Performance Comparison of Mechanical and Ferrofluidic Micropumps: Structural and Operational Perspectives
Abstract
1. Introduction
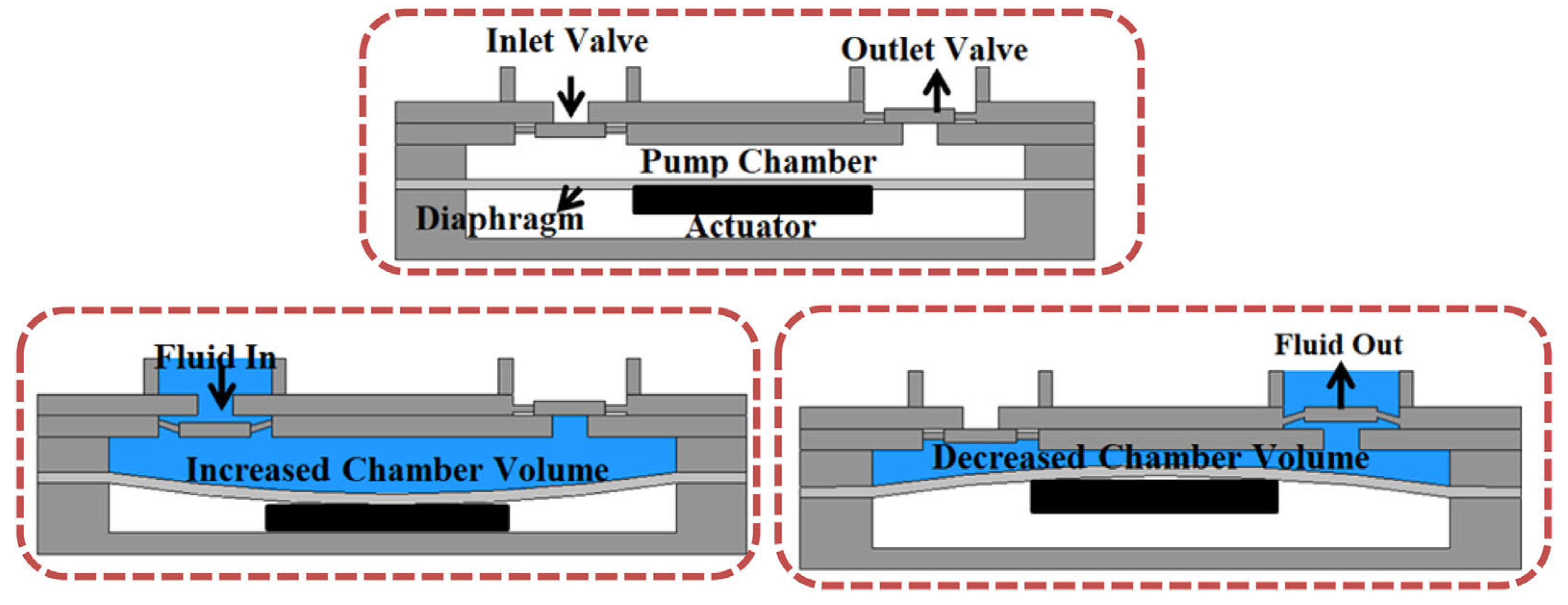
2. Mechanical Micropumps and Their Compositions
2.1. Actuator
2.2. Diaphragm
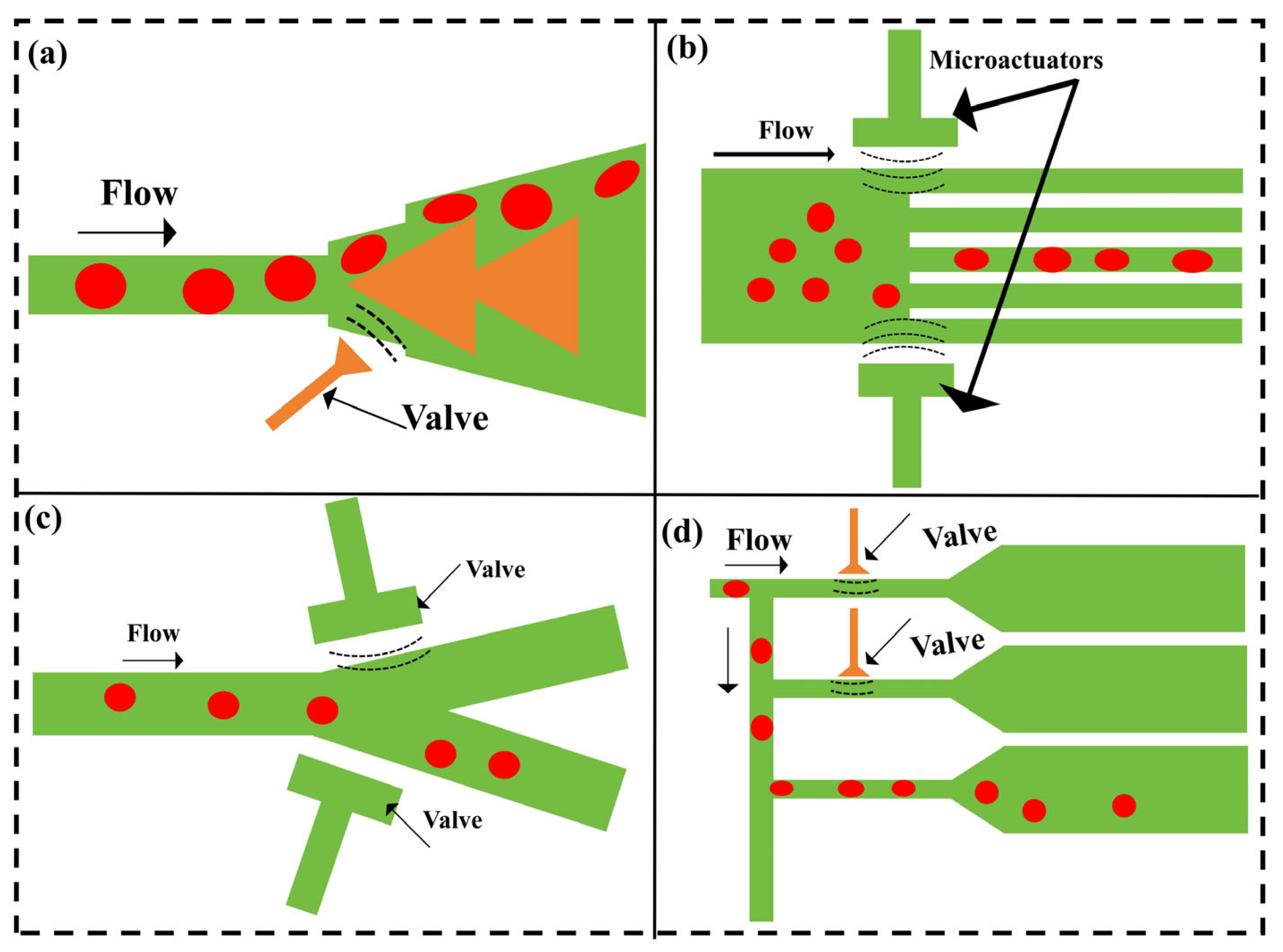
2.3. Valves
2.4. Pump Chamber and Pump Body Materials
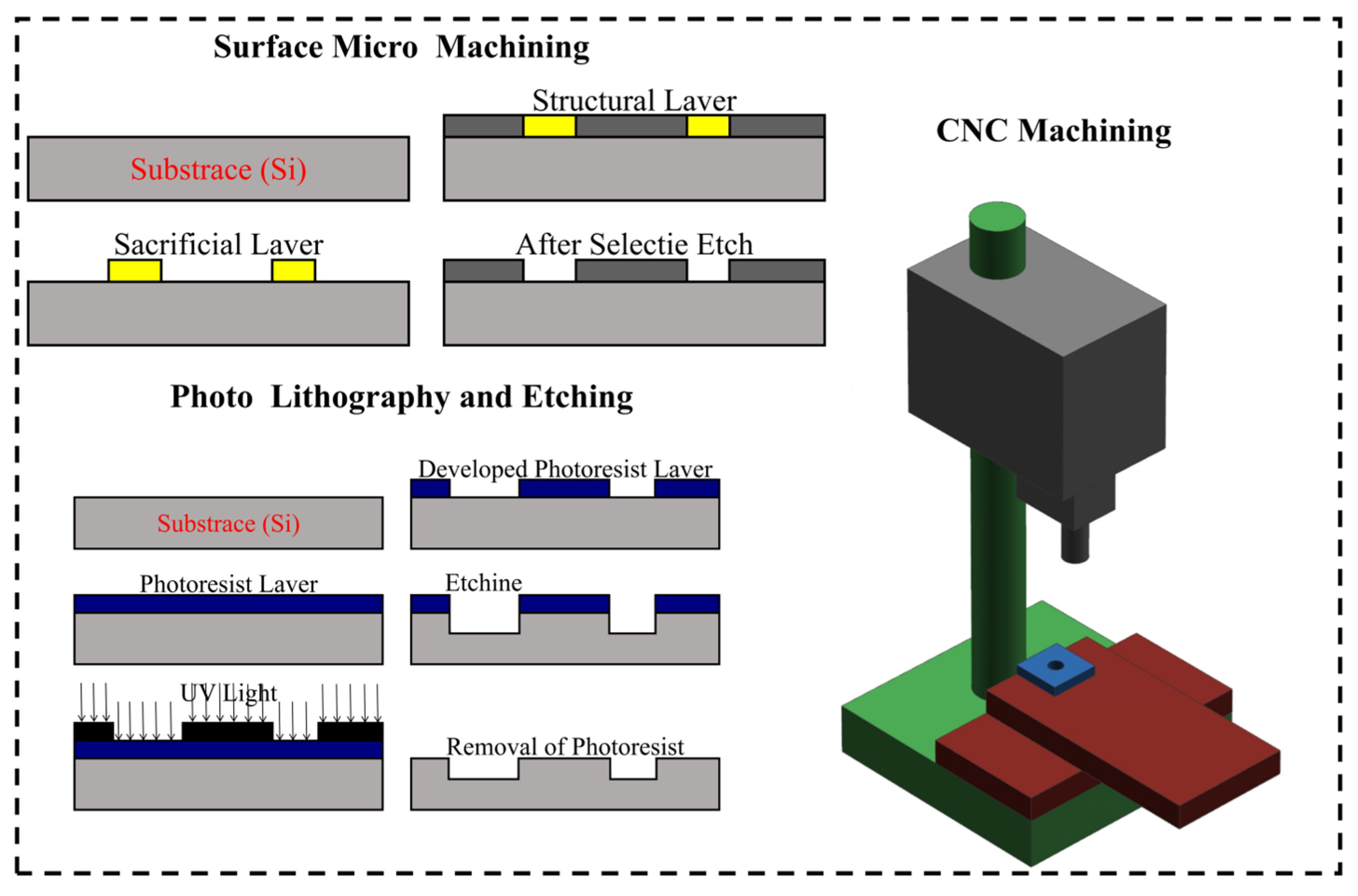
2.5. Fabrication Techniques
2.6. External Equipment of Micropump
2.7. Applications of Mechanical Micropumps
2.8. Failure of Mechanical Micropumps
2.9. Challenges of Mechanical Micropumps
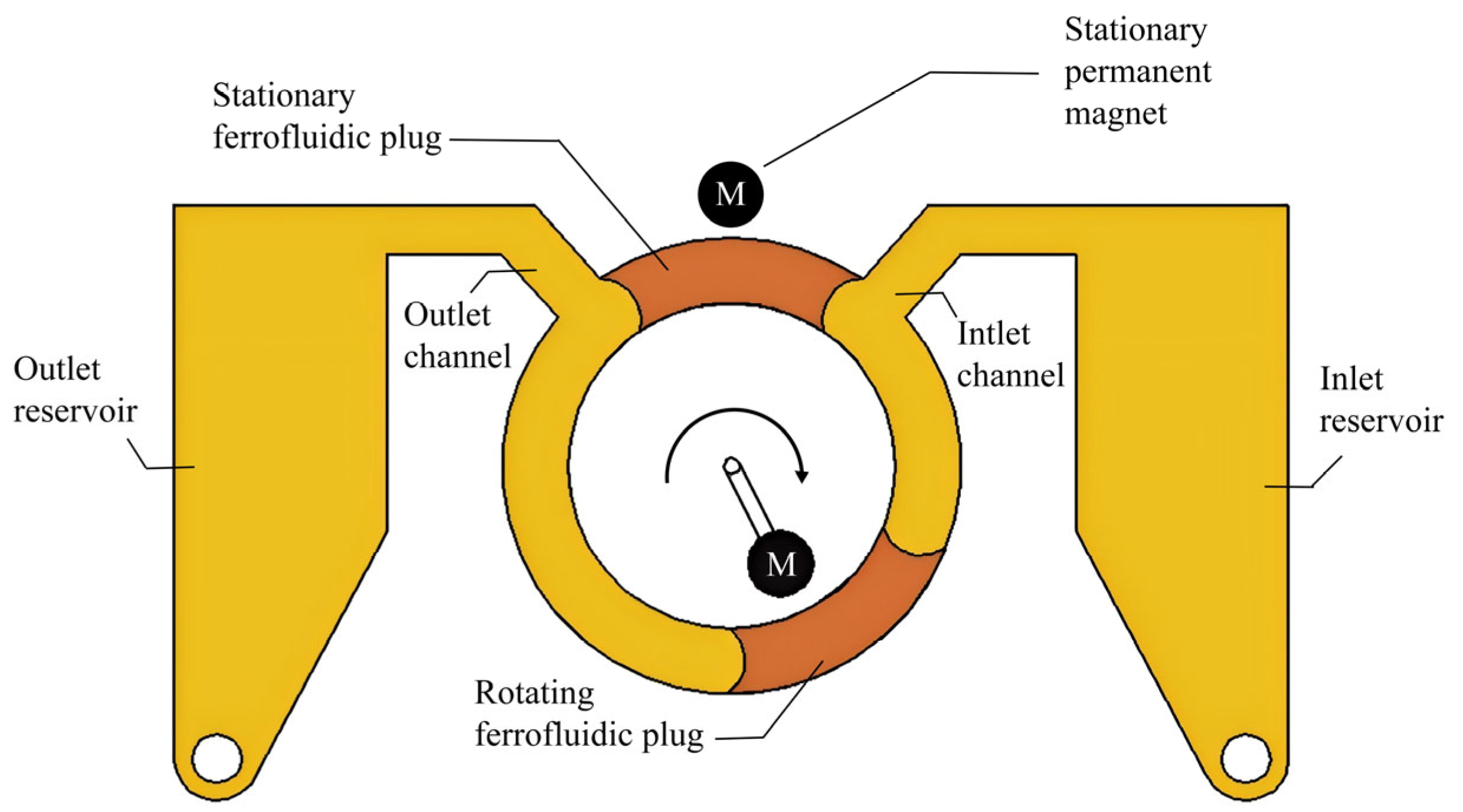
3. Composition and Development Status of Ferrofluidic Micropumps
3.1. Ferrofluid
3.2. Actuating Magnets
3.3. Valves and Sealing Means
3.4. Pump Body and Manufacturing
3.4.1. Classification of Pump Body Shapes
3.4.2. Fabrication of Ferrofluidic Micropumps
3.5. Pump Chamber and Piston of Ferrofluidic Micropumps
3.6. Failure and Restart of Ferrofluidic Micropumps
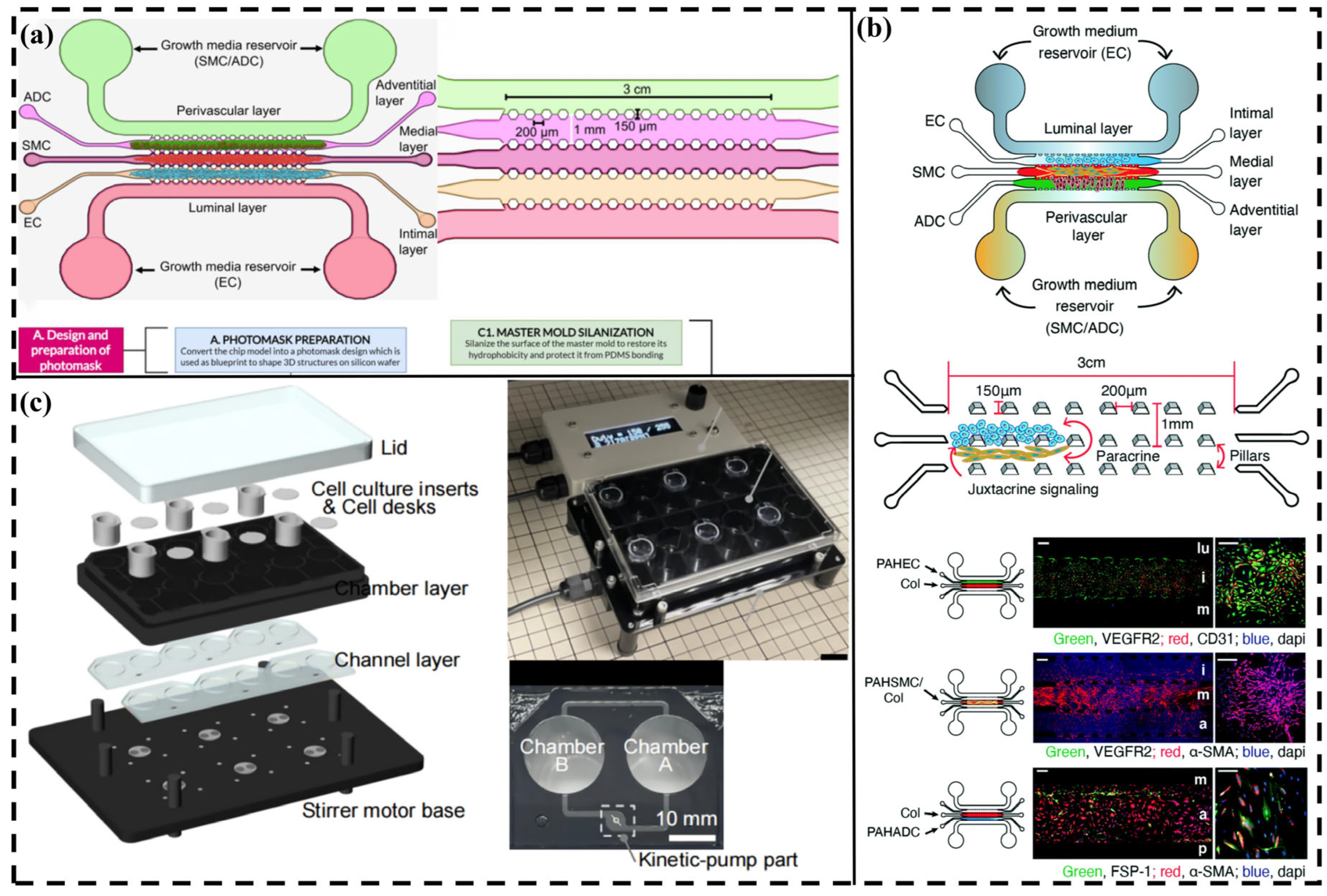
3.7. Application Fields
3.8. Performance Comparison
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PDMS | Polydimethylsiloxane |
| PMMA | Polymethyl methacrylate |
| PZT | Lead zirconate titanate |
| MEMS | Micro-Electro-Mechanical Systems |
| SMA | Shape memory alloy |
| EAP | Electroactive polymer |
| CNC | Computer numerical control |
References
- Mujtaba, J.; Liu, J.; Dey, K.K.; Li, T.; Chakraborty, R.; Xu, K. Micro-Bio-Chemo-Mechanical-Systems: Micromotors, Microfluidics, and Nanozymes for Biomedical Applications. Adv. Mater. 2021, 33, 2007465. [Google Scholar] [CrossRef] [PubMed]
- Kant, K. Microfluidic Bio-Sensors and Their Applications. Biosensors 2023, 13, 843. [Google Scholar] [CrossRef]
- Filippi, M.; Buchner, T.; Yasa, O.; Weirich, S.; Katzschmann, R.K. Microfluidic Tissue Engineering and Bio-Actuation. Adv. Mater. 2022, 34, e2108427. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Ju, Y.S. Finger-Powered Electro-Digital-Microfluidics. Methods Mol. Biol. 2017, 1572, 293–311. [Google Scholar]
- Kimura, H.; Sakai, Y.; Fujii, T. Organ/body-on-a-chip based on microfluidic technology for drug discovery. Drug Metab. Pharmacokinet. 2018, 33, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Tien, J.; Dance, Y.W. Microfluidic Biomaterials. Adv. Healthc. Mater. 2021, 10, e2001028. [Google Scholar] [CrossRef]
- Mao, K.; Min, X.; Zhang, H.; Zhang, K.; Cao, H.; Guo, Y. Paper-based microfluidics for rapid diagnostics and drug delivery. J. Control Release 2020, 322, 187–199. [Google Scholar] [CrossRef]
- Maurya, R.; Gohil, N.; Bhattacharjee, G.; Alzahrani, K.J.; Ramakrishna, S.; Singh, V. Microfluidics device for drug discovery, screening and delivery. Prog. Mol. Biol. Transl. Sci. 2022, 187, 335–346. [Google Scholar]
- Kuang, S.; Singh, N.M.; Wu, Y.; Shen, Y.; Ren, W.; Tu, L. Role of microfluidics in accelerating new space missions. Biomicrofluidics 2022, 16, 021503. [Google Scholar] [CrossRef]
- Yeung, C.K.; Koenig, P.; Countryman, S.; Thummel, K.E.; Himmelfarb, J.; Kelly, E.J. Tissue Chips in Space-Challenges and Opportunities. Clin. Transl. Sci. 2020, 13, 8–10. [Google Scholar] [CrossRef]
- Sun, H.; Hu, N.; Wang, J. Application of microfluidic technology in antibody screening. Biotechnol. J. 2022, 17, e2100623. [Google Scholar] [CrossRef]
- He, S.; Joseph, N.; Feng, S.; Jellicoe, M.; Raston, C.L. Application of microfluidic technology in food processing. Food Funct. 2020, 11, 5726–5737. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Li, Z.; Wang, H.; Li, N.; Deng, Y. Microfluidic Brain-on-a-Chip: From Key Technology to System Integration and Application. Small 2023, 19, e2304427. [Google Scholar] [CrossRef]
- Shi, Y.; Shao, X. Development of microfluidic technology in reproductive researches. Se Pu 2019, 37, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zeng, W.; Chen, R.; Ge, Y.; Zi, L.; Yang, J. Tactic movement of microalgae and its application in targeted transport: A review. Sheng Wu Gong Cheng Xue Bao 2022, 38, 578–591. [Google Scholar]
- Srisom, K.; Tittabutr, P.; Teaumroong, N.; Lapwong, Y.; Phatthanakun, R.; Sirivisoot, S. New method for arbuscular mycorrhizal fungus spore separation using a microfluidic device based on manual temporary flow diversion. Mycorrhiza 2020, 30, 789–796. [Google Scholar] [CrossRef]
- Cha, H.; Fallahi, H.; Dai, Y.; Yuan, D.; An, H.; Nguyen, N.T. Multiphysics microfluidics for cell manipulation and separation: A review. Lab Chip 2022, 22, 423–444. [Google Scholar] [CrossRef]
- El-Mayta, R.; Padilla, M.S.; Billingsley, M.M.; Han, X.; Mitchell, M.J. Testing the In Vitro and In Vivo Efficiency of mRNA-Lipid Nanoparticles Formulated by Microfluidic Mixing. J. Vis. Exp. 2023, e64810. [Google Scholar]
- Manshadi, M.K.D.; Mohammadi, M.; Monfared, L.K.; Sanati-Nezhad, A. Manipulation of micro- and nanoparticles in viscoelastic fluid flows within microfluid systems. Biotechnol. Bioeng. 2020, 117, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Ávila, B.E.; Gao, W.; Karshalev, E.; Zhang, L.; Wang, J. Cell-Like Micromotors. Acc. Chem. Res. 2018, 51, 1901–1910. [Google Scholar]
