Molecular Epidemiology of Vancomycin-Resistant Enterococci Bloodstream Infections in Germany: A Population-Based Prospective Longitudinal Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Demographic and Geographic Analyses
2.2. Molecular Analysis
2.3. Data Processing
3. Results
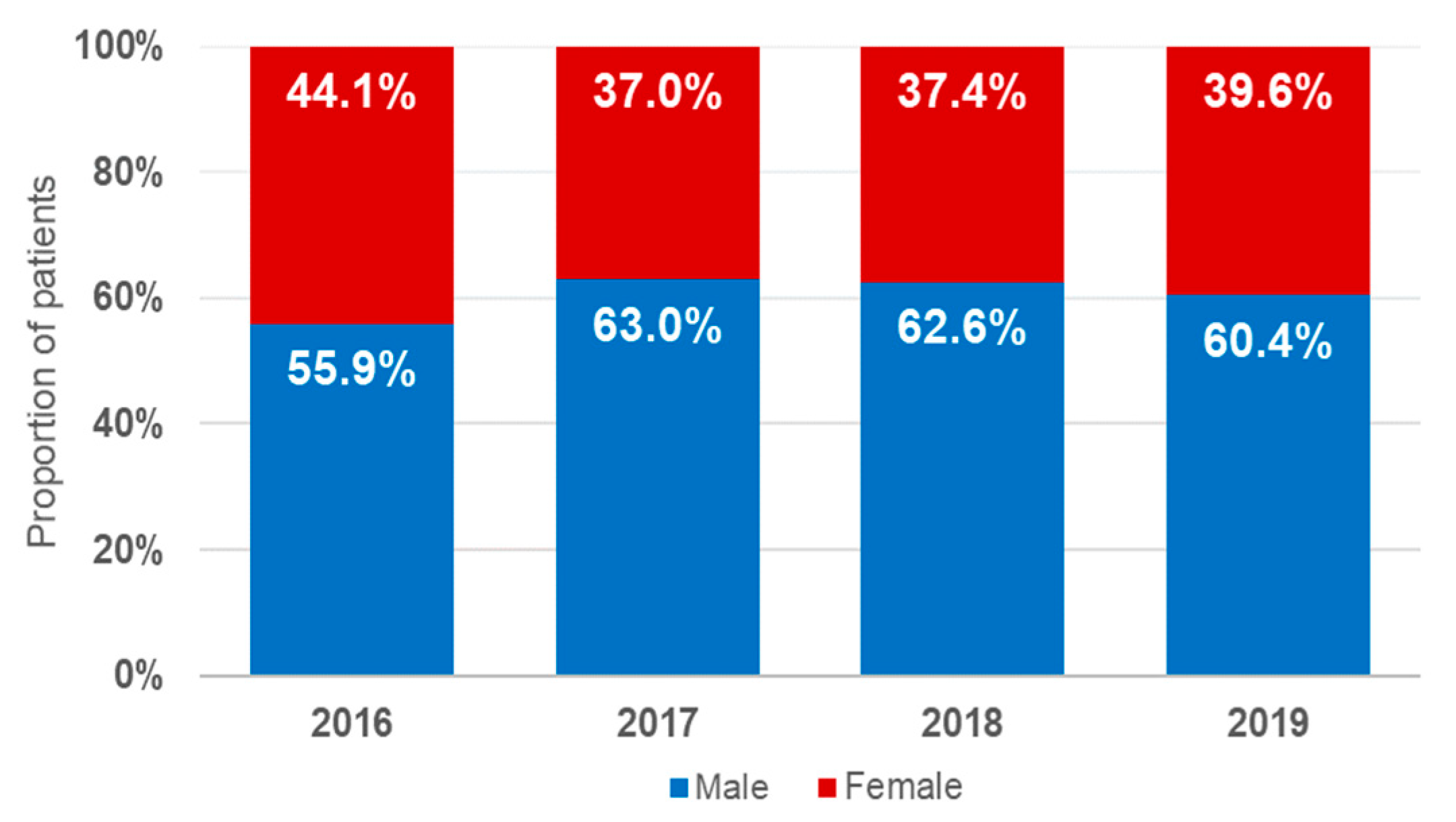
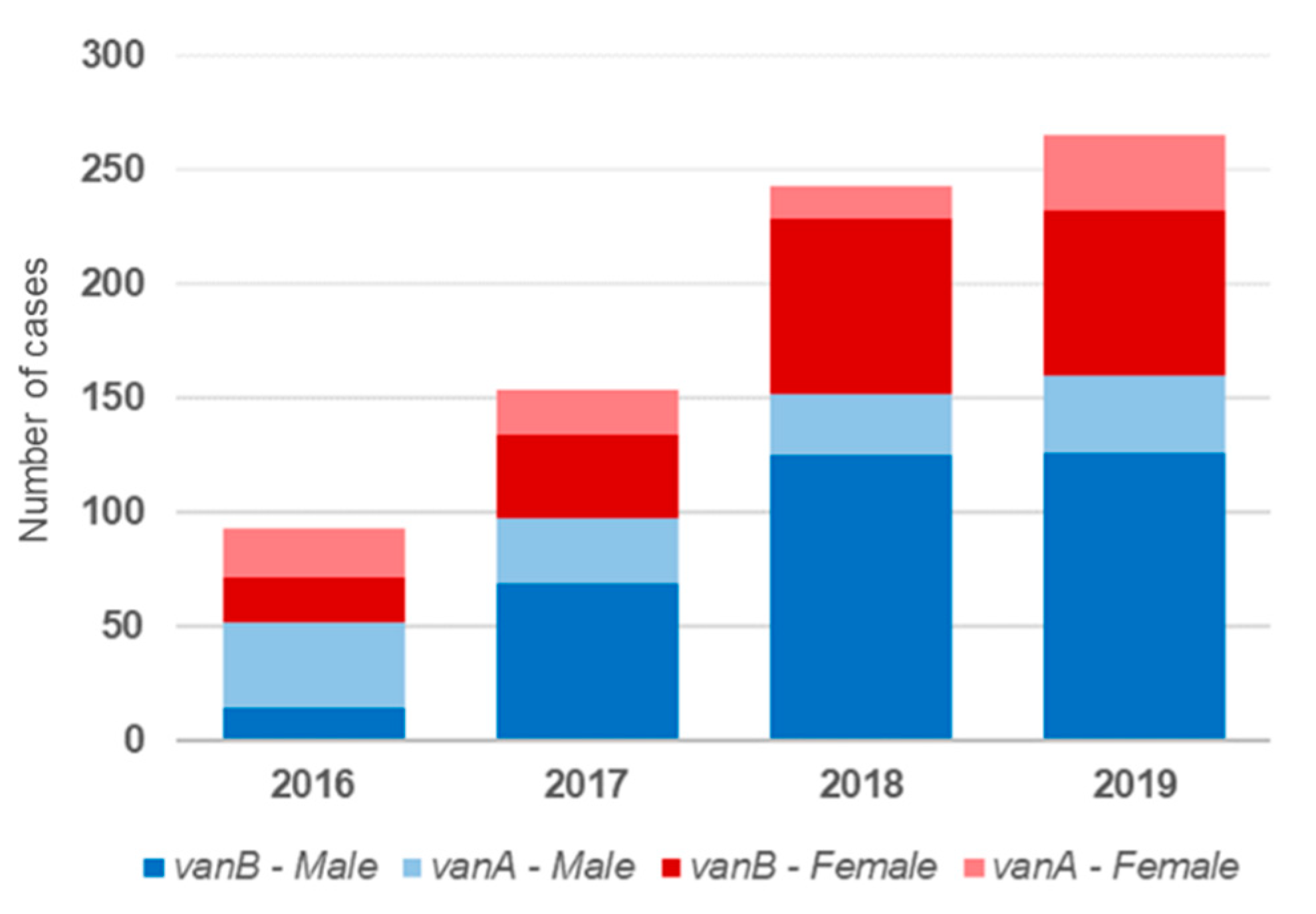
3.1. Demographic Characteristics
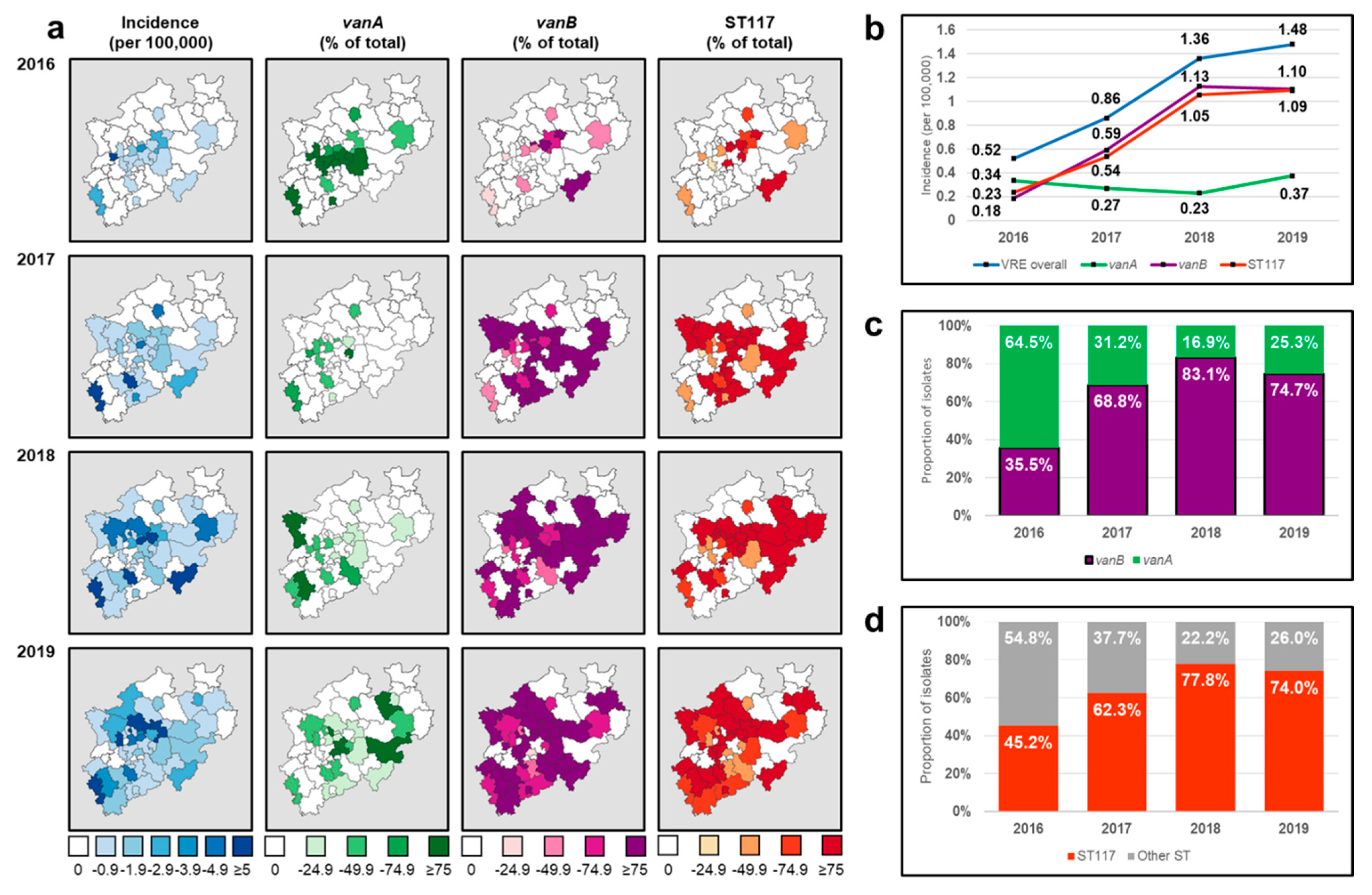
3.2. Molecular and Epidemiological Characteristics
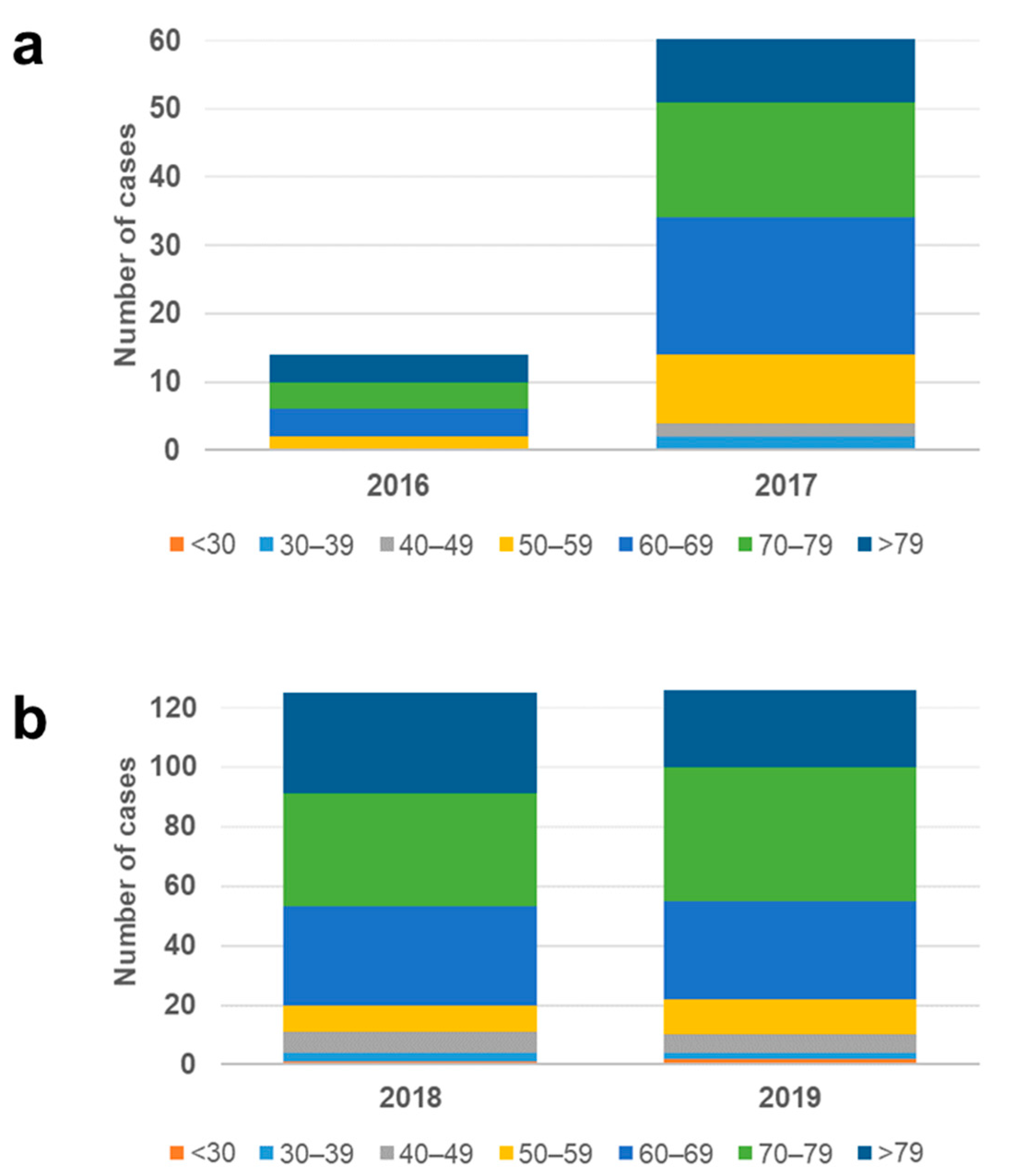
3.3. Demographic Correlation of the vanB/ST117 Increase
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Uttley, A.H.; Collins, C.H.; Naidoo, J.; George, R.C. Vancomycin-resistant enterococci. Lancet 1988, 1, 157–158. [Google Scholar] [CrossRef]
- Leclercq, R.; Derlot, E.; Duval, J.; Courvalin, P. Plasmid-Mediated Resistance to Vancomycin and Teicoplanin in Enterococcus faecium. N. Engl. J. Med. 1988, 319, 157–161. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- García-Solache, M.; Rice, L.B. The Enterococcus: A Model of Adaptability to Its Environment. Clin. Microbiol. Rev. 2019, 32, e00058-18. [Google Scholar] [CrossRef] [PubMed]
- DiazGranados, C.A.; Zimmer, S.M.; Mitchel, K.; Jernigan, J.A. Comparison of Mortality Associated with Vancomycin-Resistant and Vancomycin-Susceptible Enterococcal Bloodstream Infections: A Meta-analysis. Clin. Infect. Dis. 2005, 41, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Puchter, L.; Chaberny, I.F.; Schwab, F.; Vonberg, R.