Arbovirus Epidemiology: The Mystery of Unnoticed Epidemics in Ghana, West Africa
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
4.1. Medically Important Arboviruses in Ghana
4.1.1. Yellow Fever Virus
4.1.2. Dengue Virus
4.2. Entomological Investigations
4.3. Causes of Low Report of Arbovirus Infections in Ghana
4.3.1. Vector Competence and Vectorial Capacity
4.3.2. Misdiagnosis of Febrile Illnesses
4.3.3. Presence of Microbiota in the Mosquito Vector
4.3.4. Mosquito Immune Response
4.3.5. Use of Vector Control Tools
4.3.6. Ecology and Climate
4.3.7. Genetic Diversity of Mosquito Vectors
4.4. Other Causes of Low Report of Arbovirus Infections in Africa
5. Future Prospects and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuno, G.; Chang, G.-J.J. Biological Transmission of Arboviruses: Reexamination of and New Insights into Components, Mechanisms, and Unique Traits as Well as Their Evolutionary Trends. Clin. Microbiol. Rev. 2005, 18, 608–637. [Google Scholar] [CrossRef] [PubMed]
- Moureau, G.; Cook, S.; Lemey, P.; Nougairede, A.; Forrester, N.L.; Khasnatinov, M.; Charrel, R.N.; Firth, A.E.; Gould, E.A.; de Lamballerie, X. New Insights into Flavivirus Evolution, Taxonomy and Biogeographic History, Extended by Analysis of Canonical and Alternative Coding Sequences. PLoS ONE 2015, 10, e0117849. [Google Scholar] [CrossRef] [PubMed]
- Mordecai, E.A.; Ryan, S.J.; Caldwell, J.M.; Shah, M.M.; LaBeaud, A.D. Climate Change Could Shift Disease Burden from Malaria to Arboviruses in Africa. Lancet Planet. Health 2020, 4, e416–e423. [Google Scholar] [CrossRef]
- WHO. Global Vector Control Response 2017–2030; World Health Organization: Genèva, Switzerland, 2017.
- Agboli, E.; Zahouli, J.B.Z.; Badolo, A.; Jöst, H. Mosquito-Associated Viruses and Their Related Mosquitoes in West Africa. Viruses 2021, 13, 891. [Google Scholar] [CrossRef] [PubMed]
- Kading, R.C.; Brault, A.C.; Beckham, J.D. Global Perspectives on Arbovirus Outbreaks: A 2020 Snapshot. Trop. Med. Infect. Dis. 2020, 5, 142. [Google Scholar] [CrossRef] [PubMed]
- Braack, L.; Gouveia de Almeida, A.P.; Cornel, A.J.; Swanepoel, R.; de Jager, C. Mosquito-Borne Arboviruses of African Origin: Review of Key Viruses and Vectors. Parasites Vectors 2018, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Agboli, E.; Leggewie, M.; Altinli, M.; Schnettler, E. Mosquito-Specific Viruses—Transmission and Interaction. Viruses 2019, 11, 873. [Google Scholar] [CrossRef]
- Epelboin, Y.; Talaga, S.; Epelboin, L.; Dusfour, I. Zika Virus: An Updated Review of Competent or Naturally Infected Mosquitoes. PLoS Negl. Trop. Dis. 2017, 11, e0005933. [Google Scholar] [CrossRef]
- Paupy, C.; Delatte, H.; Bagny, L.; Corbel, V.; Fontenille, D. Aedes Albopictus, an Arbovirus Vector: From the Darkness to the Light. Microbes Infect. 2009, 11, 1177–1185. [Google Scholar] [CrossRef]
- Calzolari, M.; Gaibani, P.; Bellini, R.; Defilippo, F.; Pierro, A.; Albieri, A.; Maioli, G.; Luppi, A.; Rossini, G.; Balzani, A.; et al. Mosquito, Bird and Human Surveillance of West Nile and Usutu Viruses in Emilia-Romagna Region (Italy) in 2010. PLoS ONE 2012, 7, e38058. [Google Scholar] [CrossRef] [Green Version]
- Boorman, J.P.T.; Draper, C.C. Isolations of Arboviruses in the Lagos Area of Nigeria, and a Survey of Antibodies to Them in Man and Animals. Trans. R. Soc. Trop. Med. Hyg. 1968, 62, 269–277. [Google Scholar] [CrossRef]
- Ratovonjato, J.; Olive, M.-M.; Tantely, L.M.; Andrianaivolambo, L.; Tata, E.; Razainirina, J.; Jeanmaire, E.; Reynes, J.-M.; Elissa, N. Detection, Isolation, and Genetic Characterization of Rift Valley Fever Virus from Anopheles (Anopheles) Coustani, Anopheles (Anopheles) Squamosus, and Culex (Culex) Antennatus of the Haute Matsiatra Region, Madagascar. Vector-Borne Zoonotic Dis. 2011, 11, 753–759. [Google Scholar] [CrossRef]
- Maquart, M.; Boyer, S.; Rakotoharinome, V.M.; Ravaomanana, J.; Tantely, M.L.; Heraud, J.-M.; Cardinale, E. High Prevalence of West Nile Virus in Domestic Birds and Detection in 2 New Mosquito Species in Madagascar. PLoS ONE 2016, 11, e0147589. [Google Scholar] [CrossRef]
- Wilder-Smith, A.; Gubler, D.J.; Weaver, S.C.; Monath, T.P.; Heymann, D.L.; Scott, T.W. Epidemic Arboviral Diseases: Priorities for Research and Public Health. Lancet Infect. Dis. 2017, 17, e101–e106. [Google Scholar] [CrossRef]
- Mwanyika, G.O.; Mboera, L.E.G.; Rugarabamu, S.; Ngingo, B.; Sindato, C.; Lutwama, J.J.; Paweska, J.T.; Misinzo, G. Dengue Virus Infection and Associated Risk Factors in Africa: A Systematic Review and Meta-Analysis. Viruses 2021, 13, 536. [Google Scholar] [CrossRef]
- Bawe, L.D.; Patassi, A.A.; Kotosso, A.; Abaltou, B.; Moukaïla, A.-R.; Dandogan, D.; Wateba, M.I. Knowledge of Health Workers in Public Health Centers of the Health District of Lomé Commune on Dengue. Adv. Infect. Dis. 2021, 11, 430–440. [Google Scholar]
- Stoler, J.; al Dashti, R.; Anto, F.; Fobil, J.N.; Awandare, G.A. Deconstructing “Malaria”: West Africa as the next Front for Dengue Fever Surveillance and Control. Acta Trop. 2014, 134, 58–65. [Google Scholar] [CrossRef]
- MoH. Health Alert on Dengue Fever. Ministry of Health Republic of Ghana. 2016. Available online: https://www.moh.gov.gh/health-alert-on-dengue-fever/ (accessed on 20 April 2022).