- Yadav, A.S.; Galogahi, F.M.; Vashi, A.; Tran, D.T.; Kijanka, G.S.; Cha, H. Synthesis and active manipulation of magnetic liquid beads. Biomed. Microdevices 2024, 26, 24. [Google Scholar] [CrossRef]
- Descamps, L.; Audry, M.C.; Howard, J.; Mekkaoui, S.; Albin, C.; Barthelemy, D. Self-Assembled Permanent Micro-Magnets in a Polymer-Based Microfluidic Device for Magnetic Cell Sorting. Cells 2021, 10, 1734. [Google Scholar] [CrossRef]
- Lv, X.; Geng, Z.; Fan, Z.; Wang, S.; Pei, W.; Chen, H. An integrated method for cell isolation and migration on a chip. Sci. Rep. 2017, 7, 8963. [Google Scholar] [CrossRef]
- Ghazimirsaeed, E.; Madadelahi, M.; Dizani, M.; Shamloo, A. Secondary Flows, Mixing, and Chemical Reaction Analysis of Droplet-Based Flow inside Serpentine Microchannels with Different Cross Sections. Langmuir 2021, 37, 5118–5130. [Google Scholar] [CrossRef]
- Liu, M.; Li, N.; Cui, S.; Li, G.; Yang, F. Biochemical Reaction Acceleration by Electrokinetic Mixing in a Microfluidic Chip. J. Phys. Chem. Lett. 2022, 13, 5633–5637. [Google Scholar] [CrossRef]
- Hazra, S.; Mitra, S.; Sen, A.K. Migration and Spreading of Droplets across a Fluid-Fluid Interface in Microfluidic Coflow. Langmuir 2022, 38, 9660–9668. [Google Scholar] [CrossRef]
- Reis, N.M.; Needs, S.H.; Jegouic, S.M.; Gill, K.K.; Sirivisoot, S.; Howard, S. Gravity-Driven Microfluidic Siphons: Fluidic Characterization and Application to Quantitative Immunoassays. ACS Sens. 2021, 6, 4338–4348. [Google Scholar] [CrossRef] [PubMed]
- Kitenbergs, G.; Tatuļčenkovs, A.; Puķina, L.; Cēbers, A. Gravity effects on mixing with magnetic micro-convection in microfluidics. Eur. Phys. J. E Soft Matter 2018, 41, 138. [Google Scholar] [CrossRef] [PubMed]
- Pajic-Lijakovic, I.; Milivojevic, M. Marangoni effect and cell spreading. Eur. Biophys. J. 2022, 51, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Guo, F.; Cui, Z.; Zhou, J.; Zhai, Y.; Du, Y. Controllable and Continuous Hollow Fiber Swimmers Based on the Marangoni Effect. ACS Appl. Mater. Interfaces 2020, 12, 53503–53509. [Google Scholar] [CrossRef]
- Glieberman, A.L.; Pope, B.D.; Zimmerman, J.F.; Liu, Q.; Ferrier, J.P.; Kenty, J.H.R. Synchronized stimulation and continuous insulin sensing in a microfluidic human Islet on a Chip designed for scalable manufacturing. Lab Chip 2019, 19, 2993–3010. [Google Scholar] [CrossRef] [PubMed]
- Payne, F.W.; Ledden, B.; Lamps, G. Capabilities of Next-Generation Patch Pump: Improved Precision, Instant Occlusion Detection, and Dual-Hormone Therapy. J. Diabetes Sci. Technol. 2019, 13, 49–54. [Google Scholar] [CrossRef]
- Mohith, S.; Karanth, P.N.; Kulkarni, S.M. Recent trends in mechanical micropumps and their applications: A review. Mechatronics 2019, 60, 34–55. [Google Scholar] [CrossRef]
- Nepomnyashchy, A. Droplet on a liquid substrate: Wetting, dewetting, dynamics, instabilities. Curr. Opin. Colloid Interface Sci. 2021, 51, 101398. [Google Scholar] [CrossRef]
- Salari, A.; Navi, M.; Lijnse, T.; Dalton, C. AC Electrothermal Effect in Microfluidics: A Review. Micromachines 2019, 10, 762. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.N.; Fu, L.M. Micropumps and biomedical applications—A review. Microelectron. Eng. 2018, 195, 121–138. [Google Scholar] [CrossRef]
- Chen, P.; Chen, C.; Liu, Y.H.; Du, W.; Feng, X.J.; Liu, B.F. Fully integrated nucleic acid pretreatment, amplification, and detection on a paper chip for identifying EGFR mutations in lung cancer cells. Sens. Actuators B: Chem. 2019, 283, 472–477. [Google Scholar] [CrossRef]
- Yang, Y.T.; Ho, T.Y. Conquering the Tyranny of Number With Digital Microfluidics. Front. Chem. 2021, 9, 676365. [Google Scholar] [CrossRef]
- Farzbod, A.; Moon, H. Integration of reconfigurable potentiometric electrochemical sensors into a digital microfluidic platform. Biosens. Bioelectron. 2018, 106, 37–42. [Google Scholar] [CrossRef]
- Alistar, M.; Gaudenz, U. OpenDrop: An integrated do-it-yourself platform for personal use of biochips. Bioengineering 2017, 4, 45. [Google Scholar] [CrossRef]
- Sefton, M.V.; Lusher, H.M.; Firth, S.R.; Waher, M.U. Controlled release micropump for insulin administration. Ann. Biomed. Eng. 1979, 7, 329–343. [Google Scholar] [CrossRef] [PubMed]
- Senesh, G.; Bushi, D.; Neta, A.; Yodfat, O. Compatibility of insulin Lispro, Aspart, and Glulisine with the Solo MicroPump, a novel miniature insulin pump. J. Diabetes Sci. Technol. 2010, 4, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Rimon, M.T.I.; Hasan, M.W.; Hassan, M.F.; Cesmeci, S. Advancements in Insulin Pumps: A Comprehensive Exploration of Insulin Pump Systems, Technologies, and Future Directions. Pharmaceutics 2024, 16, 944. [Google Scholar] [CrossRef]
- Cobo, A.; Sheybani, R.; Tu, H.; Meng, E. A Wireless Implantable Micropump for Chronic Drug Infusion Against Cancer. Sens. Actuators A Phys. 2016, 239, 18–25. [Google Scholar] [CrossRef]