-P.; Bange, F.-C.; Ebadi, E. Economic burden of nosocomial infections caused by vancomycin-resistant enterococci. Antimicrob. Resist. Infect. Control 2018, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chiang, H.-Y.; Perencevich, E.N.; Nair, R.; Nelson, R.E.; Samore, M.; Khader, K.; Chorazy, M.L.; Herwaldt, L.A.; Blevins, A.; Ward, M.A.; et al. Incidence and Outcomes Associated With Infections Caused by Vancomycin-Resistant Enterococci in the United States: Systematic Literature Review and Meta-Analysis. Infect. Control Hosp. Epidemiol. 2016, 38, 203–215. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC). Antimicrobial resistance in the EU/EEA (EARS-Net)—Annual Epidemiological Report for 2019; Stockholm, Sweden, 2020; Available online: https://www.ecdc.europa.eu/en/publications-data/surveillance-antimicrobial-resistance-europe-2019 (accessed on 15 January 2021).
- Ahmed, M.O.; Baptiste, K.E. Vancomycin-Resistant Enterococci: A Review of Antimicrobial Resistance Mechanisms and Perspectives of Human and Animal Health. Microb. Drug Resist. 2018, 24, 590–606. [Google Scholar] [CrossRef] [PubMed]
- Cafini, F.; Nguyen, L.T.T.; Higashide, M.; Roman, F.; Prieto, J.; Morikawa, K. Horizontal gene transmission of the cfr gene to MRSA and Enterococcus: Role of Staphylococcus epidermidis as a reservoir and alternative pathway for the spread of linezolid resistance. J. Antimicrob. Chemother. 