Acute Pediatric Chagas Disease in Antioquia, Colombia: A Geographic Location of Suspected Oral Transmission
Abstract
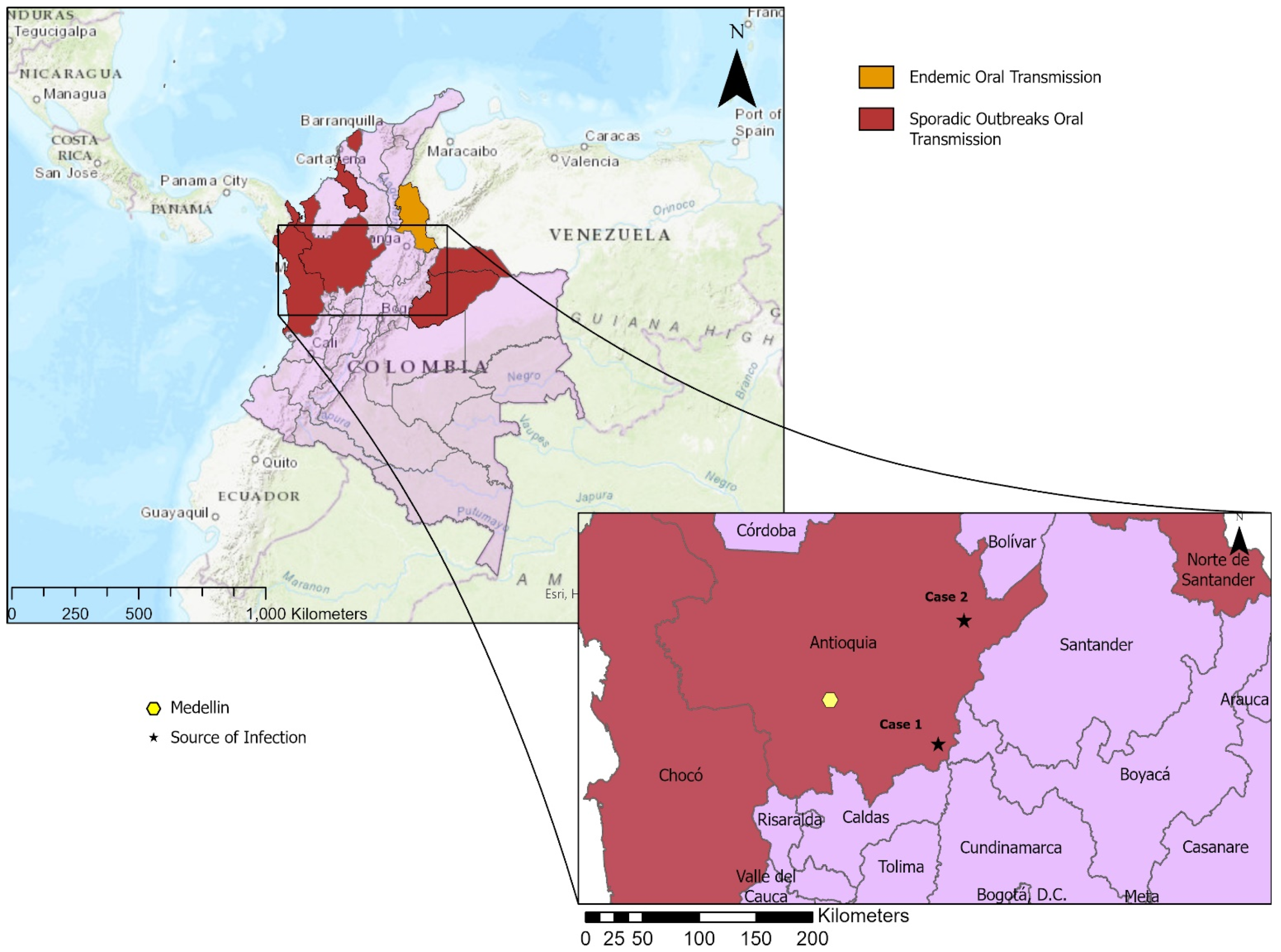
1. Introduction
2. Case Presentations
2.1. Case 1
2.2. Case 2
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Chagas Disease in Latin America: An Epidemiological Update Based on 2010 Estimates = Maladie de Chagas en Amérique Latine: Le Point Épidémiologique Basé Sur Les Estimations de 2010; World Health Organization: Geneva, Switzerland, 2015; Volume 90, pp. 33–44. [Google Scholar]
- Filigheddu, M.T.; Gorgolas, M.; Ramos, J.M. Orally-transmitted Chagas disease. Med. Clin. 2017, 148, 125–131. [Google Scholar] [CrossRef]
- Franco-Paredes, C.; Villamil-Gómez, W.E.; Schultz, J.; Henao-Martínez, A.F.; Parra-Henao, G.; Rassi, A.; Rodríguez-Morales, A.J.; Suarez, J.A. A deadly feast: Elucidating the burden of orally acquired acute Chagas disease in Latin America—Public health and travel medicine importance. Travel Med. Infect. Dis. 2020, 36, 101565. [Google Scholar] [CrossRef] [PubMed]
- Santana, R.A.G.; Guerra, M.G.V.B.; Sousa, D.R.; Couceiro, K.; Ortiz, J.V.; Oliveira, M.; Ferreira, L.S.; Souza, K.R.; Tavares, I.C.; Morais, R.F.; et al. Oral Transmission of Trypanosoma cruzi, Brazilian Amazon. Emerg. Infect. Dis. 2019, 25, 132–135. [Google Scholar] [CrossRef]
- Anez, N.; Crisante, G.; Rojas, A.; Segnini, S.; Espinoza-Alvarez, O.; Teixeira, M.M.G. Update on Chagas disease in Venezuela during the period 2003–2018. A review. Acta Trop. 2019, 203, 105310. [Google Scholar] [CrossRef]
- Esper, H.R.; Freitas, V.L.T.; Assy, J.; Shimoda, E.Y.; Berreta, O.C.P.; Lopes, M.H.; Franca, F.O.S. Fatal evolution of acute Chagas disease in a child from Northern Brazil: Factors that determine poor prognosis. Rev. Inst. Med. Trop. Sao Paulo 2019, 61, e27. [Google Scholar] [CrossRef]