- Kar, A.; Ahamad, N.; Dewani, M.; Awasthi, L.; Patil, R.; Banerjee, R. Wearable and implantable devices for drug delivery: Applications and challenges. Biomaterials 2022, 283, 121435. [Google Scholar] [CrossRef]
- Meng, E.; Hoang, T. MEMS-enabled implantable drug infusion pumps for laboratory animal research, preclinical, and clinical applications. Adv. Drug Deliv. Rev. 2012, 64, 1628–1638. [Google Scholar] [CrossRef]
- Dolete, G.; Chircov, C.; Motelica, L.; Ficai, D.; Oprea, O.C.; Gheorghe, M. Magneto-Mechanically Triggered Thick Films for Drug Delivery Micropumps. Nanomaterials 2022, 12, 3598. [Google Scholar] [CrossRef]
- Ito, T.; Ota, T.; Kono, R.; Miyaoka, Y.; Ishibashi, H.; Komori, M. Pump-Free Microfluidic Hemofiltration Device. Micromachines 2021, 12, 992. [Google Scholar] [CrossRef]
- Mu, X.; Chen, F.D.; Dang, K.M.; Brunk, M.G.K.; Li, J.; Wahn, H. Implantable photonic neural probes with 3D-printed microfluidics and applications touncaging. Front. Neurosci. 2023, 17, 1213265. [Google Scholar] [CrossRef]
- Lee, S.H.; Ahn, J.W.; Cho, Y.C.; Kim, S.N.; Lee, C.; Ku, G.W. Wirelessly Controlled Implantable System for On-demand and Pulsatile Insulin Administration. Sci. Rep. 2019, 9, 5009. [Google Scholar] [CrossRef] [PubMed]
- Attiguppe, A.P.; Chatterjee, D.; DasGupta, A. A Novel Integrated Transdermal Drug Delivery System with Micropump and Microneedle Made from Polymers. Micromachines 2022, 14, 71. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, L.; Cui, Y. A wearable, rapidly manufacturable, stability-enhancing microneedle patch for closed-loop diabetes management. Microsyst. Nanoeng. 2024, 10, 112. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Wu, L.; Wu, J.; Zheng, Y.; Zhao, H.; Jin, Q. Integrated microfluidic chip for endothelial cells culture and analysis exposed to a pulsatile and oscillatory shear stress. Lab Chip 2009, 9, 3118–3125. [Google Scholar] [CrossRef] [PubMed]
- Kumaresan, P.; Yang, C.J.; Cronier, S.A.; Blazej, R.G.; Mathies, R.A. High-throughput single copy DNA amplification and cell analysis in engineered nanoliter droplets. Anal. Chem. 2008, 80, 3522–3529. [Google Scholar] [CrossRef]
- Gao, T.; McNeill, J.M.; Oliver, V.A.; Xiao, L.; Mallouk, T.E. Geometric and Scaling Effects in the Speed of Catalytic Enzyme Micropumps. ACS Appl. Mater. Interfaces 2022, 14, 39515–39523. [Google Scholar] [CrossRef]
- Rothbauer, M.; Wartmann, D.; Charwat, V.; Ertl, P. Recent advances and future applications of microfluidic live-cell microarrays. Biotechnol. Adv. 2015, 33, 948–961. [Google Scholar] [CrossRef] [PubMed]
- Ou, X.; Chen, P.; Huang, X.; Li, S.; Liu, B.F. Microfluidic chip electrophoresis for biochemical analysis. J. Sep. Sci. 2020, 43, 258–270. [Google Scholar] [CrossRef]
- Wei, Z.; Fan, P.; Jiao, Y.; Wang, Y.; Huang, Y.; Liu, Z. Integrated microfluidic chip for on-line proteome analysis with combination of denaturing and rapid digestion of protein. Anal. Chim. Acta 2020, 1102, 1–10. [Google Scholar] [CrossRef]
- Guan, X.L.; Chang, D.P.S.; Mok, Z.X.; Lee, B. Assessing variations in manual pipetting: An under-investigated requirement of good laboratory practice. J. Mass Spectrom. Adv. Clin. Lab. 2023, 30, 25–29. [Google Scholar] [CrossRef]
- Martin, J.S.; Borges, A.R.; Beck, D.T. Peripheral conduit and resistance artery function are improved following a single, 1-h bout of peristaltic pulse external pneumatic compression. Eur. J. Appl. Physiol. 2015, 115, 2019–2029. [Google Scholar] [CrossRef]
- Li, Z.; Lowe, J.P.; Fletcher, P.J.; Carta, M.; McKeown, N.B.; Marken, F. Tuning and Coupling Irreversible Electroosmotic Water Flow in Ionic Diodes: Methylation of an Intrinsically Microporous Polyamine (PIM-EA-TB). ACS Appl. Mater. Interfaces 2023, 15, 42369–42377. [Google Scholar] [CrossRef]
- Abdelghany, A.; Yamasaki, K.; Ichikawa, Y.; Motosuke, M. Efficient nanoparticle focusing utilizing cascade AC electroosmotic flow. Electrophoresis 2022, 43, 1755–1764. [Google Scholar] [CrossRef]
- Qi, C.; Sugita, N.; Shinshi, T. A Disposable Electromagnetic Bi-Directional Micropump Utilizing a Rotating Multi-Pole Ring Magnetic Coupling. Micromachines 2022, 13, 1565. [Google Scholar] [CrossRef]
- Meng, Z.; Tayyab, M.; Lin, Z.; Raji, H.; Javanmard, M. A Smartphone-Based Disposable Hemoglobin Sensor Based on Colorimetric Analysis. Sensors 2022, 23, 394. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, T.; Gerrett, N.; Shinkawa, S.; Sato, T.; Miyake, R.; Kondo, N. Fluidic Patch Device to Sample Sweat for Accurate Measurement of Sweat Rate and Chemical Composition: A Proof-of-Concept Study. Anal. Chem. 2020, 92, 15534–15541. [Google Scholar] [CrossRef] [PubMed]
- Abate, A.R.; Weitz, D.A. Syringe-vacuum microfluidics: A portable technique to create monodisperse emulsions. Biomicrofluidics 2011, 5, 014107. [Google Scholar] [CrossRef] [PubMed]
- Laser, D.J.; Santiago, J.G. A review of micropumps. J. Micromech. Microeng. 2004, 14, R35. [Google Scholar] [CrossRef]