2016, 71, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Cormican, M.; Flamm, R.K.; Mendes, R.; Jones, R.N. Temporal and Geographic Variation in Antimicrobial Susceptibility and Resistance Patterns of Enterococci: Results from the SENTRY Antimicrobial Surveillance Program, 1997–2016. Open Forum Infect. Dis. 2019, 6, 54–62. [Google Scholar] [CrossRef]
- Simner, P.J.; Adam, H.; Baxter, M.; McCracken, M.; Golding, G.; Karlowsky, J.A.; Nichol, K.; Lagacé-Wiens, P.; Gilmour, M.; Hoban, D.J.; et al. Epidemiology of Vancomycin-Resistant Enterococci in Canadian Hospitals (CANWARD Study, 2007 to 2013). Antimicrob. Agents Chemother. 2015, 59, 4315–4317. [Google Scholar] [CrossRef] [PubMed]
- Akpaka, P.E.; Kissoon, S.; Jayaratne, P.; Wilson, C.; Golding, G.R.; Nicholson, A.M.; Lewis, D.B.; Hermelijn, S.M.; Wilson-Pearson, A.; Smith, A. Genetic characteristics and molecular epidemiology of vancomycin-resistant Enterococci isolates from Caribbean countries. PLoS ONE 2017, 12, e0185920. [Google Scholar] [CrossRef]
- Panesso, D.; Reyes, J.; Rincon, S.; Díaz, L.; Galloway-Pena, J.; Zurita, J.; Carrillo, C.; Merentes, A.; Guzmán, M.; Adachi, J.A.; et al. Molecular Epidemiology of Vancomycin-Resistant Enterococcus faecium: A Prospective, Multicenter Study in South American Hospitals. J. Clin. Microbiol. 2010, 48, 1562–1569. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, J.-X.; Li, T.; Ning, Y.-Z.; Shao, D.-H.; Liu, J.; Wang, S.-Q.; Liang, G.