- Alarcón de Noya, B.; Diaz-Bello, Z.; Colmenares, C.; Ruiz-Guevara, R.; Mauriello, L.; Zavala-Jaspe, R.; Suarez, J.A.; Abate, T.; Naranjo, L.; Paiva, M.; et al. Large urban outbreak of orally acquired acute Chagas disease at a school in Caracas, Venezuela. J. Infect. Dis. 2010, 201, 1308–1315. [Google Scholar] [CrossRef]
- Nolan, M.S.; Tonussi Mendes, J.E.; Perez Riera, A.R.; Laporta, G.Z. Oral Trypanosoma cruzi Transmission Resulting in Advanced Chagasic Cardiomyopathy in an 11-Month-Old Male. Case Rep. Infect. Dis 2020, 2020, 8828950. [Google Scholar] [CrossRef]
- Buendia, J.; Agudelo Calderon, C.; Pardo, R.; Gaitan, H.; Gomez, P.I.; Pinilla Roa, A.; Bustos, J.C.; Sanchez, C.L.; Pineda, F. Guía de Atención de la Engermedad de Chagas; Instituto Nacional de Salud: Bogotá, Colombia, 2005; p. 48.
- Velásquez-Ortiz, N.; Ramírez, J.D. Understanding the oral transmission of Trypanosoma cruzi as a veterinary and medical foodborne zoonosis. Res. Vet. Sci. 2020, 132, 448–461. [Google Scholar] [CrossRef] [PubMed]
- Rueda, K.; Trujillo, J.E.; Carranza, J.C.; Vallejo, G.A. Oral transmission of Trypanosoma cruzi: A new epidemiological scenario for Chagas’ disease in Colombia and other South American countries. Biomedica 2014, 34, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Molina, J.A.; Molina, I. Chagas disease. Lancet 2018, 391, 82–94. [Google Scholar] [CrossRef]
- Dirección de Vigilancia y Análisis del Riesgo en Salud Pública. Enfermedad de Chagas: En Busca de la Sostenibilidad; Instituto Nacional de Salud: Bogotá, Colombia, 2021; p. 30.
- Zuluaga, S.; Mejía, P.; Vélez-Mira, A.; Quintero, J.; Triana-Chávez, O.; Cantillo-Barraza, O. Updated geographical distribution and natural infection of Panstrongylus geniculatus (Latreille, 1811) in Antioquia department, Colombia. Parasite Epidemiol. Control 2021, 15, e00226. [Google Scholar] [CrossRef] [PubMed]
- Cantillo-Barraza, O.; Medina, M.; Zuluaga, S.; Valverde, C.; Motta, C.; Ladino, A.; Osorio, M.I.; Jaimes-Dueñez, J.; Triana-Chávez, O. Eco-epidemiological study reveals the importance of Triatoma dimidiata in the Trypanosoma cruzi transmission, in a municipality certified without transmission by Rhodnius prolixus in Colombia. Acta Trop. 2020, 209, 105550. [Google Scholar] [CrossRef] [PubMed]
- Alarcón, N.B.; Díaz-Bello, Z.; Colmenares, C.; Zavala-Jaspe, R.; Mauriello, L.; Díaz, M.P.; Soto, M.; Aponte, M.; Ruiz-Guevara, R.; Losada, S.; et al. Transmisión urbana de la enfermedad de Chagas en Caracas, Venezuela: Aspectos epidemiológicos, clínicos y de laboratorio. Rev. Biomed. 2009, 20, 158–164. [Google Scholar]
- Hernández, C.; Vera, M.J.; Cucunubá, Z.; Flórez, C.; Cantillo, O.; Buitrago, L.S.; González, M.S.; Ardila, S.; Dueñas, L.Z.; Tovar, R.; et al. High-Resolution Molecular Typing of Trypanosoma cruzi in 2 Large Outbreaks of Acute Chagas Disease in Colombia. J. Infect. Dis. 2016, 214, 1252–1255. [Google Scholar] [CrossRef]
- Mejía-Jaramillo, A.M.; Agudelo-Uribe, L.A.; Dib, J.C.; Ortiz, S.; Solari, A.; Triana-Chávez, O. Genotyping of Trypanosoma cruzi in a hyper-endemic area of Colombia reveals an overlap among domestic and sylvatic cycles of Chagas disease. Parasit Vectors 2014, 7, 108. [Google Scholar] [CrossRef] [PubMed]
- Rincón-Acevedo, C.Y.; Parada-García, A.S.; Olivera, M.J.; Torres-Torres, F.; Zuleta-Dueñas, L.P.; Hernández, C.; Ramírez, J.D. Clinical and Epidemiological Characterization of Acute Chagas Disease in Casanare, Eastern Colombia, 2012–2020. Front. Med. 2021, 8, 681635. [Google Scholar] [CrossRef]
- Añez, N.; Carrasco, H.; Parada, H.; Crisante, G.; Rojas, A.; Gonzalez, N.; Ramirez, J.L.; Guevara, P.; Rivero, C.; Borges, R.; et al. Acute Chagas’ disease in western Venezuela: A clinical, seroparasitologic, and epidemiologic study. Am. J. Trop Med. Hyg. 1999, 60, 215–222. [Google Scholar] [CrossRef]
- Alarcón de Noya, B.; Díaz-Bello, Z.; Colmenares, C.; Ruiz-Guevara, R.; Mauriello, L.; Muñoz-Calderón, A.; Noya, O. Update on oral Chagas disease outbreaks in Venezuela: Epidemiological, clinical and diagnostic approaches. Mem. Do Inst. Oswaldo Cruz 2015, 110, 377–386. [Google Scholar] [CrossRef]
- Barreto-de-Albuquerque, J.; Silva-dos-Santos, D.; Pérez, A.R.; Berbert, L.R.; de Santana-van-Vliet, E.; Farias-de-Oliveira, D.A.; Moreira, O.C.; Roggero, E.; de Carvalho-Pinto, C.E.; Jurberg, J.; et al. Trypanosoma cruzi Infection through the Oral Route Promotes a Severe Infection in Mice: New Disease Form from an Old Infection? PLoS Negl. Trop. Dis. 2015, 9, e0003849. [Google Scholar] [CrossRef]
- Parada, H.; Carrasco, H.A.; Anez, N.; Fuenmayor, C.; Inglessis, I. Cardiac involvement is a constant finding in acute Chagas’ disease: A clinical, parasitological and histopathological study. Int. J. Cardiol. 1997, 60, 49–54. [Google Scholar] [CrossRef]
- Marchiol, A.; Forsyth, C.; Bernal, O.; Valencia Hernández, C.; Cucunubá, Z.; Pachón Abril, E.; Vera Soto, M.J.; Batista, C. Increasing access to comprehensive care for Chagas disease: Development of a patient-centered model in Colombia. Rev. Panam Salud Publica 2017, 41, e153. [Google Scholar] [CrossRef][Green Version]
- Olivera, M.J.; Fory, J.A.; Porras, J.F.; Buitrago, G. Prevalence of Chagas disease in Colombia: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0210156. [Google Scholar] [CrossRef]
- Llau, A.F.; Tejada, C.E.; Ahmed, N.U. Chagas Disease Prevalence in Colombia: A Meta-Analysis and Systematic Review. Vector Borne Zoonotic Dis. 2019, 19, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Acero, C.T.; Peñata, J.N.; González, C.; León, C.; Ortiz, M.; Pacheco, J.C.; Monterrosa, E.; Luna, A.; Caldera, D.R.; Espitia-Pérez, L. New Scenarios of Chagas Disease Transmission in Northern Colombia. J. Parasitol. Res. 2017, 2017, 3943215. [Google Scholar] [CrossRef]
- Olivera, M.J.; Porras Villamil, J.F.; Toquica Gahona, C.C.; Rodríguez Hernández, J.M. Barriers to Diagnosis Access for Chagas Disease in Colombia. J. Parasitol. Res. 2018, 2018, 4940796. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Parra, A.G.; Pinilla-Alfonso, M.Y.; Abadía-Barrero, C.E. Sociocultural dynamics that influence Chagas disease health care in Colombia. Soc. Sci. Med. 2018, 215, 142–150. [Google Scholar] [CrossRef]
- Rey León, J.A. La justicia social en salud y su relación con la enfermedad de Chagas. Rev. Cuba. Salud Pública 2021, 46, e1264. [Google Scholar]
- Shikanai-Yasuda, M.A.; Carvalho, N.B. Oral transmission of Chagas disease. Clin. Infect. Dis. 2012, 54, 845–852. [Google Scholar] [CrossRef]
- Quirós-Gómez, Ó.; Jaramillo, N.; Angulo, V.; Parra-Henao, G. Triatoma dimidiata in Colombia: Distribution, ecology and epidemiological importance. Biomedica 2017, 37, 274–285. [Google Scholar] [CrossRef]
- Stimpert, K.K.; Montgomery, S.P. Physician Awareness of Chagas Disease, USA. Emerg. Infect. Dis. J. 2010, 16, 871. [Google Scholar] [CrossRef]
- Marques, J.; Mendoza, I.; Noya, B.; Acquatella, H.; Palacios, I.; Marques-Mejias, M. ECG manifestations of the biggest outbreak of Chagas disease due to oral infection in Latin-America. Arq. Bras. Cardiol. 2013, 101, 249–254. [Google Scholar] [CrossRef]
- Hopkins, T.; Gonçalves, R.; Mamani, J.; Courtenay, O.; Bern, C. Chagas disease in the Bolivian Chaco: Persistent transmission indicated by childhood seroscreening study. Int. J. Infect. Dis. 2019, 86, 175–177. [Google Scholar] [CrossRef]
- Nolan, M.S.; Murray, K.O.; Mejia, R.; Hotez, P.J.; Villar Mondragon, M.J.; Rodriguez, S.; Palacios, J.R.; Murcia Contreras, W.E.; Lynn, M.K.; Torres, M.E.; et al. Elevated Pediatric Chagas Disease Burden Complicated by Concomitant Intestinal Parasites and Malnutrition in El Salvador. Trop. Med. Infect. Dis. 2021, 6, 72. [Google Scholar] [CrossRef]
- Salazar-Schettino, P.M.; Cabrera-Bravo, M.; Vazquez-Antona, C.; Zenteno, E.; Alba-Alvarado, M.; Gutierrez, E.T.; Gomez, Y.G.; Perera-Salazar, M.G.; Torre, G.G.; Bucio-Torres, M.I. Chagas Disease in Mexico: Report of 14 Cases of Chagasic Cardiomyopathy in Children. Tohoku J. Exp. Med. 2016, 240, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Schettino, P.M.; Perera, R.; Ruiz-Hernandez, A.L.; Bucio Torres, M.I.; Zamora-Gonzalez, C.; Cabrera-Bravo, M.; Harnden, A. Chagas disease as a cause of symptomatic chronic myocardopathy in Mexican children. Pediatr Infect. Dis. J. 2009, 28, 1011–1013. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, S.M.; Avelis, C.M.; Asti, L.; Hertenstein, D.L.; Ndeffo-Mbah, M.; Galvani, A.; Lee, B.Y. The economic value of identifying and treating Chagas disease patients earlier and the impact on Trypanosoma cruzi transmission. PLoS Negl. Trop. Dis. 2018, 12, e0006809. [Google Scholar] [CrossRef] [PubMed]
| Clinical Presentations of Chagas Disease | Case 1 | Case 2 |
|---|---|---|
| Age | 10 years old | 5 years old |
| Suspected place of infection | Doradal (Antioquia) | Remedios (Antioquia) |
| Acute phase | ||
| Fever | Present | Present |
| Malaise | Present | Present |
| Myalgia | Present | Absent |
| Headache | Absent | Present |
| Adynamia | Present | Absent |
| Gastrointestinal involvement | Present | Present |
| Hyporexia | Present | Present |
| Lymphadenomegaly | Present | Absent |
| Romaña sign | Absent | Absent |
| Chagoma | Absent | Absent |
| ECG abnormalities | Absent | Absent |
| Myocarditis | Absent | Absent |
| Leukopenia | Absent | Present |
| Blood abnormalities 1 | Present | Absent |
| Rash | Present | Absent |
| Hepatosplenomegaly 2 | Present | Present |
| Liver impairment 2 | Absent | Present |
| Intermediate phase | ||
| Heart conduction abnormalities | Absent | Absent |
| Regional LV wall motion abnormalities | Absent | Absent |
| Chronic phase | ||
| Cardiomegaly | Absent | Present |
| Megacolon | Absent | Absent |
| Megaesophagus | Absent | Absent |
| Blood detection | ||
| Trypanosoma cruzi observation | Not observed | Observed |
| Antibodies | IgG positive | Negative |
| PCR detection | Positive | Positive |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gual-Gonzalez, L.; Arango-Ferreira, C.; Lopera-Restrepo, L.C.; Cantillo-Barraza, O.; Marín, D.V.; Bustamante, N.R.; Triana-Chavez, O.; Nolan, M.S. Acute Pediatric Chagas Disease in Antioquia, Colombia: A Geographic Location of Suspected Oral Transmission. Microorganisms 2022, 10, 8. https://doi.org/10.3390/microorganisms10010008
Gual-Gonzalez L, Arango-Ferreira C, Lopera-Restrepo LC, Cantillo-Barraza O, Marín DV, Bustamante NR, Triana-Chavez O, Nolan MS. Acute Pediatric Chagas Disease in Antioquia, Colombia: A Geographic Location of Suspected Oral Transmission. Microorganisms. 2022; 10(1):8. https://doi.org/10.3390/microorganisms10010008
Chicago/Turabian StyleGual-Gonzalez, Lídia, Catalina Arango-Ferreira, Laura Camila Lopera-Restrepo, Omar Cantillo-Barraza, Daniela Velásquez Marín, Natalia Restrepo Bustamante, Omar Triana-Chavez, and Melissa S. Nolan. 2022. "Acute Pediatric Chagas Disease in Antioquia, Colombia: A Geographic Location of Suspected Oral Transmission" Microorganisms 10, no. 1: 8. https://doi.org/10.3390/microorganisms10010008
APA StyleGual-Gonzalez, L., Arango-Ferreira, C., Lopera-Restrepo, L. C., Cantillo-Barraza, O., Marín, D. V., Bustamante, N. R., Triana-Chavez, O., & Nolan, M. S. (2022). Acute Pediatric Chagas Disease in Antioquia, Colombia: A Geographic Location of Suspected Oral Transmission. Microorganisms, 10(1), 8. https://doi.org/10.3390/microorganisms10010008