- Mohith, S.; Karanth, N.; Kulkarni, S.M. Analysis of annularly excited bossed diaphragm for performance enhancement of mechanical micropump. Sens. Actuators A Phys. 2022, 335, 113381. [Google Scholar]
- Bohm, S.; Phi, H.B.; Dittrich, L.; Runge, E. Chip-integrated non-mechanical microfluidic pump driven by electrowetting on dielectrics. Lab Chip 2024, 24, 2893–2905. [Google Scholar] [CrossRef]
- Zhang, C.; Xing, D.; Li, Y. Micropumps, microvalves, and micromixers within PCR microfluidic chips: Advances and trends. Biotechnol. Adv. 2007, 25, 483–514. [Google Scholar] [CrossRef]
- Asadi Dereshgi, H.; Dal, H.; Yildiz, M.Z. Piezoelectric micropumps: State of the art review. Microsyst. Technol. 2021, 27, 4127–4155. [Google Scholar] [CrossRef]
- Forouzandeh, F.; Arevalo, A.; Alfadhel, A.; Borkholder, D.A. A review of peristaltic micropumps. Sens. Actuators A Phys. 2021, 326, 112602. [Google Scholar] [CrossRef] [PubMed]
- Gebreyesus, E.A.; Park, A.; Guldberg, R.E.; Ong, K.G. In vitromagnetohydrodynamics system for modulating cell migration. Biomed. Phys. Eng. Express 2023, 9, 025007. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wang, K.; Huang, P.; Liu, D.; Guan, Y. Single-Cell Isolation Microfluidic Chip Based on Thermal Bubble Micropump Technology. Sensors 2023, 23, 3623. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.G.; Wu, D.; Li, W.X.; Li, D.C.; Zhou, X. Design and research of ferromagnetic fluid micropump with double rotating magnetic sources. Phys. Lett. A 2024, 519, 129718. [Google Scholar] [CrossRef]
- Ahn, J.J.; Oh, J.G.; Choi, B. A novel type of a microfluidic system using ferrofluidics for an application of μ-TAS. Microsyst. Technol. 2004, 10, 622–627. [Google Scholar] [CrossRef]
- Iverson, B.D.; Garimella, S.V. Recent advances in microscale pumping technologies: A review and evaluation. Microfluid. Nanofluidics 2008, 5, 145–174. [Google Scholar] [CrossRef]
- Amirouche, F.; Zhou, Y.; Johnson, T. Current micropump technologies and their biomedical applications. Microsyst. Technol. 2009, 15, 647–666. [Google Scholar] [CrossRef]
- Gusenbauer, M.; Mazza, G.; Posnicek, T.; Brandl, M.; Schrefl, T. Magnetically actuated circular displacement micropump. Int. J. Adv. Manuf. Technol. 2018, 95, 3575–3588. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Pham, M.; Goo, N.S. Development of a peristaltic micropump for bio-medical applications based on mini LIPCA. J. Bionic Eng. 2008, 5, 135–141. [Google Scholar] [CrossRef]
- Bussmann, A.; Thalhofer, T.; Hoffmann, S.; Daum, L.; Surendran, N.; Hayden, O. Microfluidic Cell Transport with Piezoelectric Micro Diaphragm Pumps. Micromachines 2021, 12, 1459. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, W.; Chen, X. Study on the performance of piezoelectric micro pump for insulin injection. Zhongguo Yi Liao Qi Xie Za Zhi 2015, 39, 64–67. [Google Scholar]
- Bao, Q.; Zhang, J.; Tang, M.; Huang, Z.; Lai, L.; Huang, J. A Novel PZT Pump with Built-in Compliant Structures. Sensors 2019, 19, 1301. [Google Scholar] [CrossRef]
- Uhlig, S.; Gaudet, M.; Langa, S.; Schimmanz, K.; Conrad, H.; Kaiser, B. Electrostatically Driven In-Plane Silicon Micropump for Modular Configuration. Micromachines 2018, 9, 190. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Miao, X.; Lu, H.; Liu, S.; Yang, Z. High-Efficiency 3D-Printed Three-Chamber Electromagnetic Peristaltic Micropump. Micromachines 2023, 14, 257. [Google Scholar] [CrossRef]
- So, H.; Pisano, A.P.; Seo, Y.H. Caterpillar locomotion-inspired valveless pneumatic micropump using a single teardrop-shaped elastomeric membrane. Lab Chip 2014, 14, 2240–2248. [Google Scholar] [CrossRef]
- Zeng, Y.; Shin, M.; Wang, T. Programmable active droplet generation enabled by integrated pneumatic micropumps. Lab Chip 2013, 13, 267–273. [Google Scholar] [CrossRef]
- Ameri, A.R.; Imanparast, A.; Passandideh-Fard, M.; Mousavi Shaegh, S.A. A whole-thermoplastic microfluidic chip with integrated on-chip micropump, bioreactor and oxygenator for cell culture applications. Anal. Chim. Acta 2022, 1221, 340093. [Google Scholar] [CrossRef] [PubMed]
- Munas, F.R.; Melroy, G.; Abeynayake, C.B.; Chathuranga, H.L.; Amarasinghe, R.; Kumarage, P.; Dau, V.T.; Dao, D.V. Development of PZT Actuated Valveless Micropump. Sensors 2018, 18, 1302. [Google Scholar] [CrossRef]
- Kan, J.; Xuan, M.; Yang, Z.; Wu, Y.; Wu, B.; Cheng, G. Analysis and test of piezoelectric micropump for drug delivery. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi 2005, 22, 809–813. [Google Scholar] [PubMed]
- Johnston, I.D.; Davis, J.B.; Richter, R.; Herbert, G.I.; Tracey, M.C. Elastomer-glass micropump employing active throttles. Analyst 2004, 129, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Amrani, I.; Cheriet, A.; Feliachi, M. Design and experimental investigation of a bi-directional valveless electromagnetic micro-pump. Sens. Actuators A Phys. 2018, 272, 310–317. [Google Scholar] [CrossRef]
- Amer, S.; Badawy, W. An integrated platform for bio-analysis and drug delivery. Curr. Pharm. Biotechnol. 2005, 6, 57–64. [Google Scholar] [CrossRef]