-W. Molecular characterization of resistance, virulence and clonality in vancomycin-resistant Enterococcus faecium and Enterococcus faecalis: A hospital-based study in Beijing, China. Infect. Genet. Evol. 2015, 33, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; García-Cobos, S.; Ruijs, G.J.H.M.; Kampinga, G.A.; Arends, J.P.; Borst, D.M.; Möller, L.V.; Holman, N.D.; Schuurs, T.A.; Van Coppenraet, L.E.B.; et al. Epidemiology of Extended-Spectrum β-Lactamase-Producing E. coli and Vancomycin-Resistant Enterococci in the Northern Dutch–German Cross-Border Region. Front. Microbiol. 2017, 8, 1914. [Google Scholar] [CrossRef]
- Rangberg, A.; Larsen, A.L.; Kacelnik, O.; Sæther, H.S.; Bjørland, M.; Ringstad, J.; Jonassen, C.M. Molecular analysis and epidemiological typing of Vancomycin-resistant Enterococcus outbreak strains. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Robert Koch Institut (RKI). Epidemiologisches Bulletin. Eigenschaften, Häufigkeit und Verbreitung von Vancomycin-Resistenten Enterokokken (VRE) in Deutschland; Update 2015/2016; RKI: Berlin, Germany, 2017. [Google Scholar]
- Robert Koch Institut (RKI). Epidemiologisches Bulletin. Verbreitung, Krankheitslast, Therapieoptionen und Prävention von Infektionen mit Vancomycin-Resistenten Enterokokken; RKI: Berlin, Germany, 2019; Available online: https://www.rki.de/DE/Content/Infekt/EpidBull/Archiv/2019/Ausgaben/27_19.pdf?__blob=publicationFile (accessed on 9 December 2021).