- Buchwald, A.G.; Hayden, M.H.; Dadzie, S.K.; Paull, S.H.; Carlton, E.J. Aedes-Borne Disease Outbreaks in West Africa: A Call for Enhanced Surveillance. Acta Trop. 2020, 209, 105468. [Google Scholar] [CrossRef]
- Boorman, J.; Porterfield, J. A Small Outbreak of Yellow Fever in the Gold Coast. Trans. R. Soc. Trop. Med. Hyg. 1957, 51, 439–449. [Google Scholar] [CrossRef]
- Fabiyi, A. Yellow Fever at Tema, Ghana, 1959: A Serological Survey by Complement Fixation. Ann. Trop. Med. Parasitol. 1961, 55, 235–241. [Google Scholar] [CrossRef]
- Agadzi, V.K.; Boatin, B.A.; Appawu, M.A.; Mingle, J.A.; Addy, P.A. Yellow Fever in Ghana, 1977–1980. Bull. World Health Organ. 1984, 62, 577–583. [Google Scholar]
- Addy, P.A.; Minami, K.; Agadzi, V.K. Recent Yellow Fever Epidemics in Ghana (1969–1983). East Afr. Med. J. 1986, 63, 422–434. [Google Scholar]
- WHO. Yellow Fever Cases Reported in Ghana, 1950–2004; World Health Organization: Genève, Switzerland, 2005.
- Disaster Relief Emergency Fund (DREF). Ghana: Yellow Fever Outbreak. 2011. Available online: https://reliefweb.int/report/ghana/ghana-yellow-fever-outbreak-dref-operation-n%C2%B0-mdrgh005-final-report (accessed on 10 February 2022).
- WHO. Yellow Fever Situation Report; World Health Organization: Genève, Switzerland, 2016.
- WHO. Yellow Fever-Ghana; World Health Organization: Genève, Switzerland, 2021.
- WHO. Regional Office in Africa. Weekly Bulletins on Outbreaks and Other Emergencies. Week 17: 18–24 April 2022; World Health Organization: Genève, Switzerland, 2022.
- Huhtamo, E.; Uzcátegui, N.Y.; Siikamäki, H.; Saarinen, A.; Piiparinen, H.; Vaheri, A.; Vapalahti, O. Molecular Epidemiology of Dengue Virus Strains from Finnish Travelers. Emerg. Infect. Dis. 2008, 14, 80–83. [Google Scholar] [CrossRef]
- Stoler, J.; Fobil, J.N.; Bonney, J.H.K.; Owusu-Agyei, S.; Delimini, R.K.; Awandare, G.A.; Oduro, A.R. Evidence of Recent Dengue Exposure Among Malaria Parasite-Positive Children in Three Urban Centers in Ghana. Am. J. Trop. Med. Hyg. 2015, 92, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Sherman, K.E.; Rouster, S.D.; Kong, L.X.; Shata, T.M.; Archampong, T.; Kwara, A.; Aliota, M.T.; Blackard, J.T. Zika Virus Exposure in an HIV-Infected Cohort in Ghana. JAIDS J. Acquir. Immune Defic. Syndr. 2018, 78, e35–e38. [Google Scholar] [CrossRef] [PubMed]
- Ofosu-Appiah, L.; Kutame, R.; Ayensu, B.; Bonney, J.; Boateng, G.; Adade, R.; Opare, D.; Odoom, J. Detection of Dengue Virus in Samples from Suspected Yellow Fever Cases in Ghana. Microbiol. Res. J. Int. 2018, 24, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Narkwa, P.W.; Mutocheluh, M.; Kwofie, T.B.; Owusu, M.; Annan, A.; Ali, I.; Boamah, J.K. Dengue Virus Exposure among Blood Donors in Ghana. J. Med. Biomed. Sci. 2016, 5, 30–35. [Google Scholar] [CrossRef]
- Pappoe-Ashong, P.J.; Ofosu-Appiah, L.H.; Mingle, J.A.; Jassoy, C. Seroprevalence of Dengue Virus Infections in Ghana. East Afr. Med. J. 2018, 95, 2132–2140. [Google Scholar]
- Bonney, J.H.K.; Hayashi, T.; Dadzie, S.; Agbosu, E.; Pratt, D.; Nyarko, S.; Asiedu-Bekoe, F.; Ido, E.; Sarkodie, B.; Ohta, N.; et al. Molecular Detection of Dengue Virus in Patients Suspected of Ebola Virus Disease in Ghana. PLoS ONE 2018, 13, e0208907. [Google Scholar] [CrossRef]
- Amoako, N.; Duodu, S.; Dennis, F.E.; Bonney, J.H.K.; Asante, K.P.; Ameh, J.; Mosi, L.; Hayashi, T.; Agbosu, E.E.; Pratt, D.; et al. Detection of Dengue Virus among Children with Suspected Malaria, Accra, Ghana. Emerg. Infect. Dis. 2018, 24, 1544–1547. [Google Scholar] [CrossRef]
- Manu, S.K.; Bonney, J.H.K.; Pratt, D.; Abdulai, F.N.; Agbosu, E.E.; Frimpong, P.O.; Adiku, T.K. Arbovirus Circulation among Febrile Patients at the Greater Accra Regional Hospital, Ghana. BMC Res. Notes 2019, 12, 332. [Google Scholar] [CrossRef]
- Aniakwaa-Bonsu, E.; Amoako-Sakyi, D.; Dankwa, K.; Prah, J.K.; Nuvor, S.V. Seroprevalence of Dengue Viral Infection among Adults Attending the University of Cape Coast Hospital. Adv. Infect. Dis. 2021, 11, 60–72. [Google Scholar] [CrossRef]
- Wang, W.; Sarkodie, F.; Danso, K.; Addo-Yobo, E.; Owusu-Ofori, S.; Allain, J.-P.; Li, C. Seroprevalence of West Nile Virus in Ghana. Viral Immunol. 2009, 22, 17–22. [Google Scholar] [CrossRef]
- Ankrah, G.A.; Bonney, J.H.K.; Agbosu, E.E.; Pratt, D.; Adiku, T.K. Serological Evidence of Zika Virus Infection in Febrile Patients at Greater Accra Regional Hospital, Accra Ghana. BMC Res. Notes 2019, 12, 326. [Google Scholar] [CrossRef]
- Woodruff, A.W.; Bowen, E.T.W.; Platt, G.S. Viral Infections in Travellers from Tropical Africa. Br. Med. J. 1978, 1, 956–958. [Google Scholar] [CrossRef]
- Akuffo, R.; Brandful, J.A.M.; Zayed, A.; Adjei, A.; Watany, N.; Fahmy, N.T.; Hughes, R.; Doman, B.; Voegborlo, S.V.; Aziati, D.; et al. Crimean-Congo Hemorrhagic Fever Virus in Livestock Ticks and Animal Handler Seroprevalence at an Abattoir in Ghana. BMC Infect. Dis. 2016, 16, 324. [Google Scholar] [CrossRef]
- Appawu, M.; Dadzie, S.; Abdul, H.; Asmah, H.; Boakye, D.; Wilson, M.; Ofori-adjei, D. Surveillance of Viral Haemorrhagic Fevers in Ghana: Entomological Assessment of the Risk of Transmission in the Northern Regions. Ghana Med. J. 2006, 40, 137–141. [Google Scholar] [CrossRef]
- Amoa-Bosompem, M.; Kobayashi, D.; Murota, K.; Faizah, A.N.; Itokawa, K.; Fujita, R.; Osei, J.H.N.; Agbosu, E.; Pratt, D.; Kimura, S.; et al. Entomological Assessment of the Status and Risk of Mosquito-Borne Arboviral Transmission in Ghana. Viruses 2020, 12, 147. [Google Scholar] [CrossRef]