- Mahnama, A.; Nourbakhsh, A.; Ghorbaniasl, G. A survey on the applications of implantable micropump systems in drug delivery. Curr. Drug Deliv. 2014, 11, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Okura, N.; Nakashoji, Y.; Koshirogane, T.; Kondo, M.; Tanaka, Y.; Inoue, K. A compact and facile microfluidic droplet creation device using a piezoelectric diaphragm micropump for droplet digital PCR platforms. Electrophoresis 2017, 38, 2666–2672. [Google Scholar] [CrossRef] [PubMed]
- Kotb, Y.; Elgamal, I.; Serry, M. Shape Memory Alloy Capsule Micropump for Drug Delivery Applications. Micromachines (Basel) 2021, 12, 520. [Google Scholar] [CrossRef]
- Lin, J.L. Design and fabrication of pneumatically actuated valveless pumps. Micromachines 2021, 13, 16. [Google Scholar] [CrossRef]
- Machauf, A.; Yael, N.; Uri, D. A membrane micropump electrostatically actuated across the working fluid. J. Micromech. Microeng. 2005, 15, 2309. [Google Scholar]
- Lee, K.S.; Kim, B.; Shannon, M.A. Development of a peristaltic gas micropump with a single chamber and multiple electrodes. J. Micromech. Microeng. 2013, 23, 095006. [Google Scholar] [CrossRef]
- Tahmasebipour, M.; Paknahad, A.A. Unidirectional and bidirectional valveless electromagnetic micropump with PDMS-Fe3O4 nanocomposite magnetic membrane. J. Micromech. Microeng. 2019, 29, 075014. [Google Scholar] [CrossRef]
- Lin, Y.Q.; Yang, X.L.; Jin, T.; Wang, J.Y.; Yi, S.C.; Wang, Y. A Compact, High-Performance, and Deformation-Resilient Trielectrode Electrostatic Soft Pump for Soft Robotics. Adv. Intell. Syst. 2025, 7, 2400423. [Google Scholar] [CrossRef]
- Nicola, B.A.; Popescu, M.N.; Gáspár, S. Substrate-Controlled Bidirectional Pumping by a Bienzymatic Micropump. ACS Appl. Mater. Interfaces 2024, 16, 59556–59566. [Google Scholar] [CrossRef]
- Werner, E.M.; Lam, B.X.; Hui, E.E. Phase-Optimized Peristaltic Pumping by Integrated Microfluidic Logic. Micromachines 2022, 13, 1784. [Google Scholar] [CrossRef] [PubMed]
- Bui, G.T.; Wang, J.H.; Lin, J.L. Optimization of Micropump Performance Utilizing a Single Membrane with an Active Check Valve. Micromachines 2017, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Li, B.; Yang, J. A pneumatic PDMS micropump with in-plane check valves for disposable microfluidic systems. Microelectron. Eng. 2012, 99, 28–32. [Google Scholar] [CrossRef]
- Al-Halhouli, A.T.; Demming, S.; Dietzel, A.; Büttgenbach, S. Design, Fabrication, and Characterization of a Continuous Flow Micropump System. ASME J. Therm. Sci. Eng. Appl. 2016, 8, 021006. [Google Scholar] [CrossRef]
- Xi, H.D.; Zheng, H.; Guo, W.; Gañán-Calvo, A.M.; Ai, Y.; Tsao, C.W. Active droplet sorting in microfluidics: A review. Lab Chip 2017, 17, 751–771. [Google Scholar] [CrossRef]
- Agnihotri, S.N.; Raveshi, M.R.; Nosrati, R.; Bhardwaj, R.; Neild, A. Droplet splitting in microfluidics: A review. Phys. Fluids 2025, 37, 051304. [Google Scholar] [CrossRef]
- Abate, A.R.; Weitz, D.A. Single-layer membrane valves for elastomeric microfluidic devices. Appl. Phys. Lett. 2008, 92, 243509. [Google Scholar] [CrossRef]
- Abate, A.R.; Agresti, J.J.; Weitz, D.A. Microfluidic sorting with high-speed single-layer membrane valves. Appl. Phys. Lett. 2010, 96, 203509. [Google Scholar] [CrossRef]
- Agnihotri, S.N.; Raveshi, M.R.; Bhardwaj, R.; Neild, A. Microvalves for integrated selective droplet generation, splitting and merging on a chip. Microfluid. Nanofluidics 2021, 25, 88. [Google Scholar] [CrossRef]
- Nguyen, Q.M.; Abouezzi, J.; Ristroph, L. Early turbulence and pulsatile flows enhance diodicity of Tesla’s macrofluidic valve. Nat. Commun. 2021, 12, 2884. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.D.; Zhang, Z.; Yang, J.; Yang, J.; Li, D. An electromagnetically actuated tube type micropump with a one-way valve. Int. J. Appl. Electromagn. Mech. 2018, 56, 1–9. [Google Scholar] [CrossRef]
- Cesmeci, S.; Hassan, R.; Baniasadi, M. A Comparative Evaluation of Magnetorheological Micropump Designs. Micromachines 2022, 13, 764. [Google Scholar] [CrossRef]
- Jie, M.; Qi, Z.X.; Yu, W.X.; Ma, T.F.; Zhao, Y.J.; Cai, L.T. Design and Performance Test of Four-Chamber Series–Parallel Piezoelectric Pump. Coatings 2024, 14, 1348. [Google Scholar] [CrossRef]
- Grover, W.H.; Skelley, A.M.; Liu, C.N.; Lagally, E.T.; Mathies, R.A. Monolithic membrane valves and diaphragm pumps for practical large-scale integration into glass microfluidic devices. Sens. Actuators B Chem. 2003, 89, 315–323. [Google Scholar] [CrossRef]
- Raveshi, M.R.; Agnihotri, S.N.; Sesen, M.; Bhardwaj, R.; Neild, A. Selective droplet splitting using single layer microfluidic valves. Sens. Actuators B Chem. 2019, 292, 233–240. [Google Scholar] [CrossRef]
- Roy, E.; Galas, J.C.; Veres, T. Thermoplastic elastomers for microfluidics: Towards a high-throughput fabrication method of multilayered microfluidic devices. Lab Chip 2011, 11, 3193–3196. [Google Scholar] [CrossRef]