- Falgenhauer, L.; Fritzenwanker, M.; Imirzalioglu, C.; Steul, K.; Scherer, M.; Heudorf, U.; Chakraborty, T. Near-ubiquitous presence of a vancomycin-resistant Enterococcus faecium ST117/CT71/vanB -clone in the Rhine-Main metropolitan area of Germany. Antimicrob. Resist. Infect Control. 2019, 8, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Xanthopoulou, K.; Peter, S.; Tobys, D.; Behnke, M.; Dinkelacker, A.G.; Eisenbeis, S.; Falgenhauer, J.; Falgenhauer, L.; Fritzenwanker, M.; Gölz, H.; et al. Vancomycin-resistant Enterococcus faecium colonizing patients on hospital admission in Germany: Prevalence and molecular epidemiology. J. Antimicrob. Chemother. 2020, 75, 2743–2751. [Google Scholar] [CrossRef]
- Eisenberger, D.; Tuschak, C.; Werner, M.; Bogdan, C.; Bollinger, T.; Hossain, H.; Friedrich, P.; Hussein, Z.; Pöhlmann, C.; Würstl, B.; et al. Whole-genome analysis of vancomy-cin-resistant Enterococcus faecium causing nosocomial outbreaks suggests the occurrence of few endemic clonal lineages in Bavaria, Germany. J. Antimicrob. Chemother. 2020, 75, 1398–1404. [Google Scholar] [CrossRef]
- Homan, W.L.; Tribe, D.; Poznanski, S.; Li, M.; Hogg, G.; Spalburg, E.; van Embden, J.D.A.; Willems, R.J.L. Multilocus Sequence Typing Scheme for Enterococcus faecium. J. Clin. Microbiol. 2002, 40, 1963–1971. [Google Scholar] [CrossRef] [PubMed]
- Ayobami, O.; Willrich, N.; Reuss, A.; Eckmanns, T.; Markwart, R. The ongoing challenge of vancomycin-resistant Enterococcus faecium and Enterococcus faecalis in Europe: An epidemiological analysis of bloodstream infections. Emerg. Microbes Infect. 2020, 9, 1180–1193. [Google Scholar] [CrossRef]
