019, 22, 121–130. [Google Scholar] [CrossRef]
- Witzler, J.J.P.; Pinto, R.A.; de Valdez, G.F.; de Castro, A.D.; Cavallini, D.C.U. Development of a potential probiotic lozenge containing Enterococcus faecium CRL 183. LWT 2017, 77, 193–199. [Google Scholar] [CrossRef]
- Marcial-Coba, M.S.; Saaby, L.; Knøchel, S.; Nielsen, D.S. Dark chocolate as a stable carrier of microencapsulated Akkermansia muciniphila and Lactobacillus casei. FEMS Microbiol. Lett. 2019, 366 (Suppl. S1), i24–i29. [Google Scholar] [CrossRef] [PubMed]
- Gadhiya, D.; Patel, A.; Prajapati, J.B. Current trend and future prospective of functional probiotic milk chocolates and related products-a review. Czech J. Food Sci. 2015, 33, 295–301. [Google Scholar] [CrossRef]
- Rajam, R.; Kumar, S.B.; Prabhasankar, P.; Anandharamakrishnan, C. Microencapsulation of Lactobacillus plantarum MTCC 5422 in fructooligosaccharide and whey protein wall systems and its impact on noodle quality. J. Food Sci. Technol. 2015, 52, 4029–4041. [Google Scholar] [CrossRef]
- Kalkan, S.; Mustafa, O.T.A.Ğ.; Köksal, E.İ.; Bozkurt, N.Ş. Production of functional Turkish noodle (Erişte) supplementary probiotic and determining of some quality properties. Food Health 2020, 6, 140–150. [Google Scholar] [CrossRef]
- Silva, E.K.; Zabot, G.L.; Bargas, M.A.; Meireles, M.A.A. Microencapsulation of lipophilic bioactive compounds using prebiotic carbohydrates: Effect of the degree of inulin polymerization. Carbohydr. Polym. 2016, 152, 775–783. [Google Scholar] [CrossRef]
- Eratte, D.; Dowling, K.; Barrow, C.J.; Adhikari, B.P. In-vitro digestion of probiotic bacteria and omega-3 oil co-microencapsulated in whey protein isolate-gum Arabic complex coacervates. Food Chem. 2017, 227, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Enache, I.M.; Vasile, A.M.; Enachi, E.; Barbu, V.; Stănciuc, N.; Vizireanu, C. Co-microencapsulation of anthocyanins from black currant extract and lactic acid bacteria in biopolymeric matrices. Molecules 2020, 25, 1700. [Google Scholar] [CrossRef] [PubMed]
- Niño-Vásquez, I.A.; Muñiz-Márquez, D.; Ascacio-Valdés, J.A.; Contreras-Esquivel, J.C.; Aguilar, C.N.; Rodríguez-Herrera, R.; Flores-Gallegos, A.C. Co-microencapsulation: A promising multi-approach technique for enhancement of functional properties. Bioengineered 2022, 13, 5168–5189. [Google Scholar] [CrossRef]
- Kvakova, M.; Bertkova, I.; Stofilova, J.; Savidge, T.C. Co-encapsulated synbiotics and immobilized probiotics in human health and gut Microbiota modulation. Foods 2021, 10, 1297. [Google Scholar] [CrossRef]
- Massounga Bora, A.F.; Li, X.; & Liu, L. Physicochemical and Functional Characterization of Newly Designed Biopolymeric-Based Encapsulates with Probiotic Culture and Charantin. Foods 2021, 10, 2677. [Google Scholar] [CrossRef]
- Hao, L.; Shan, Q.; Wei, J.; Ma, F.; Sun, P. Lactoferrin: Major physiological functions and applications. Curr. Protein Pept. Sci. 2019, 20, 139–144. [Google Scholar] [CrossRef]
- Desiderato, C.K.; Sachsenmaier, S.; Ovchinnikov, K.V.; Stohr, J.; Jacksch, S.; Desef, D.N.; Crauwels, P.; Egert, M.; Diep, D.B.; Goldbeck, O.; et al. Identification of Potential Probiotics Producing Bacteriocins Active against Listeria monocytogenes by a Combination of Screening Tools. Int. J. Mol. Sci. 2021, 22, 8615. [Google Scholar] [CrossRef]
- Huq, T.; Fraschini, C.; Khan, A.; Riedl, B.; Bouchard, J.; Lacroix, M. Alginate based nanocomposite for microencapsulation of probiotic: Effect of cellulose nanocrystal (CNC) and lecithin. Carbohydr. Polym. 2017, 168, 61–69. [Google Scholar] [CrossRef]
- Mawad, A.; Helmy, Y.A.; Shalkami, A.G.; Kathayat, D.; Rajashekara, G.E. E. coli Nissle microencapsulation in alginate-chitosan nanoparticles and its effect on Campylobacter jejuni in vitro. Appl. Microbiol. Biotechnol. 2018, 102, 10675–10690. [Google Scholar] [CrossRef] [PubMed]
- Lotfipour, F.; Shahi, S.; Farjami, A.; Salatin, S.; Mahmoudian, M.; Dizaj, S.M. Safety and Toxicity Issues of Therapeutically Used Nanoparticles from the Oral Route. BioMed Res. Int. 2021, 2021. [Google Scholar] [CrossRef]
- Centurion, F.; Basit, A.W.; Liu, J.; Gaisford, S.; Rahim, M.A.; Kalantar-Zadeh, K. Nanoencapsulation for probiotic delivery. ACS Nano 2021, 15, 18653–18660. [Google Scholar] [CrossRef] [PubMed]
- Marketsand Markets (2022). Microcapsule Market by Technology (Spray, Emulsion and Dripping), End-user Industry (Pharmaceuticals & Healthcare, Food, Household & Personal Care, Textiles, Agrochemicals), Shell Material, Core Material & Region—Global Forecast to 2026. Available online: https://www.marketsandmarkets.com/Market-Reports/microcapsule-market-24415649.html (accessed on 29 September 2022).