- Mohan, R.; Schudel, B.R.; Desai, A.V.; Yearsley, J.D.; Apblett, C.A.; Kenisl, P.J.A. Design considerations for elastomeric normally closed microfluidic valves. Sens. Actuators B Chem. 2011, 160, 1216–1223. [Google Scholar] [CrossRef]
- Vashi, A.; Sreejith, K.R.; Nguyen, N.T. Lab-on-a-Chip Technologies for Microgravity Simulation and Space Applications. Micromachines 2022, 14, 116. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Ayan, B.; Shayan, M.; Rando, T.A.; Huang, N.F. Skeletal muscle-on-a-chip in microgravity as a platform for regeneration modeling and drug screening. Stem Cell Rep. 2024, 19, 1061–1073. [Google Scholar] [CrossRef]
- Krakos, A. Lab-on-chip technologies for space research—Current trends and prospects. Mikrochim. Acta 2023, 191, 31. [Google Scholar] [CrossRef]
- Shi, J.J.; Wu, D.; Liu, T.Z.; Hao, S.J.; Meng, B.C.; Li, S.L. Comparative of Forensic DNA Identification Using Cell Lysis Method and Magnetic Beads Method. Fa Yi Xue Za Zhi 2023, 39, 45–49, (In English and Chinese). [Google Scholar]
- Peng, X.Y.L.; Peng, L.; Guo, Y. Manipulating nanoliter fluid circuits on an all-glass chip by the magnetic field. iScience 2023, 26, 107659. [Google Scholar] [CrossRef]
- Park, C.Y.; Park, Y.H.; Kim, Y.S.; Song, H.J.; Kim, J.D. Permanent magnet actuation for magnetic bead-based DNA extraction. Biomed. Eng. Online 2018, 17 (Suppl. S2), 143. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.K.; Bryan, M.T.; Gilbert, A.D.; Ogrin, F.Y.; Myers, T.O. A new class of magnetically actuated pumps and valves for microfluidic applications. Sci. Rep. 2018, 8, 933. [Google Scholar] [CrossRef]
- Yuan, S.Q.; Yang, S.; He, X.H.; Deng, Z.D.; Cai, S.C. Design and experimental study of a novel three-way diffuser/nozzle elements employed in valveless piezoelectric micropumps. J. Braz. Soc. Mech. Sci. Eng. 2015, 37, 221–222. [Google Scholar] [CrossRef]
- Zhu, C.L.; Shu, X.L.; Liu, D.C.; Du, X.H.; Li, L.X.; Pan, Q.S. Resonant-Type Piezoelectric Pump Driven by Piezoelectric Stacks and a Rhombic Micro Displacement Amplifier. Micromachines 2023, 14, 1764. [Google Scholar] [CrossRef] [PubMed]
- Bouzid, A.; Negassa, M.E.A.B.; Pirastesh, P.; Charrier, J.; Soulimane, S. Figure of merit study for valveless piezoelectric micropump designed to handle liquid and gas fluid in medical and environmental applications. J. Serbian Soc. For. Comput. Mech. 2024, 18, 29–44. [Google Scholar] [CrossRef]
- Guan, Y.F.; Meng, X.X.; Liu, Y.S.; Bai, M.Y.; Xu, F.Q. Modeling, Vibration Analysis and Fabrication of Micropumps Based on Piezoelectric Transducers. Int. J. Acoust. Vib. 2020, 25, 383–391. [Google Scholar] [CrossRef]
- Gidde, R.R.; Pawar, P.M.; Ronge, B.P.; Dhamgaye, V.P. Design optimization of an electromagnetic actuation based valveless micropump for drug delivery application. Microsyst. Technol. 2019, 25, 509–519. [Google Scholar] [CrossRef]
- Huang, Y.F.; Tsou, C.H.; Hsu, C.J.; Lin, Y.C.; Ono, T.; Tsai, Y.C. Metallic glass thin film integrated with flexible membrane for electromagnetic micropump application. Jpn. J. Appl. Phys. 2020, 59, SIIK03. [Google Scholar] [CrossRef]
- Gidde, R.R.; Pawar, P.M.; Dhamgaye, V.P. Fully coupled modeling and design of a piezoelectric actuation based valveless micropump for drug delivery application. Microsyst. Technol. 2020, 26, 633–645. [Google Scholar] [CrossRef]
- Aggarwal, R.; Agarwal, M.P.; Avasthi, R. Indian Journal of Endocrinology and Metabolism; ESICON 2016 Abstracts; National Institutes of Health: Bethesda, MD, USA, 2017; Volume 21, pp. S1–S90. [Google Scholar]
- Anheuer, D.; Karacan, B.; Herzog, L.; Weigel, N.; Meyer-Nieberg, S.; Gebhardt, T. Framework for Microdosing Odors in Virtual Reality for Psychophysiological Stress Training. Sensors 2024, 24, 7046. [Google Scholar] [CrossRef] [PubMed]
- Tjulkins, F.; Sebastian, R.; Guillerm, T.; Clover, A.J.P.; Hu, Y.; Lyness, A. Towards Micropump- and Microneedle-based Drug Delivery using Micro Transdermal Interface Platforms (MicroTIPs). Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2022, 2022, 3020–3023. [Google Scholar]
- Plano, D.; Kibler, S.; Rudolph, N.; Zett, O.; Dressman, J. Silicon-Based Piezo Micropumps Enable Fully Flexible Drug Delivery Patterns. J. Pharm. Sci. 2024, 113, 1555–1565. [Google Scholar] [CrossRef] [PubMed]
- Silverio, V.; Canane, P.A.G.; Martins, T.A.; Afonso, R.; Cardoso, S.; Batista, E. Development of a microfluidic electroosmosis pump on a chip for steady and continuous fluid delivery. Biomed. Tech. 2022, 68, 79–90. [Google Scholar] [CrossRef]
- Holman, J.B.; Zhu, X.; Cheng, H. Piezoelectric micropump with integrated elastomeric check valves: Design, performance characterization and primary application for 3D cell culture. Biomed. Microdevices 2023, 25, 5. [Google Scholar] [CrossRef]
- Zoupanou, S.; Chiriacò, M.S.; Tarantini, I.; Ferrara, F. Innovative 3D Microfluidic Tools for On-Chip Fluids and Particles Manipulation: From Design to Experimental Validation. Micromachines 2021, 12, 104. [Google Scholar] [CrossRef]
- Lin, L.; Chung, C.K. PDMS Microfabrication and Design for Microfluidics and Sustainable Energy Application: Review. Micromachines 2021, 12, 1350. [Google Scholar] [CrossRef]