- Joannides, J.; Dzodzomenyo, M.; Azerigyik, F.; Agbosu, E.E.; Pratt, D.; Nyarko Osei, J.H.; Pwalia, R.; Amlalo, G.K.; Appawu, M.; Takashi, H.; et al. Species Composition and Risk of Transmission of Some Aedes-Borne Arboviruses in Some Sites in Northern Ghana. PLoS ONE 2021, 16, e0234675. [Google Scholar] [CrossRef]
- Nimo-Paintsil, S.C.; Mosore, M.; Addo, S.O.; Lura, T.; Tagoe, J.; Ladzekpo, D.; Addae, C.; Bentil, R.E.; Behene, E.; Dafeamekpor, C.; et al. Ticks and Prevalence of Tick-Borne Pathogens from Domestic Animals in Ghana. Parasites Vectors 2022, 15, 86. [Google Scholar] [CrossRef]
- Kobayashi, D.; Ohashi, M.; Osei, J.H.N.; Agbosu, E.; Opoku, M.; Agbekudzi, A.; Joannides, J.; Fujita, R.; Sasaki, T.; Bonney, J.H.K.; et al. Detection of a Novel Putative Phlebovirus and First Isolation of Dugbe Virus from Ticks in Accra, Ghana. Ticks Tick. Borne. Dis. 2017, 8, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Amoa-Bosompem, M.; Kobayashi, D.; Faizah, A.N.; Kimura, S.; Antwi, A.; Agbosu, E.; Pratt, D.; Ohashi, M.; Bonney, J.H.K.; Dadzie, S.; et al. Screening for Tick-Borne and Tick-Associated Viruses in Ticks Collected in Ghana. Arch. Virol. 2022, 167, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Adam, A.; Jassoy, C. Epidemiology and Laboratory Diagnostics of Dengue, Yellow Fever, Zika, and Chikungunya Virus Infections in Africa. Pathogens 2021, 10, 1324. [Google Scholar] [CrossRef] [PubMed]
- CDC. Yellow Fever. Available online: https://www.cdc.gov/globalhealth/newsroom/topics/yellowfever/index.html (accessed on 19 March 2021).
- Captain-Esoah, M.; Kweku Baidoo, P.; Frempong, K.K.; Adabie-Gomez, D.; Chabi, J.; Obuobi, D.; Kwame Amlalo, G.; Balungnaa Veriegh, F.; Donkor, M.; Asoala, V.; et al. Biting Behavior and Molecular Identification of Aedes Aegypti (Diptera: Culicidae) Subspecies in Some Selected Recent Yellow Fever Outbreak Communities in Northern Ghana. J. Med. Entomol. 2020, 57, 1239–1245. [Google Scholar] [CrossRef]
- Tchouassi, D.P.; Marklewitz, M.; Chepkorir, E.; Zirkel, F.; Agha, S.B.; Tigoi, C.C.; Koskei, E.; Drosten, C.; Borgemeister, C.; Torto, B.; et al. Sand Fly–Associated Phlebovirus with Evidence of Neutralizing Antibodies in Humans, Kenya. Emerg. Infect. Dis. 2019, 25, 681–690. [Google Scholar] [CrossRef]
- Oerther, S.; Jöst, H.; Heitmann, A.; Lühken, R.; Krüger, A.; Steinhausen, I.; Brinker, C.; Lorentz, S.; Marx, M.; Schmidt-Chanasit, J.; et al. Phlebotomine Sand Flies in Southwest Germany: An Update with Records in New Locations. Parasites Vectors 2020, 13, 173. [Google Scholar] [CrossRef]
- Elliott, R.M.; Brennan, B. Emerging Phleboviruses. Curr. Opin. Virol. 2014, 5, 50–57. [Google Scholar] [CrossRef]
- Tesh, R. The Genus Phlebovirus And Its Vectors. Annu. Rev. Entomol. 1988, 33, 169–181. [Google Scholar] [CrossRef]
- Charrel, R.N.; Gallian, P.; Navarro-Marí, J.-M.; Nicoletti, L.; Papa, A.; Sánchez-Seco, M.P.; Tenorio, A.; de Lamballerie, X. Emergence of Toscana Virus in Europe. Emerg. Infect. Dis. 2005, 11, 1657–1663. [Google Scholar] [CrossRef]
- Amaro, F.; Zé-Zé, L.; Alves, M.J.; Börstler, J.; Clos, J.; Lorenzen, S.; Becker, S.C.; Schmidt-Chanasit, J.; Cadar, D. Co-Circulation of a Novel Phlebovirus and Massilia Virus in Sandflies, Portugal. Virol. J. 2015, 12, 174. [Google Scholar] [CrossRef]
- Fontenille, D.; Ba, Y.; Digoutte, J.P.; Leclerc, A.; Zeller, H.G.; Mondo, M.; Traore-Lamizana, M.; Trouillet, J. First Isolations of Arboviruses from Phlebotomine Sand Flies in West Africa. Am. J. Trop. Med. Hyg. 1994, 50, 570–574. [Google Scholar] [CrossRef]
- Geevarghese, G.; Arankalle, V.A.; Jadi, R.; Kanojia, P.C.; Joshi, M.V.; Mishra, A.C. Detection of Chandipura Virus from Sand Flies in the Genus Sergentomyia (Diptera: Phlebotomidae) at Karimnagar District, Andhra Pradesh, India. J. Med. Entomol. 2005, 42, 495–496. [Google Scholar] [CrossRef]
- Doe, E.D.; Kwakye-Nuako, G.; Addo, S.O.; Egyir-Yawson, A. Identification of Sand Flies (Diptera: Psychodidae) Collected from Cutaneous Leishmaniasis Endemic Focus in the Ho Municipality, Ghana. Int. Ann. Sci. 2020, 10, 33–44. [Google Scholar] [CrossRef]
- Mayton, E.H.; Tramonte, A.R.; Wearing, H.J.; Christofferson, R.C. Age-Structured Vectorial Capacity Reveals Timing, Not Magnitude of within-Mosquito Dynamics Is Critical for Arbovirus Fitness Assessment. Parasites Vectors 2020, 13, 310. [Google Scholar] [CrossRef]
- Beerntsen, B.T.; James, A.A.; Christensen, B.M. Genetics of Mosquito Vector Competence. Microbiol. Mol. Biol. Rev. 2000, 64, 115–137. [Google Scholar] [CrossRef]
- Gould, E.; Pettersson, J.; Higgs, S.; Charrel, R.; de Lamballerie, X. Emerging Arboviruses: Why Today? One Health 2017, 4, 1–13. [Google Scholar] [CrossRef]
- Amoa-Bosompem, M.; Kobayashi, D.; Itokawa, K.; Murota, K.; Faizah, A.N.; Azerigyik, F.A.; Hayashi, T.; Ohashi, M.; Bonney, J.H.K.; Dadzie, S.; et al. Determining Vector Competence of Aedes Aegypti from Ghana in Transmitting Dengue Virus Serotypes 1 and 2. Parasites Vectors 2021, 14, 1–12. [Google Scholar] [CrossRef]
- Ayorinde, A.F.; Oyeyiga, A.M.; Nosegbe, N.O.; Folarin, O.A. A Survey of Malaria and Some Arboviral Infections among Suspected Febrile Patients Visiting a Health Centre in Simawa, Ogun State, Nigeria. J. Infect. Public Health 2016, 9, 52–59. [Google Scholar] [CrossRef]