- Dey, G. Non-dairy Probiotic Foods: Innovations and market trends. In Innovations in Technologies for Fermented Food and Beverage Industries; Springer: Cham, Switzerland, 2018; pp. 159–173. [Google Scholar]
| Probiotic Strains | Encapsulation Facts | Storage Conditions | Viability (At the End Storage) | Capsule Size (µm) | Notes | Author(s) |
|---|---|---|---|---|---|---|
| L. casei 01 L. acidophilus LA-5 B. lactis BB-12 | Thai herbal plants (cashew flower, yanang, pennywort, and green tea) | 4 °C for 30 days |
| Not reported |
| [22] |
| B. longum LMG 13197 | Vegetal BM 297 and inulin Freeze-drying | 4° C for 6 weeks |
| Not reported |
| [24] |
| L. paracasei subsp. Paracasei E6 | Whey protein isolate (WPI) and gum Arabic Complex coacervation | 4 °C for 45 days |
| Not reported |
| [25] |
| L. acidophilus NCFM L. delbrueckii subsp. bulgaricus S. thermophilus | Polymerized whey protein (PWP) Compared to Sodium alginate | 4 °C for 9 weeks |
| Not reported |
| [26] |
| L. acidophilus LA-5 | Whey protein concentrate (WPC) and mixture of polysaccharides (sodium alginate, λ-carrageenan, inulin, lentinan, and glucose) | 4 °C for 35 days |
| Not reported |
| [28] |
| L. acidophilus ATTC-4356 | Sodium alginate and carrageenan | 4 °C for 18 days |
| -ALG beads 714-Carrageenan 726 |
| [30] |
| Bifidobacterium BB-12 L. bulgaricus (LB) S. thermophiles (ST) | Sweet whey (SW) and inulin (SWI) Spray drying | 4 °C for 28 days |
| Not reported |
| [31] |
| S. thermophilus (ST) and L. bulgaricus (LB) NCDC 263 | Sodium alginate (ALG) Extrusion, spray drying | - |
| 82.00–149.37 |
| [32] |
| Saccharomyces cerevisiae var. boulardii | Sodium alginate, extrusion | 4 °C for 21 days |
| Not reported |
| [33] |
| L. acidophilus LA-5 | Polymerized whey protein (PWP) | 4 °C for 10 weeks |
| Average ~744 |
| [34] |
| L. lactis Gh1 | Gum Arabic Synsepalum dulcificum (miracle fruit: seed, pulp, and leaf) Spray drying | 4 °C for 21 days |
| Not reported |
| [35] |
| Mix: Lactobacillus delbrueckii ssp. bulgaricus (1.0%), Bifidobacterium bifidum (6.0%), Streptococcus salivarius ssp. thermophilus, (80.0%), Lactobacillus acidophilus | Whey, whey protein concentrate, and sodium alginate. Spray and freeze drying | 4 °C for 28 days Tested under simulated GI conditions |
| Spray drying: 5.06–7.23 Freeze drying: 2.98–3.62 |
| [37] Whey-based beverage |
| L. acidophilus La-5 | Cottonseed vegetable fat Spray chilling |
|
| 78 ± 4 |
| [38]
|
| Probiotic | Encapsulation Facts | Storage Conditions | Viability (At the End Storage) | CapsuleSize (µm) | Notes | Author(s) |
|---|---|---|---|---|---|---|
| ALG Pineapple juice | 4 °C for 45 days |
| Not reported. |
| [49] |
| CHI/ALG+ inulin Apple juice | 4 or 25 °C for 90 days |
| With inulin: 1.40 ± 0.08 mmWithout inulin: 1.39 ± 0.06 mm |
| [50] |
| ALG and poly-L-lysine Mixed fruit | 4 and 8 °C for 8 weeks. |
| Not reported. |
| [51] |
| Maltodextrins and inulin Passion fruit juice | 4 or 25 °C for 30 days |
| 10.65 and 16.52 μm |
| [52] |
| Calcium Alginate Sour cherry juice (SCJ) | 4 °C or 25 °C for 4 weeks |
| Not reported |
| [53] |
| ALG, Persian Gum FOS and inulin Orange juice | 4 °C for 6 weeks |
| ALG: 860–1130 μmALG + PG: 340–370 μm+FOS: 350–430 μm+Inulin: 460–560 μm |
| [56] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sbehat, M.; Mauriello, G.; Altamimi, M. Microencapsulation of Probiotics for Food Functionalization: An Update on Literature Reviews. Microorganisms 2022, 10, 1948. https://doi.org/10.3390/microorganisms10101948
Sbehat M, Mauriello G, Altamimi M. Microencapsulation of Probiotics for Food Functionalization: An Update on Literature Reviews. Microorganisms. 2022; 10(10):1948. https://doi.org/10.3390/microorganisms10101948
Chicago/Turabian StyleSbehat, Maram, Gianluigi Mauriello, and Mohammad Altamimi. 2022. "Microencapsulation of Probiotics for Food Functionalization: An Update on Literature Reviews" Microorganisms 10, no. 10: 1948. https://doi.org/10.3390/microorganisms10101948
APA StyleSbehat, M., Mauriello, G., & Altamimi, M. (2022). Microencapsulation of Probiotics for Food Functionalization: An Update on Literature Reviews. Microorganisms, 10(10), 1948. https://doi.org/10.3390/microorganisms10101948