- Du, Z.; Sun, W.; Mi, S. Multi-step PDMS curing and a controlled separation method for mass manufacturing of high-performance and user-friendly micro-devices: Valved micropumps. Lab Chip 2024, 24, 843–853. [Google Scholar] [CrossRef]
- Akram, M.M.; Aghajani, A.; Shi, W.; Gosselin, B. A closed-loop micropump and flow meter for high-precision drug delivery in an implantable neural probe. In Proceedings of the 2024 46th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 15–19 July 2024; pp. 1–4. [Google Scholar]
- Ahmadianyazdi, A.; Miller, I.J.; Folch, A. Tunable resins with PDMS-like elastic modulus for stereolithographic 3D-printing of multimaterial microfluidic actuators. Lab Chip 2023, 23, 4019–4032. [Google Scholar] [CrossRef]
- Spencer, W.J.; Corbett, W.T.; Dominguez, L.R.; Shafer, B. D, An electronically controlled piezoelectric insulin pump and valves. IEEE Trans. Sonics Ultrason. 1978, 25, 153–156. [Google Scholar] [CrossRef]
- Calvo-López, A.; Ymbern, O.; Puyol, M.; Alonso-Chamarro, J. Soluble reactive phosphorous determination in wastewater treatment plants by automatic microanalyzers. Talanta 2021, 221, 121508. [Google Scholar] [CrossRef] [PubMed]
- Dhwaj, A.; Roy, N.; Jaiswar, A.; Prabhakar, A.; Verma, D. 3D-Printed Impedance Micropump for Continuous Perfusion of the Sample and Nutrient Medium Integrated with a Liver-On-Chip Prototype. ACS Omega 2022, 7, 40900–40910. [Google Scholar] [CrossRef]
- Kim, J.; Kim, S.; Choi, J.; Koo, C. A 3D Miniaturized Glass Magnetic-Active Centrifugal Micropump Fabricated by SLE Process and Laser Welding. Micromachines 2022, 13, 1331. [Google Scholar] [CrossRef]
- Alexandre-Franco, M.F.; Kouider, R.; Kassir Al-Karany, R.; Cuerda-Correa, E.M.; Al-Kassir, A. Recent Advances in Polymer Science and Fabrication Processes for Enhanced Microfluidic Applications: An Overview. Micromachines 2024, 15, 1137. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, H.; Li, Q.; Deng, Y.; Ye, Z.; Gui, L. Texture-structure-based liquid metal filling for blind-end microchannels and its application on multi-layer chips. RSC Adv. 2023, 13, 24228–24236. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Huang, F.; Wang, B.; Li, B.; Lin, Q. A planar PDMS micropump using in-contact minimized-leakage check valves. J. Micromech. Microeng. 2010, 20, 095033. [Google Scholar] [CrossRef]
- Regmi, S.; Poudel, C.; Adhikari, R.; Luo, K.Q. Applications of Microfluidics and Organ-on-a-Chip in Cancer Research. Biosensors 2022, 12, 459. [Google Scholar] [CrossRef]
- Lan, Z.; Chen, R.; Zou, D.; Zhao, C.X. Microfluidic Nanoparticle Separation for Precision Medicine. Adv. Sci. 2025, 12, e2411278. [Google Scholar] [CrossRef]
- Abu-Dawas, S.; Alawami, H.; Zourob, M.; Ramadan, Q. Design and Fabrication of Low-Cost Microfluidic Chips and Microfluidic Routing System for Reconfigurable Multi-(Organ-on-a-Chip) Assembly. Micromachines 2021, 12, 1542. [Google Scholar] [CrossRef]
- Vante, A.B.; Kanish, T.C. Fluid-structure interaction and experimental studies of passive check valve based piezoelectric micropump for biomedical applications. Adv. Mater. Process. Technol. 2024, 10, 2095–2121. [Google Scholar] [CrossRef]
- Liu, X.P.; Li, X.Q.; Wang, M.; Cao, S.Q.; Wang, X.F.; Liu, G.J. A High-Performance Piezoelectric Micropump with Multi-Chamber in Series. Appl. Sci. 2022, 12, 4483. [Google Scholar] [CrossRef]
- Lv, W.C.; Ni, J.F.; Xuan, W.P.; Li, Y.X.; Huang, X.Y.; Sun, L.L. A simulation and experimental study of a valveless piezoelectric micropump based on the synthetic jet principle. Nanotechnol. Precis. Eng. 2025, 8, 023009. [Google Scholar] [CrossRef]
- Chuang, W.C.; Lee, H.L.; Chang, P.Z.; Hu, Y.C. Review on the modeling of electrostatic MEMS. Sensors 2010, 10, 6149–6171. [Google Scholar] [CrossRef]
- Surendran, N.; Durasiewicz, C.P.; Hoffmann, T.; Wille, A.; Bussmann, A.B.; Richter, M. Microfluidic Delivery of High Viscosity Liquids Using Piezoelectric Micropumps for Subcutaneous Drug Infusion Applications. IEEE Open J. Eng. Med. Biol. 2024, 5, 21–31. [Google Scholar] [CrossRef]
- Bussmann, A.B.; Grünerbel, L.M.; Durasiewicz, C.P.; Thalhofer, T.A.; Wille, A.; Richter, M. Microdosing for drug delivery application—A review. Sens. Actuators A Phys. 2021, 330, 112820. [Google Scholar] [CrossRef]
- Ochoa, M.; Ziaie, B. A fermentation-powered thermopneumatic pump for biomedical applications. Lab Chip 2012, 12, 4044–4048. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, K.; Liu, X.; Zhang, Y.S. Blood-Vessel-on-a-Chip Platforms for Evaluating Nanoparticle Drug Delivery Systems. Curr. Drug Metab. 2018, 19, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Lu, Y.; Zhang, F.; Liu, Q. Electronically powered drug delivery devices: Considerations and challenges. Expert Opin. Drug Deliv. 2022, 19, 1636–1649. [Google Scholar] [CrossRef]
- You, R.; Fu, X.; Duan, X. Acoustofluidic Based Wireless Micropump for Portable Drug Delivery Applications. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2021, 2021, 1276–1279. [Google Scholar]