- Stoler, J.; Awandare, G.A. Febrile Illness Diagnostics and the Malaria-Industrial Complex: A Socio-Environmental Perspective. BMC Infect. Dis. 2016, 16, 683. [Google Scholar] [CrossRef]
- Sow, A.; Loucoubar, C.; Diallo, D.; Faye, O.; Ndiaye, Y.; Senghor, C.S.; Dia, A.T.; Faye, O.; Weaver, S.C.; Diallo, M.; et al. Concurrent Malaria and Arbovirus Infections in Kedougou, Southeastern Senegal. Malar. J. 2016, 15, 47. [Google Scholar] [CrossRef]
- Hogan, B.; Eibach, D.; Krumkamp, R.; Sarpong, N.; Dekker, D.; Kreuels, B.; Maiga-Ascofaré, O.; Gyau Boahen, K.; Wiafe Akenten, C.; Adu-Sarkodie, Y.; et al. Malaria Coinfections in Febrile Pediatric Inpatients: A Hospital-Based Study From Ghana. Clin. Infect. Dis. 2018, 66, 1838–1845. [Google Scholar] [CrossRef]
- Reyes, J.I.L.; Suzuki, Y.; Carvajal, T.; Muñoz, M.N.M.; Watanabe, K. Intracellular Interactions Between Arboviruses and Wolbachia in Aedes Aegypti. Front. Cell. Infect. Microbiol. 2021, 11, 690087. [Google Scholar] [CrossRef]
- Gesto, J.S.M.; Ribeiro, G.S.; Rocha, M.N.; Dias, F.B.S.; Peixoto, J.; Carvalho, F.D.; Pereira, T.N.; Moreira, L.A. Reduced Competence to Arboviruses Following the Sustainable Invasion of Wolbachia into Native Aedes Aegypti from Southeastern Brazil. Sci. Rep. 2021, 11, 10039. [Google Scholar] [CrossRef]
- McFarlane, M.; Arias-Goeta, C.; Martin, E.; O’Hara, Z.; Lulla, A.; Mousson, L.; Rainey, S.M.; Misbah, S.; Schnettler, E.; Donald, C.L.; et al. Characterization of Aedes Aegypti Innate-Immune Pathways That Limit Chikungunya Virus Replication. PLoS Negl. Trop. Dis. 2014, 8, e2994. [Google Scholar] [CrossRef]
- Lee, W.-S.; Webster, J.A.; Madzokere, E.T.; Stephenson, E.B.; Herrero, L.J. Mosquito Antiviral Defense Mechanisms: A Delicate Balance between Innate Immunity and Persistent Viral Infection. Parasites Vectors 2019, 12, 165. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y. Post-Transcriptional Suppression of Gene Expression in Xenopus Embryos by Small Interfering RNA. Nucleic Acids Res. 2002, 30, 1664–1669. [Google Scholar] [CrossRef] [PubMed]
- Leggewie, M.; Schnettler, E. RNAi-Mediated Antiviral Immunity in Insects and Their Possible Application. Curr. Opin. Virol. 2018, 32, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Swevers, L.; Kolliopoulou, A.; Smagghe, G. Arboviruses and the Challenge to Establish Systemic and Persistent Infections in Competent Mosquito Vectors: The Interaction With the RNAi Mechanism. Front. Physiol. 2019, 10, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Vargas, I.; Scott, J.C.; Poole-Smith, B.K.; Franz, A.W.E.; Barbosa-Solomieu, V.; Wilusz, J.; Olson, K.E.; Blair, C.D. Dengue Virus Type 2 Infections of Aedes Aegypti Are Modulated by the Mosquito’s RNA Interference Pathway. PLoS Pathog. 2009, 5, e1000299. [Google Scholar] [CrossRef] [PubMed]
- Schnettler, E.; Donald, C.L.; Human, S.; Watson, M.; Siu, R.W.C.; McFarlane, M.; Fazakerley, J.K.; Kohl, A.; Fragkoudis, R. Knockdown of PiRNA Pathway Proteins Results in Enhanced Semliki Forest Virus Production in Mosquito Cells. J. Gen. Virol. 2013, 94, 1680–1689. [Google Scholar] [CrossRef]
- Tripet, F.; Aboagye-Antwi, F.; Hurd, H. Ecological Immunology of Mosquito–Malaria Interactions. Trends Parasitol. 2008, 24, 219–227. [Google Scholar] [CrossRef]
- Alonso, P.; Engels, D.; Reeder, J. Renewed Push to Strengthen Vector Control Globally. Lancet 2017, 389, 2270–2271. [Google Scholar] [CrossRef]
- Achee, N.L.; Grieco, J.P.; Vatandoost, H.; Seixas, G.; Pinto, J.; Ching-NG, L.; Martins, A.J.; Juntarajumnong, W.; Corbel, V.; Gouagna, C.; et al. Alternative Strategies for Mosquito-Borne Arbovirus Control. PLoS Negl. Trop. Dis. 2019, 13, e0006822. [Google Scholar]
- Binka, F.N.; Kubaje, A.; Adjuik, M.; Williams, L.A.; Lengeler, C.; Maude, G.H.; Armah, G.E.; Kajihara, B.; Adiamah, J.H.; Smith, P.G. Impact of Permethrin Impregnated Bednets on Child Mortality in Kassena-Nankana District, Ghana: A Randomized Controlled Trial. Trop. Med. Int. Health 1996, 1, 147–154. [Google Scholar] [CrossRef]
- Ahorlu, C.S.; Adongo, P.; Koenker, H.; Zigirumugabe, S.; Sika-Bright, S.; Koka, E.; Tabong, P.T.-N.; Piccinini, D.; Segbaya, S.; Olapeju, B.; et al. Understanding the Gap between Access and Use: A Qualitative Study on Barriers and Facilitators to Insecticide-Treated Net Use in Ghana. Malar. J. 2019, 18, 417. [Google Scholar] [CrossRef] [Green Version]
- Axame, W.K.; Kweku, M.; Amelor, S.; Kye-Duodu, G.; Agboli, E.; Agbemafle, I.; Takramah, W.; Tarkang, E.; Binka, F.N. Ownership and Utilization of Long Lasting Insecticide Treated Nets ( LLIN ) and Factors Associated to Non-Utilization Among Pregnant Women in Ho Municipality of Ghana. Cent. African J. Public Health 2016, 2, 35–42. [Google Scholar]
- Addy, P.A.K.; Esena, R.K.; Atuahene, S.K.N. Possible Contributing Factors to the Paucity of Yellow Fever Epidemics in the Ashanti Region of Ghana, West Africa. East Afr. Med. J. 1996, 73, 3–9. [Google Scholar]
- Ng’ang’a, P.N.; Aduogo, P.; Mutero, C.M. Strengthening Community and Stakeholder Participation in the Implementation of Integrated Vector Management for Malaria Control in Western Kenya: A Case Study. Malar. J. 2021, 20, 155. [Google Scholar] [CrossRef]
- WHO. A Toolkit for Integrated Vector Management in Sub-Saharan Africa; WHO/HTM/NTD/VEM/2016.02; World Health Organization: Genève, Switzerland, 2016.
- Zahouli, J.B.Z.; Koudou, B.G.; Müller, P.; Malone, D.; Tano, Y.; Utzinger, J. Urbanization Is a Main Driver for the Larval Ecology of Aedes Mosquitoes in Arbovirus-Endemic Settings in South-Eastern Côte d’Ivoire. PLoS Negl. Trop. Dis. 2017, 11, e0005751. [Google Scholar] [CrossRef]
- Schmidt-Chanasit, J.; Agboli, E.; Jöst, H. Special Issue “Mosquito-Borne Virus Ecology. ” Viruses 2022, 14, 357. [Google Scholar] [CrossRef]
- MoFA. Agriculture in Ghana Facts and Figures (2015); MoFA: Singapore, 2016.
- Amarasinghe, A.; Kuritsky, J.N.; William Letson, G.; Margolis, H.S. Dengue Virus Infection in Africa. Emerg. Infect. Dis. 2011, 17, 1349–1354. [Google Scholar] [CrossRef] [PubMed]
- Im, J.; Balasubramanian, R.; Ouedraogo, M.; Wandji Nana, L.R.; Mogeni, O.D.; Jeon, H.J.; van Pomeren, T.; Haselbeck, A.; Lim, J.K.; Prifti, K.; et al. The Epidemiology of Dengue Outbreaks in 2016 and 2017 in Ouagadougou, Burkina Faso. Heliyon 2020, 6, e04389. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, A.M.; Randolph, S.E. Drivers, Dynamics, and Control of Emerging Vector-Borne Zoonotic Diseases. Lancet 2012, 380, 1946–1955. [Google Scholar] [CrossRef]
- Afrane, Y.A.; Klinkenberg, E.; Drechsel, P.; Owusu-Daaku, K.; Garms, R.; Kruppa, T. Does Irrigated Urban Agriculture Influence the Transmission of Malaria in the City of Kumasi, Ghana? Acta Trop. 2004, 89, 125–134. [Google Scholar] [CrossRef]
- Ye-Ebiyo, Y.; Pollack, R.J.; Spielman, A. Enhanced Development in Nature of Larval Anopheles Arabiensis Mosquitoes Feeding on Maize Pollen. Am. J. Trop. Med. Hyg. 2000, 63, 90–93. [Google Scholar] [CrossRef]
- Latreille, A.C.; Milesi, P.; Magalon, H.; Mavingui, P.; Atyame, C.M. High Genetic Diversity but No Geographical Structure of Aedes Albopictus Populations in Réunion Island. Parasites Vectors 2019, 12, 597. [Google Scholar] [CrossRef]
- Li, S.; Jiang, F.; Lu, H.; Kang, X.; Wang, Y.; Zou, Z.; Wen, D.; Zheng, A.; Liu, C.; Liu, Q.; et al. Mosquito Diversity and Population Genetic Structure of Six Mosquito Species From Hainan Island. Front. Genet. 2020, 11, 602863. [Google Scholar] [CrossRef]
- Fernando, H.S.D.; Hapugoda, M.; Perera, R.; Black IV, W.C.; De Silva, B.G.D.N.K. Mitochondrial Metabolic Genes Provide Phylogeographic Relationships of Global Collections of Aedes Aegypti (Diptera: Culicidae). PLoS ONE 2020, 15, e0235430. [Google Scholar] [CrossRef]
- Sylla, M.; Bosio, C.; Urdaneta-Marquez, L.; Ndiaye, M.; Black, W.C. Gene Flow, Subspecies Composition, and Dengue Virus-2 Susceptibility among Aedes Aegypti Collections in Senegal. PLoS Negl. Trop. Dis. 2009, 3, e408. [Google Scholar] [CrossRef]
- McBride, C.S.; Baier, F.; Omondi, A.B.; Spitzer, S.A.; Lutomiah, J.; Sang, R.; Ignell, R.; Vosshall, L.B. Evolution of Mosquito Preference for Humans Linked to an Odorant Receptor. Nature 2014, 515, 222–227. [Google Scholar] [CrossRef]
- Carpenter, S.; Groschup, M.H.; Garros, C.; Felippe-Bauer, M.L.; Purse, B.V. Culicoides Biting Midges, Arboviruses and Public Health in Europe. Antiviral Res. 2013, 100, 102–113. [Google Scholar] [CrossRef] [Green Version]
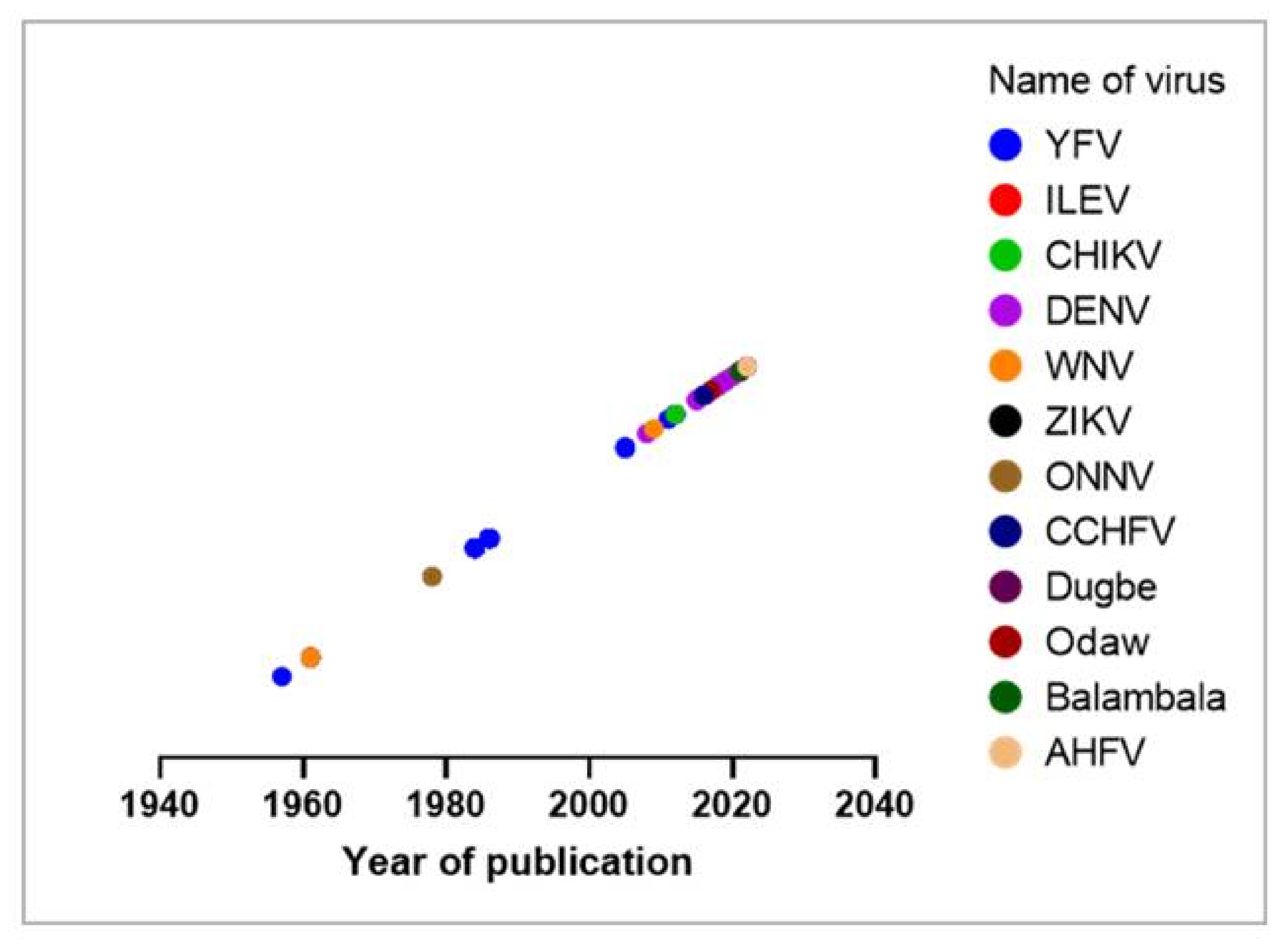
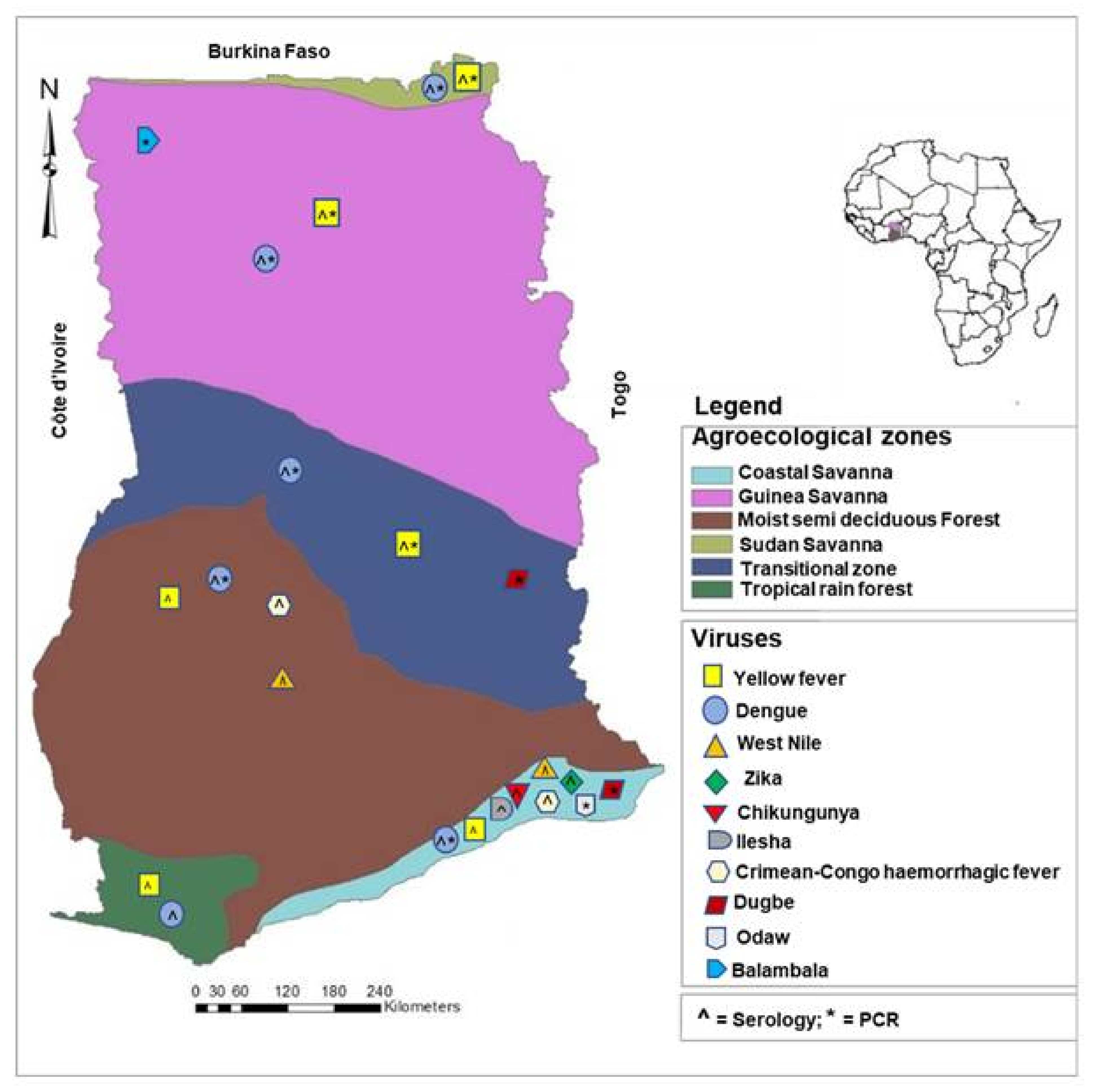
| Year of Sampling/ Detection | Study Design | Number of People/Sample/Cases | Frequencies (Positives/ Deaths) | Region (Place) | Detection Method | Reference |
|---|---|---|---|---|---|---|
| Yellow fever (Flaviviridae) | Human cases | |||||
| 1955 | Cross-sectional | 12 cases, 155 total sera | 3 confirmed | Brong Ahafo (Kintampo) | Histology, Serology | [21] |
| 1959 | Cross-sectional | 76 sera | 38.3% CFR | Greater Accra (Tema) | Serology—Complement fixation | [22] |
| 1963 | Case report | 3 cases | NI | Ashanti (/Kumasi), Northern (Damongo) | NI | [23] |
| 1969 | Case report | 5 cases 303 cases | 3 deaths 72 deaths | Northen (Tamale) Upper East (Bolgatanga) | NI | [23] |
| 1970 | Case report Case report | 11 cases NI | 14 deaths 60 deaths | Eastern (Akwatia) Eastern (Asikasu) | NI Serology, Histopathology | [23] [24] |
| 1970–1975 | Case report | 12 cases | 7 deaths | Brong Ahafo (Dormaa Ahenkro, Berekum, Hwidiem) | NI | [23] |
| 1977–1978 | Cross-sectional | 136 cases | 34 deaths | Upper East (Jirapa) | Serology, Histopathology | [23] |
| 1978–1979 | Cross-sectional | 239 cases 340 cases | 56 deaths 52 deaths | Eastern (Maase, Somanya, Akuse, Akosombo, Nkwakwa, Asamankese) Volta (Hohoe, Kpandu) | Histopathology Serology, Histopathology | [23] |
| 1979–1980 | Cross-sectional | 104 cases | 41 deaths (39% CFR) | Brong Ahafo (Wenchi, Techiman, Hwidiem, Berekum, Dormaa Ahenkro) Eastern (Akwatia) | Serology, Histopathology | [23] |
| 1979 | Case report | NI | 4 deaths | Greater Accra | Serology, Histopathology | [25] |
| 1980 | Case report | 6 cases 2 cases | 4 deaths 2 deaths | Brong Ahafo Volta | Serology, Histopathology | [24] |
| Yellow fever (Flaviviridae) | Human cases | |||||
| 1981 | Case report | 6 cases | 3 deaths | Whole Ghana | Serology, Histopathology | [24] |
| 1982 | Case report | 7 cases | 5 deaths | Whole Ghana | Serology, Histopathology | [24] |
| 1983 | Case report | 205 cases 53 cases 55 cases 12 cases | 120 deaths 36 deaths 8 deaths 12 deaths | Northern (Bole, Damongo, Gambaga, Tamale) Upper East (Bawku, Bolgatanga) Upper West (Wa, Tumu) Brong Ahafo (Kintampo) | Serology, Histopathology | [24] |
| 1983–1984 | Case report | 372 cases | 201 deaths | Upper regions (Jirapa, Wa, Bolgatanga, Navrongo, Nandom, Jirapa) | Serology, Histopathology | [25] |
| 1993–1994 | Case report | 118 cases | 26 deaths | Upper West (Jirapa) | Serology, Histopathology | [25] |
| 1996–1997 | Case report | 33 cases | 5 deaths | Upper East (Bolgatanga etc.), Northern (Mamprusi) | Serology, Histopathology | [25] |
| 2005 | Case report | 3 cases | None | Upper West (Jirapa, Wa, Nadowli) | NI | [26] |
| 2011 | Case report | 2 cases 3 cases | 1 death None | Northern region/Sawla Kalba Greater Accra (Ledzokuku) | NI | [26] |
| 2016 | Case report | 4 suspected | None | Brong Ahafo, Volta | NI | [27] |
| 2021 | Case report | 70 cases | 35 deaths (17% CFR). PCR positive | Savannah, Upper West, Bono, and Oti | Serology—IgM, PCR, Plaque reduction neutralization assay. | [28] |
| 2022 | Case report | 71 cases | 71 IgM positive | Whole Ghana (13 regions) | Serology | [29] |
| Dengue (Flaviviridae) | Human cases | |||||
| 1959 | Cross-sectional | 76 sera | Not specific | Greater Accra (Tema) | Serology | [22] |
| 2005 | Cross-sectional | 11 isolates | 1 PCR positive | Ghana-Finland | Serology—IgG/IgM, RT-PCR | [30] |
| 2011–2014 | Cross-sectional | 218 children | 3.2% IgM, 21.6% IgG | Greater Accra (Kpeshie), Brong Ahafo (Kintampo), Upper East (Navrongo) | Serology—IgG/IgM, RT-PCR | [31] |
| 2012–2014 | Cross-sectional | 236 sera | 87.2% antibody prevalence | Greater Accra (Korlebu) | Serology, Plaque reduction neutralization tests (PRNT) | [32] |
| 2013 | Cross-sectional | 360 sera | 1.9% IgM, 3.6% IgG | Whole Ghana (ten regions) | Serology—IgG, IgM, RT-PCR | [33] |
| 2013–2015 | Cross-sectional | 188 sera | 82 (43.6%) IgG | Ashanti (Agogo, Kumasi), Brong Ahafo (Techiman) | Serology—IgG, IgM, RT-PCR | [34] |
| 2014 | Cross-sectional | 417 sera | 29.7% IgG | Whole Ghana (ten regions) | Serology—IgG | [35] |
| 2014–2016 | Cross-sectional | 150 patients | 32 IgM positive, 4 PCR positives | Greater Accra, Central, Upper East | Serology—IgG, IgM, RT-PCR | [36] |
| 2016–2017 | Cross-sectional | 700 children | 2 PCR positives, IgG/IgM positive | Greater Accra, Brong Ahafo | Serology—IgG, IgM, RT-PCR | [37] |
| 2016–2017 | Cross-sectional | 260 febrile patients | 69.23% antibody positive | Greater Accra | Serology—IgG, IgM, NS1, RT-PCR | [38] |
| 2019 | Cross-sectional | 270 participants | 12.6% IgG, 2.2% IgM positives | Central (Cape Coast, Komenda) | Serology—IgG/IgM | [39] |
| Human cases | ||||||
| West Nile (Flaviviridae) | ||||||
| 1959 | Cross-sectional | 76 sera | Not specific | Greater Accra (Tema) | Serology—Complement fixation | [22] |
| 2009 * | Cross-sectional | 1324 plasma | Children = 4.8% IgG, 2.4% IgM positive | Ashanti (Kumasi) | Serology—IgG/IgM, PCR | [40] |
| Zika (Flaviviridae) | ||||||
| 2012–2014 | Cross-sectional | 236 sera | 12.9% antibody positive | Greater Accra (Korlebu) | Serology—IgG/IgM, PRNT | [32] |
| 2016–2017 | Cross-sectional | 160 patients | 20.6% antibody positive | Greater Accra | Serology—IgG/IgM | [41] |
| Chikungunya (Togaviridae) | ||||||
| 1959 | Cross-sectional | 76 sera | Not specific | Greater Accra (Tema) | Serology—Complement fixation | [22] |
| 2016–2017 | Cross-sectional | 260 patients | 27.69% antibody positive | Greater Accra | Serology-NS1/IgG/IgM, RT-PCR | [38] |
| Onyongnyong (Togaviridae) | ||||||
| 1954 | Cross-sectional | 86 travelers to Britain | 3 seropositive | NI | Serology—Complement fixation, Hemagglutination inhibition, Immunofluorescence assay | [42] |
| Ilesha (Peribunyaviridae) | ||||||
| 1959 | Cross-sectional | 76 sera | 28.5% (30–44 years) positive | Greater Accra (Tema) | Serology—Complement fixation | [22] |
| Crimean–Congo hemorrhagic fever (Nairoviridae) | ||||||
| 2011 | Longitudinal study | 188 sera | 5.7% seroprevalence | Greater Accra, Ashanti (Kumasi) | Serology—IgG/IgM | [43] |
| Yellow fever (Flaviviridae) | Mosquito surveillance | |||||
| 1955 | Cross-sectional | 299 mosquitoes | No arbovirus detected | Brong Ahafo (Kintampo) | Serology, Histology | [21] |
| 1999–2000 | Cross-sectional | 2804 households | No arbovirus detected | Northern (Damongo), Upper East (Bolgatanga), Upper West (Jirapa, Tumu) | RT-PCR | [44] |
| Dengue (Flaviviridae) | ||||||
| 1999–2000 | Cross-sectional | 2804 households | No arbovirus detected | Northern (Damongo), Upper East (Bolgatanga), Upper West (Jirapa, Tumu) | RT-PCR | [44] |
| 2015–2016 | Cross-sectional | 36 mosquitoes per pool | Only mosquito-specific virus detected | Greater Accra. Volta. Western. Ashanti. Upper West. Savannah. | RT-PCR | [45] |
| 2018–2019 | Cross-sectional | 1930 Aedes mosquitoes, 75 pools. | No arbovirus detected | Northern (Larabanga, Mole) | RT-PCR | [46] |