- Markwart, R.; Willrich, N.; Haller, S.; Noll, I.; Koppe, U.; Werner, G.; Eckmanns, T.; Reuss, A. The rise in vancomycin-resistant Enterococcus faecium in Germany: Data from the German Antimicrobial Resistance Surveillance (ARS). Antimicrob. Resist. Infect. Control. 2019, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Robert Koch Institut (RKI). Epidemiologisches Bulletin. Eigenschaften, Häufigkeit und Verbreitung von Vancomycin-Resistenten Enterokokken (VRE) in Deutschland; Update 2017/2018; RKI: Berlin, Gremany, 2019; Available online: https://www.rki.de/DE/Content/Infekt/EpidBull/Archiv/2019/Ausgaben/35_19.pdf?__blob=publicationFile (accessed on 9 December 2021).
- Vehreschild, M.J.G.T.; Haverkamp, M.; Biehl, L.M.; Lemmen, S.; Fätkenheuer, G. Vancomycin-resistant enterococci (VRE): A reason to isolate? Infect. 2019, 47, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Schönfeld, V.; Diercke, M.; Gilsdorf, A.; Eckmanns, T.; Walter, J. Evaluation of the statutory surveillance system for invasive MRSA infections in Germany, 2016–2017. BMC Public Health 2018, 18, 1063. [Google Scholar] [CrossRef] [PubMed]