- Rasekh, M.; Harrison, S.; Schobesberger, S.; Ertl, P.; Balachandran, W. Reagent storage and delivery on integrated microfluidic chips for point-of-care diagnostics. Biomed. Microdevices 2024, 26, 28. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, J.; Yin, W.; Wang, A.; Zheng, J.; Wang, Y. Microscale tissue engineering of liver lobule models: Advancements and applications. Front. Bioeng. Biotechnol. 2023, 11, 1303053. [Google Scholar] [CrossRef]
- Sarkar, T.; Nguyen, T.; Moinuddin, S.M.; Stenmark, K.R.; Nozik, E.S.; Saha, D. A Protocol for Fabrication and on-Chip Cell Culture to Recreate PAH-Afflicted Pulmonary Artery on a Microfluidic Device. Micromachines 2022, 13, 1483. [Google Scholar] [CrossRef]
- Al-Hilal, T.A.; Keshavarz, A.; Kadry, H.; Lahooti, B.; Al-Obaida, A.; Ding, Z. Pulmonary-arterial-hypertension (PAH)-on-a-chip: Fabrication, validation and application. Lab Chip 2020, 21, 3334–3345. [Google Scholar] [CrossRef] [PubMed]
- Shinha, K.; Nihei, W.; Nakamura, H.; Goto, T.; Kawanishi, T.; Ishida, N. A Kinetic Pump Integrated Microfluidic Plate (KIM-Plate) with High Usability for Cell Culture-Based Multiorgan Microphysiological Systems. Micromachines 2021, 12, 1007. [Google Scholar] [CrossRef] [PubMed]
- Noh, K.H.; Kang, H.M.; Oh, S.J.; Lee, J.Y.; Kim, D.H.; Kim, M. A new experimental model to study human drug responses. Biofabrication 2020, 12, 045029. [Google Scholar] [CrossRef]
- Kim, S.; Kim, J.; Joung, Y.H.; Ahn, S.; Park, C.; Choi, J. Monolithic 3D micromixer with an impeller for glass microfluidic systems. Lab Chip 2020, 20, 4474–4485. [Google Scholar] [CrossRef]
- Kalinowski, S.; Kościelniak, P.; Wierzbicka, E.; Koronkiewicz, S. A Multi-Pumping Gradient Calibration Module for Potentiometric Determination of Nitrate in Surface Water. Molecules 2023, 28, 493. [Google Scholar] [CrossRef]
- Park, J.H.; Seo, M.Y.; Ham, Y.B.; Yun, S.N.; Kim, D.I. A study on high-output piezoelectric micropumps for application in DMFC. J. Electroceram. 2013, 30, 102–107. [Google Scholar] [CrossRef]
- Michael, I.J.; Kumar, S.; Oh, J.M.; Kim, D.; Kim, J.; Cho, Y.K. Surface-Engineered Paper Hanging Drop Chip for 3D Spheroid Culture and Analysis. ACS Appl. Mater. Interfaces 2018, 10, 33839–33846. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, J.; Wang, X.; Feng, L.; Wu, J.; Zhu, X. Organ-on-a-chip: Recent breakthroughs and future prospects. Biomed. Eng. Online 2020, 19, 9. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Foo, G.W.; Aggarwal, N.; Chang, M.W. Organ-on-chip technology: Opportunities and challenges. Biotechnol. Notes 2024, 5, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Chliara, M.A.; Elezoglou, S.; Zergioti, I. Bioprinting on Organ-on-Chip: Development and Applications. Biosensors 2022, 12, 1135. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Lv, X.; Li, X.; Rcheulishvili, N.; Chen, Y.; Li, Z. Microfluidic actuated and controlled systems and application for lab-on-chip in space life science. Space Sci. Technol. 2023, 3, 0008. [Google Scholar] [CrossRef]
- Thorsen, T.; Maerkl, S.J.; Quake, S.R. Microfluidic large-scale integration. Science 2002, 298, 580–584. [Google Scholar] [CrossRef]
- Khandelwal, A.; Athreya, N.; Tu, M.Q.; Janavicius, L.L.; Yang, Z.; Milenkovic, O. Self-assembled microtubular electrodes for on-chip low-voltage electrophoretic manipulation of charged particles and macromolecules. Microsyst. Nanoeng. 2022, 8, 27. [Google Scholar] [CrossRef]
- Prakash, J.; Dharmendra, T.; Bég, O.A. Comparative study of hybrid nanofluids in microchannel slip flow induced by electroosmosis and peristalsis. Appl. Nanosci. 2020, 10, 1693–1706. [Google Scholar] [CrossRef]
- Xu, H.; Hang, Y.; Wu, Z.; Lei, X.; Deng, J.; Yang, J. Capillary-driven microchip integrated with nickel phosphide hybrid-modified electrode for the electrochemical detection of glucose. Anal. Chim. Acta 2024, 1316, 342882. [Google Scholar] [CrossRef]
- Wu, D.; Shi, B.; Li, B.; Wu, W. A Novel Self-Activated Mechanism for Stable Liquid Transportation Capable of Continuous-Flow and Real-time Microfluidic PCRs. Micromachines 2019, 10, 350. [Google Scholar] [CrossRef]
- Ma, H.K.; Chen, R.H.; Hsu, Y.H. Development of a piezoelectric-driven miniature pump for biomedical applications. Sens. Actuators A Phys. 2015, 234, 23–33. [Google Scholar] [CrossRef]
- Liu, B.D.; Ma, C.X.; Yang, J.H.; Li, D.S.; Liu, H.B. Study on the Heat Source Insulation of a Thermal Bubble-Driven Micropump with Induction Heating. Micromachines 2021, 12, 1040. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.G.; Dong, L.T.; Wang, M.; Li, X.Q.; Liu, X.P.; Liu, G.J. A miniature piezoelectric pump with high performance. AIP Adv. 2022, 12, 065316. [Google Scholar] [CrossRef]
- Motohashi, T.; Ogawa, N.; Akai, H.; Shintake, J. Peristaltic micropump using polyvinyl chloride gels with micropatterned surface. Sci. Rep. 2022, 12, 22608. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Affane, H.; