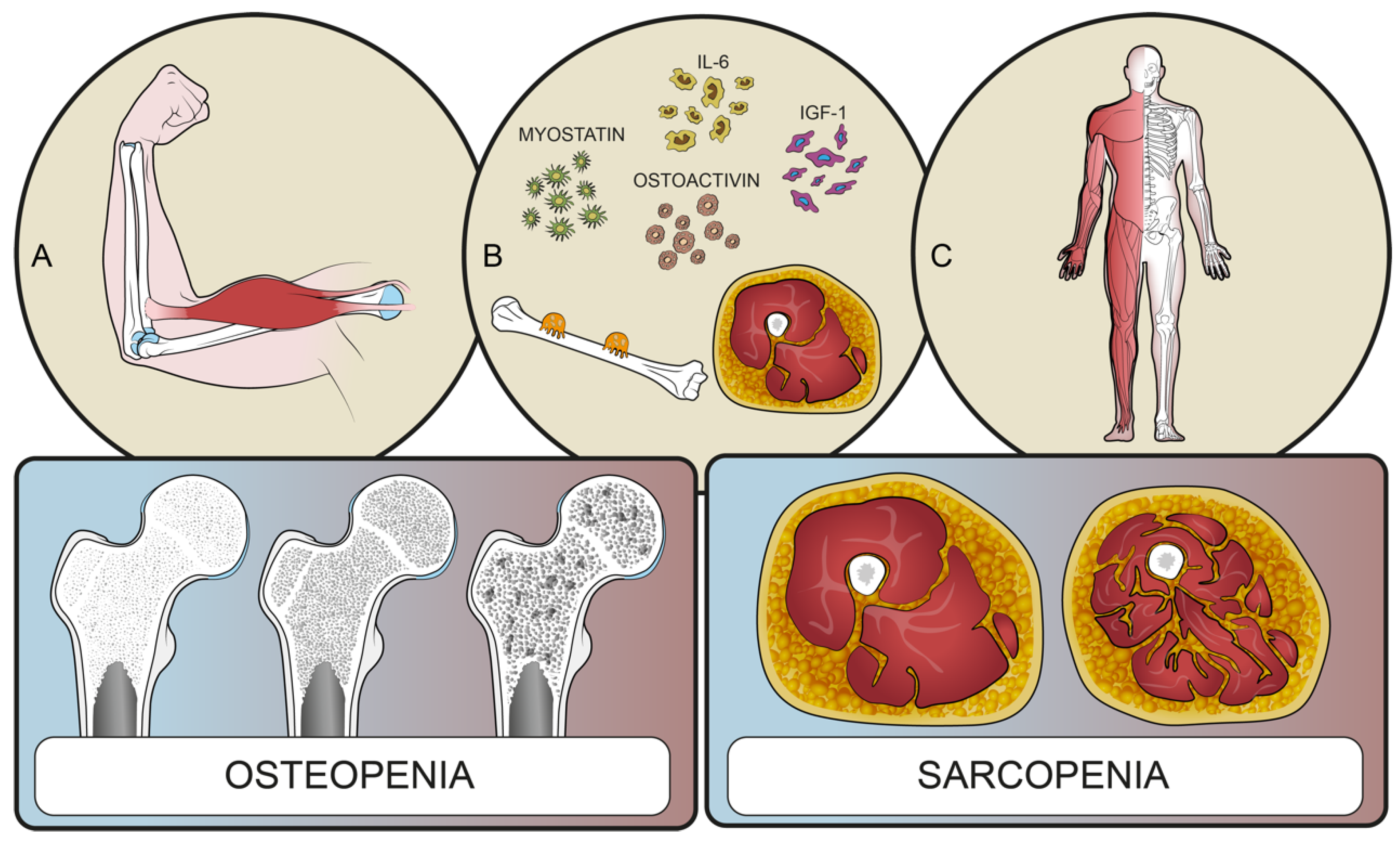
Osteopenia and Sarcopenia as Potential Risk Factors for Surgical Site Infection after Posterior Lumbar Fusion: A Retrospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sample
2.2. Data Collection
2.3. Statistical Analysis
3. Results
3.1. Demographic Data
3.2. High vs. Low PLVI Patients
3.3. High vs. Low M-Score Patients
3.4. Infectious Status
3.5. Multivariate Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lonjon, G.; Dauzac, C.; Fourniols, E.; Guigui, P.; Bonnomet, F.; Bonnevialle, P. Early Surgical Site Infections in Adult Spinal Trauma: A Prospective, Multicentre Study of Infection Rates and Risk Factors. Orthop. Traumatol. Surg. Res. 2012, 98, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Cizik, A.M.; Hamilton, D.; Chapman, J.R. Predicting Surgical Site Infection after Spine Surgery: A Validated Model Using a Prospective Surgical Registry. Spine J. 2014, 14, 2112–2117. [Google Scholar] [CrossRef] [PubMed]
- Koutsoumbelis, S.; Hughes, A.P.; Girardi, F.P.; Cammisa, F.P.; Finerty, E.A.; Nguyen, J.T.; Gausden, E.; Sama, A.A. Risk Factors for Postoperative Infection Following Posterior Lumbar Instrumented Arthrodesis. J. Bone Jt. Surg. 2011, 93, 1627–1633. [Google Scholar] [CrossRef] [PubMed]
- Tykocki, T.; Poniatowski, Ł.A.; Czyz, M.; Wynne-Jones, G. Oblique Corpectomy in the Cervical Spine. Spinal Cord 2017, 56, 426–435. [Google Scholar] [CrossRef]
- Pirkle, S.; Reddy, S.; Bhattacharjee, S.; Shi, L.L.; Lee, M.J. Chronic Opioid Use Is Associated With Surgical Site Infection After Lumbar Fusion. Spine 2020, 45, 837–842. [Google Scholar] [CrossRef]
- Puvanesarajah, V.; Nourbakhsh, A.; Hassanzadeh, H.; Shimer, A.L.; Shen, F.H.; Singla, A. Readmission Rates, Reasons, and Risk Factors in Elderly Patients Treated With Lumbar Fusion for Degenerative Pathology. Spine 2016, 41, 1933–1938. [Google Scholar] [CrossRef]
- Kubaszewski, L.; Wojdasiewicz, P.; Rozek, M.; Slowinska, I.E.; Romanowska-Próchnicka, K.; Slowinski, R.; Poniatowski, L.A.; Gasik, R. Syndromes with Chronic Non-Bacterial Osteomyelitis in the Spine. Reumatologia 2015, 53, 328–336. [Google Scholar] [CrossRef]
- Greco, E.A.; Pietschmann, P.; Migliaccio, S. Osteoporosis and Sarcopenia Increase Frailty Syndrome in the Elderly. Front. Endocrinol. 2019, 10, 255. [Google Scholar] [CrossRef]
- Flexman, A.M.; Charest-Morin, R.; Stobart, L.; Street, J.; Ryerson, C.J. Frailty and Postoperative Outcomes in Patients Undergoing Surgery for Degenerative Spine Disease. Spine J. 2016, 16, 1315–1323. [Google Scholar] [CrossRef]
- Reid, D.B.C.; Daniels, A.H.; Ailon, T.; Miller, E.; Sciubba, D.M.; Smith, J.S.; Shaffrey, C.I.; Schwab, F.; Burton, D.; Hart, R.A.; et al. Frailty and Health-Related Quality of Life Improvement Following Adult Spinal Deformity Surgery. World Neurosurg. 2018, 112, e548–e554. [Google Scholar] [CrossRef]
- De la Garza Ramos, R.; Goodwin, C.R.; Jain, A.; Abu-Bonsrah, N.; Fisher, C.G.; Bettegowda, C.; Sciubba, D.M. Development of a Metastatic Spinal Tumor Frailty Index (MSTFI) Using a Nationwide Database and Its Association with Inpatient Morbidity, Mortality, and Length of Stay After Spine Surgery. World Neurosurg. 2016, 95, 548–555.e4. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Landi, F.; Schneider, S.M.; Zúñiga, C.; Arai, H.; Boirie, Y.; Chen, L.K.; Fielding, R.A.; Martin, F.C.; Michel, J.; et al. Prevalence of and Interventions for Sarcopenia in Ageing Adults: A Systematic Review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014, 43, 48–759. [Google Scholar] [CrossRef] [PubMed]
- Puvanesarajah, V.; Jain, A.; Kebaish, K.; Shaffrey, C.I.; Sciubba, D.M.; De La Garza-Ramos, R.; Khanna, A.J.; Hassanzadeh, H. Poor Nutrition Status and Lumbar Spine Fusion Surgery in the Elderly: Readmissions, Complications, and Mortality. Spine 2017, 42, 979–983. [Google Scholar] [CrossRef] [PubMed]
- Babu, J.M.; Kalagara, S.; Durand, W.; Antoci, V.; Deren, M.E.; Cohen, E. Sarcopenia as a Risk Factor for Prosthetic Infection After Total Hip or Knee Arthroplasty. J. Arthroplast. 2019, 34, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Johnell, O.; Oden, A.; Jonsson, B.; De Laet, C.; Dawson, A. Risk of Hip Fracture According to the World Health Organization Criteria for Osteopenia and Osteoporosis. Bone 2000, 27, 585–590. [Google Scholar] [CrossRef]
- Khalid, S.I.; Nunna, R.S.; Maasarani, S.; Belmont, E.; Deme, P.; Chilakapati, S.; Eldridge, C.; Singh, R.; Bagley, C.A.; Adogwa, O. Association of Osteopenia and Osteoporosis with Higher Rates of Pseudarthrosis and Revision Surgery in Adult Patients Undergoing Single-Level Lumbar Fusion. Neurosurg. Focus 2020, 49, 1–7. [Google Scholar] [CrossRef]
- Bjerke, B.T.; Zarrabian, M.; Aleem, I.S.; Fogelson, J.L.; Currier, B.L.; Freedman, B.A.; Bydon, M.; Nassr, A. Incidence of Osteoporosis-Related Complications Following Posterior Lumbar Fusion. Glob. Spine J. 2018, 8, 563–569. [Google Scholar] [CrossRef]
- Ensrud, K.E.; Ewing, S.K.; Taylor, B.C.; Fink, H.A.; Cawthon, P.M.; Stone, K.L.; Hillier, T.A.; Cauley, J.A.; Hochberg, M.C.; Rodondi, N.; et al. Comparison of 2 Frailty Indexes for Prediction of Falls, Disability, Fractures, and Death in Older Women. Arch. Intern. Med. 2008, 168, 382–389. [Google Scholar] [CrossRef]
- Gielen, E.; Bergmann, P.; Bruyère, O.; Cavalier, E.; Delanaye, P.; Goemaere, S.; Kaufman, J.M.; Locquet, M.; Reginster, J.Y.; Rozenberg, S.; et al. Osteoporosis in Frail Patients: A Consensus Paper of the Belgian Bone Club. Calcif. Tissue Int. 2017, 101, 111–131. [Google Scholar] [CrossRef]
- Coin, A.; Perissinotto, E.; Enzi, G.; Zamboni, M.; Inelmen, E.M.; Frigo, A.C.; Manzato, E.; Busetto, L.; Buja, A.; Sergi, G. Predictors of Low Bone Mineral Density in the Elderly: The Role of Dietary Intake, Nutritional Status and Sarcopenia. Eur. J. Clin. Nutr. 2007, 62, 802–809. [Google Scholar] [CrossRef] [Green Version]
- Verschueren, S.; Gielen, E.; O’Neill, T.W.; Pye, S.R.; Adams, J.E.; Ward, K.A.; Wu, F.C.; Szulc, P.; Laurent, M.; Claessens, F.; et al. Sarcopenia and Its Relationship with Bone Mineral Density in Middle-Aged and Elderly European Men. Osteoporos. Int. 2013, 24, 87–98. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Liu, Y.; Tian, Q.; Papasian, C.J.; Hu, T.; Deng, H.W. Relationship of Sarcopenia and Body Composition with Osteoporosis. Osteoporos. Int. 2016, 27, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Reginster, J.Y.; Beaudart, C.; Buckinx, F.; Bruyère, O. Osteoporosis and Sarcopenia: Two Diseases or One? Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 31. [Google Scholar] [CrossRef] [PubMed]
- Tagliaferri, C.; Wittrant, Y.; Davicco, M.J.; Walrand, S.; Coxam, V. Muscle and Bone, Two Interconnected Tissues. Ageing Res. Rev. 2015, 21, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Girgis, C.M.; Mokbel, N.; DiGirolamo, D.J. Therapies for Musculoskeletal Disease: Can We Treat Two Birds with One Stone? Curr. Osteoporos. Rep. 2014, 12, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Laurent, M.R.; Dubois, V.; Claessens, F.; Verschueren, S.M.; Vanderschueren, D.; Gielen, E.; Jardí, F. Muscle-Bone Interactions: From Experimental Models to the Clinic? A Critical Update. Mol. Cell. Endocrinol. 2016, 432, 14–36. [Google Scholar] [CrossRef]