- Wijesuriya, T.M.; Perry, P.; Pryce, T.; Boehm, J.; Kay, I.; Flexman, J.; Coombs, G.; Ingram, P.R. Low Vancomycin MICs and Fecal Densities Reduce the Sensitivity of Screening Methods for Vancomycin Resistance in Enterococci. J. Clin. Microbiol. 2014, 52, 2829–2833. [Google Scholar] [CrossRef] [PubMed][Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Correa-Martínez, C.L.; Jurke, A.; Schmitz, J.; Schaumburg, F.; Kampmeier, S.; Mellmann, A. Molecular Epidemiology of Vancomycin-Resistant Enterococci Bloodstream Infections in Germany: A Population-Based Prospective Longitudinal Study. Microorganisms 2022, 10, 130. https://doi.org/10.3390/microorganisms10010130
Correa-Martínez CL, Jurke A, Schmitz J, Schaumburg F, Kampmeier S, Mellmann A. Molecular Epidemiology of Vancomycin-Resistant Enterococci Bloodstream Infections in Germany: A Population-Based Prospective Longitudinal Study. Microorganisms. 2022; 10(1):130. https://doi.org/10.3390/microorganisms10010130
Chicago/Turabian StyleCorrea-Martínez, Carlos L., Annette Jurke, Janne Schmitz, Frieder Schaumburg, Stefanie Kampmeier, and Alexander Mellmann. 2022. "Molecular Epidemiology of Vancomycin-Resistant Enterococci Bloodstream Infections in Germany: A Population-Based Prospective Longitudinal Study" Microorganisms 10, no. 1: 130. https://doi.org/10.3390/microorganisms10010130
APA StyleCorrea-Martínez, C. L., Jurke, A., Schmitz, J., Schaumburg, F., Kampmeier, S., & Mellmann, A. (2022). Molecular Epidemiology of Vancomycin-Resistant Enterococci Bloodstream Infections in Germany: A Population-Based Prospective Longitudinal Study. Microorganisms, 10(1), 130. https://doi.org/10.3390/microorganisms10010130