| Zika (Flaviviridae) | ||||||
| 2018–2019 | Cross-sectional | 1930 Aedes mosquitoes, 75 pools. | No arbovirus detected | Northern (Larabanga, Mole) | RT-PCR | [46] |
| West Nile (Flaviviridae) | ||||||
| 2015–2016 | Cross-sectional | 36 mosquitoes per pool | Only mosquito-specific virus detected | Greater Accra. Volta. Western. Ashanti. Upper West. Savannah. | RT-PCR | [45] |
| Chikungunya (Togaviridae) | ||||||
| 2015–2016 | Cross-sectional | 36 mosquitoes per pool | Only mosquito-specific virus detected | Greater Accra. Volta. Western. Ashanti. Upper West. Savannah. | RT-PCR | [45] |
| 2018–2019 | Cross-sectional | 1930 Aedes mosquitoes, 75 pools. | No arbovirus detected | Northern (Larabanga, Mole) | RT-PCR | [46] |
| Tick surveillance | ||||||
| Crimean-Congo haemorrhagic fever (Nairoviridae) | ||||||
| 2011 | Longitudinal | 144 ticks, 97 pools | 5 positive pools | Greater Accra, Ashanti (Kumasi) | Serology—IgG/IgM, RT-PCR | [43] |
| 2016–2017 | Cross-sectional (domestic dogs, goats, and cattle) | 2016 ticks, 912 pools | No CCHFV detected | Greater Accra (Accra), Northern (Tamale) | RT-PCR | [47] |
| Dugbe (Nairoviridae) | ||||||
| 2015 | Cross-sectional (domestic dogs and cattle) | 153 ticks, 29 pools | 2 positive pools | Greater Accra (Mobore) | RT-PCR | [48] |
| 2016 | Cross-sectional (domestic dogs and cattle) | 354 ticks, 93 pools | 1 positive pool | Volta (Hohoe) | RT-PCR | [49] |
| Odaw (Phenuiviridae) | ||||||
| 2015 | Cross-sectional (domestic dogs and cattle) | 153 ticks, 29 pools | 4 positive pools | Greater Accra (Pokuase, Korle-Gonno) | RT-PCR | [48] |
| 2016 | Cross-sectional (domestic dogs and cattle) | 354 ticks, 93 pools | 4 positive pools | Greater Accra (Accra) | RT-PCR | [49] |
| Balambala (Phenuiviridae) | ||||||
| 2016 | Cross-sectional (domestic dogs and cattle) | 354 ticks, 93 pools | 2 positive pools | Upper west (Jirapa) | RT-PCR | [49] |
| Alkhurma haemorrhagic fever (Flaviviridae) | ||||||
| 2016–2017 | Cross-sectional (domestic dogs, goats, and cattle) | 2016 ticks, 912 pools | No AHFV detected | Greater Accra (Accra), Northern (Tamale) | RT-PCR | [47] |
| Sand fly/Biting midges surveillance | ||||||
| No available record | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agboli, E.; Tomazatos, A.; Maiga-Ascofaré, O.; May, J.; Lühken, R.; Schmidt-Chanasit, J.; Jöst, H. Arbovirus Epidemiology: The Mystery of Unnoticed Epidemics in Ghana, West Africa. Microorganisms 2022, 10, 1914. https://doi.org/10.3390/microorganisms10101914
Agboli E, Tomazatos A, Maiga-Ascofaré O, May J, Lühken R, Schmidt-Chanasit J, Jöst H. Arbovirus Epidemiology: The Mystery of Unnoticed Epidemics in Ghana, West Africa. Microorganisms. 2022; 10(10):1914. https://doi.org/10.3390/microorganisms10101914
Chicago/Turabian StyleAgboli, Eric, Alexandru Tomazatos, Oumou Maiga-Ascofaré, Jürgen May, Renke Lühken, Jonas Schmidt-Chanasit, and Hanna Jöst. 2022. "Arbovirus Epidemiology: The Mystery of Unnoticed Epidemics in Ghana, West Africa" Microorganisms 10, no. 10: 1914. https://doi.org/10.3390/microorganisms10101914
APA StyleAgboli, E., Tomazatos, A., Maiga-Ascofaré, O., May, J., Lühken, R., Schmidt-Chanasit, J., & Jöst, H. (2022). Arbovirus Epidemiology: The Mystery of Unnoticed Epidemics in Ghana, West Africa. Microorganisms, 10(10), 1914. https://doi.org/10.3390/microorganisms10101914