- Ebbeling, L.; Grabo, D.J.; Shashaty, M.; Dua, R.; Sonnad, S.S.; Sims, C.A.; Pascual, J.L.; Schwab, C.W.; Holena, D.N. Psoas:Lumbar Vertebra Index: Central Sarcopenia Independently Predicts Morbidity in Elderly Trauma Patients. Eur. J. Trauma Emerg. Surg. 2014, 40, 57. [Google Scholar] [CrossRef]
- Saad, M.M.; Ahmed, A.T.; Mohamed, K.E.; Habba, M.R. Role of Lumbar Spine Signal Intensity Measurement by MRI in the Diagnosis of Osteoporosis in Post-Menopausal Women. Egypt. J. Radiol. Nucl. Med. 2019, 50, 1–7. [Google Scholar] [CrossRef]
- Hoenig, J.M.; Heisey, D.M. The Abuse of Power: The Pervasive Fallacy of Power Calculations for Data Analysis. Am. Stat. 2001, 55, 19–24. [Google Scholar] [CrossRef]
- Abdul-Jabbar, A.; Takemoto, S.; Weber, M.H.; Hu, S.S.; Mummaneni, P.V.; Deviren, V.; Ames, C.P.; Chou, D.; Weinstein, P.R.; Burch, S.; et al. Surgical Site Infection in Spinal Surgery: Description of Surgical and Patient-Based Risk Factors for Postoperative Infection Using Administrative Claims Data. Spine 2012, 37, 1340–1345. [Google Scholar] [CrossRef]
- Lai, Q.; Song, Q.; Guo, R.; Bi, H.; Liu, X.; Yu, X.; Zhu, J.; Dai, M.; Zhang, B. Risk Factors for Acute Surgical Site Infections after Lumbar Surgery: A Retrospective Study. J. Orthop. Surg. Res. 2017, 12, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bokshan, S.L.; Han, A.L.; De Passe, J.M.; Eltorai, A.E.M.; Marcaccio, S.E.; Palumbo, M.A.; Daniels, A.H. Effect of Sarcopenia on Postoperative Morbidity and Mortality after Thoracolumbar Spine Surgery. Orthopedics 2016, 39, e1159–e1164. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, H.M.; Schultz, L.; Mossa-Basha, F.; Griffith, B.; Chang, V. Morphometrics as a Predictor of Perioperative Morbidity after Lumbar Spine Surgery. Neurosurg. Focus 2015, 39, E5. [Google Scholar] [CrossRef] [PubMed]
- Mannion, A.F.; Fekete, T.F.; Porchet, F.; Haschtmann, D.; Jeszenszky, D.; Kleinstück, F.S. The Influence of Comorbidity on the Risks and Benefits of Spine Surgery for Degenerative Lumbar Disorders. Eur. Spine J. 2014, 23, 66–71. [Google Scholar] [CrossRef]
- Martin, C.T.; Pugely, A.J.; Gao, Y.; Mendoza-Lattes, S.A.; Weinstein, S.L. The Impact of Renal Impairment on Short-Term Morbidity Risk Following Lumbar Spine Surgeries. Spine 2015, 40, 909–916. [Google Scholar] [CrossRef]
- Martin, C.T.; Gao, Y.; Duchman, K.R.; Pugely, A.J. The Impact of Current Smoking and Smoking Cessation on Short-Term Morbidity Risk after Lumbar Spine Surgery. Spine 2016, 41, 577–584. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European Consensus on Definition and Diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [Green Version]


| Characteristics | Total | Low M Score | High M Score | p Value | Low PLVI | High PLVI | p Value | Non-SSI | SSI | p Value |
|---|---|---|---|---|---|---|---|---|---|---|
| n | 308 | 213 | 95 | 153 | 155 | 282 | 26 | |||
| Age at surgery (y. mean.±SD) | 63.8 ± 6.2 | 64 ± 6.4 | 62.3 ± 5.36 | 0.05 * | 65.3 ± 6.38 | 62.3 ± 5.7 | 0.012 * | 63.6 ± 5.98 | 65.9 ± 7.96 | 0.002 * |
| Gender (F) | 160 | 94 | 38 | 0.54 | 112 | 48 | <0.01* | 142 | 18 | 0.08 |
| Diabetes mellitus (yes. n) | 28 | 20 | 8 | 0.04 * | 14 | 14 | 1 | 4 | 24 | 0.27 |
| Charlson Comorbidity Index (n. mean.± SD) | 2.57 ± 3.6 | 2.55 ± 1.34 | 2.54 ± 1.64 | 0.98 | 2.77 ± 1.58 | 2.32 ± 1.02 | 0.015 * | 2.49 ± 1.35 | 3.38 ± 1.9 | <0.01 * |
| American Society of Anesthesiology score (n. mean.± SD) | 2.03 ± 0.6 | 2.06 ± 0.58 | 1.91 ± 0.61 | 0.07 | 2.06 ± 0.58 | 2.02 ± 0.6 | 0.32 | 2.01 ± 0.56 | 2.31 ± 0.74 | 0.07 |
| Body mass index (n. mean.± SD) | 26.5 ± 6.2 | 26.7 ± 3.6 | 25.8 ± 3.5 | 0.07 | 27 ± 3.5 | 25.9 ± 3.6 | 0.85 | 26.6 ± 3.6 | 26.5 ± 4.2 | 0.98 |
| Smoking (yes. n) | 74 | 44 | 30 | 0.73 | 28 | 46 | 0.016 * | 64 | 10 | 0.08 |
| Length of stay (day. mean.± SD) | 11.1 ± 12.7 | 12.3 ± 15.9 | 8.6 ± 2.17 | 0.14 | 12.23 ± 17.1 | 10.1 ± 5.5 | 0.57 | 9.78 ± 4.8 | 25.4 ± 38.3 | 0.27 |
| Operative time (min. mean± SD) | 193.3 ± 59 | 190 ± 180 | 204 ± 190 | 0.06 | 185.1 ± 62.5 | 197.4 ± 57.3 | 0.25 | 192 ± 59.1 | 208 ± 58.1 | 0.24 |
| PLVI (mean.± SD) | 0.71 ± 0.18 | 0.72 ± 1.19 | 0.7 ± 0.19 | 0.5 | 0.55 ± 0.1 | 0.88 ± 0.2 | <0.01 * | 0.71 ± 0.2 | 0.75 ± 0.62 | 0.24 |
| PLVI (low n) | 153 | 110 | 43 | 0.6 | 141 | 12 | 0.7 | |||
| M score (mean.± SD) | 0 ± 128 | −0.47 ± 42.9 | 1.27 ± 8.1 | <0.01 * | 0.06 ± 1.02 | −0.06 ± 1 | 0.36 | 0.03 ± 1.02 | −0.20 ± 0.62 | 0.29 |
| M score (low n) | 213 | 135 | 78 | 0.6 | 193 | 20 | 0.04 * | |||
| Infection (n. %) | 8.4% | 76.9% | 22.9% | 0.04 * |
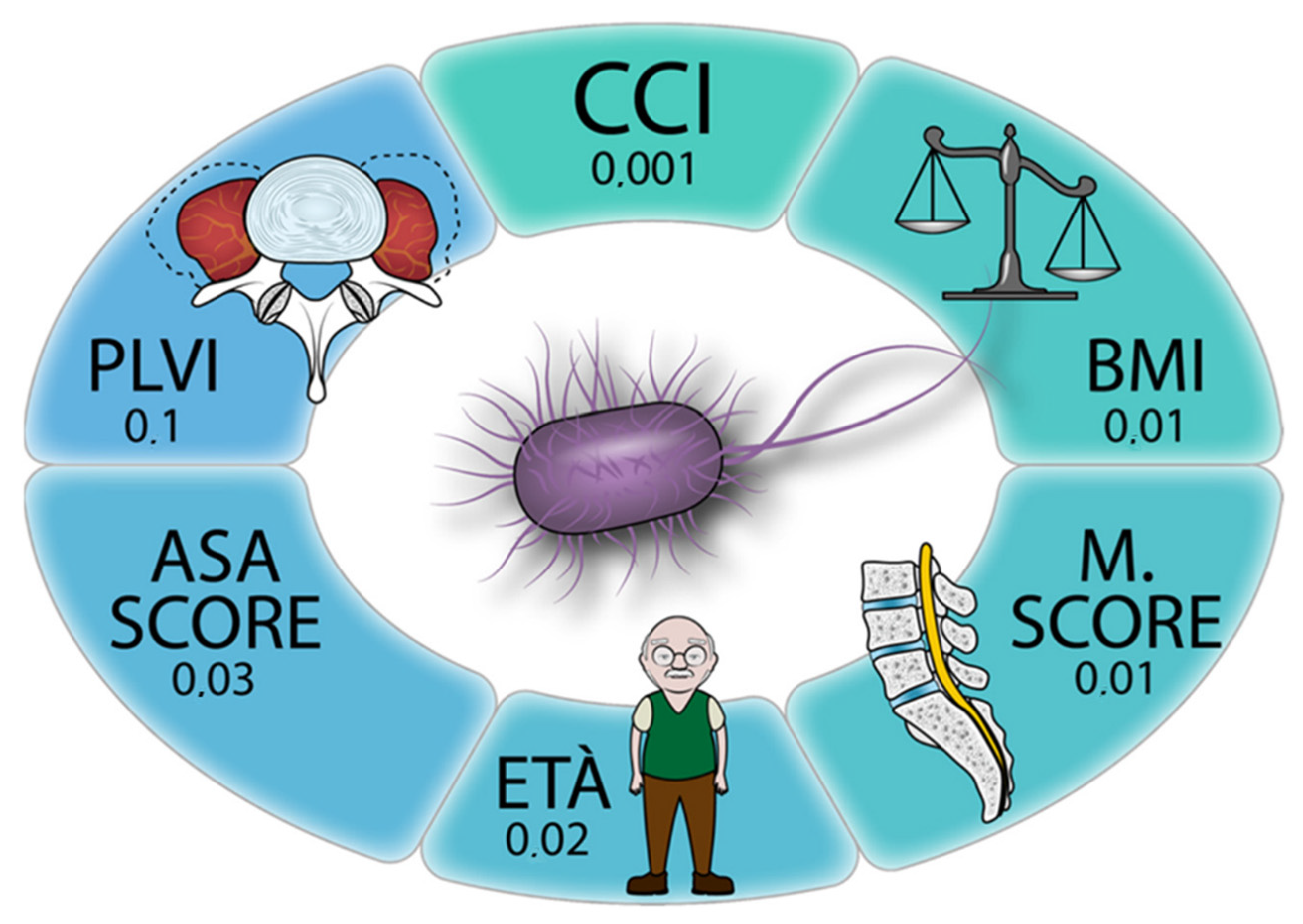
| Estimate | 95% CI Lower | 95% CI Upper | p Value | |
|---|---|---|---|---|
| Age at surgery | −0.00254 | −0.41195 | −0.03125 | 0.02 |
| Gender (F) | 0.03353 | −0.05728 | 0.12435 | 0.47 |
| Length of stay | 0.00617 | 0.00351 | 0.00884 | <0.001 |
| Diabetes mellitus (yes) | −0.02197 | −0.17905 | 0.13531 | 0.78 |
| Charlson Comorbidity Index | 0.06126 | 0.02435 | 0.09818 | 0.001 |
| American Society of Anesthesiology score | −0.08545 | −0.16355 | −000734 | 0.03 |
| Body mass index | 0.01468 | 0.00317 | 0.02619 | 0.01 |
| Smoking (yes) | −0.03029 | −0.11970 | 0.05912 | 0.5 |
| PLVI | 0.30726 | −0.06288 | 0.67739 | 0.10 |
| M score | −0.16560 | −0.30195 | −0.02925 | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruffilli, A.; Manzetti, M.; Cerasoli, T.; Barile, F.; Viroli, G.; Traversari, M.; Salamanna, F.; Fini, M.; Faldini, C. Osteopenia and Sarcopenia as Potential Risk Factors for Surgical Site Infection after Posterior Lumbar Fusion: A Retrospective Study. Microorganisms 2022, 10, 1905. https://doi.org/10.3390/microorganisms10101905
Ruffilli A, Manzetti M, Cerasoli T, Barile F, Viroli G, Traversari M, Salamanna F, Fini M, Faldini C. Osteopenia and Sarcopenia as Potential Risk Factors for Surgical Site Infection after Posterior Lumbar Fusion: A Retrospective Study. Microorganisms. 2022; 10(10):1905. https://doi.org/10.3390/microorganisms10101905
Chicago/Turabian StyleRuffilli, Alberto, Marco Manzetti, Tosca Cerasoli, Francesca Barile, Giovanni Viroli, Matteo Traversari, Francesca Salamanna, Milena Fini, and Cesare Faldini. 2022. "Osteopenia and Sarcopenia as Potential Risk Factors for Surgical Site Infection after Posterior Lumbar Fusion: A Retrospective Study" Microorganisms 10, no. 10: 1905. https://doi.org/10.3390/microorganisms10101905
APA StyleRuffilli, A., Manzetti, M., Cerasoli, T., Barile, F., Viroli, G., Traversari, M., Salamanna, F., Fini, M., & Faldini, C. (2022). Osteopenia and Sarcopenia as Potential Risk Factors for Surgical Site Infection after Posterior Lumbar Fusion: A Retrospective Study. Microorganisms, 10(10), 1905. https://doi.org/10.3390/microorganisms10101905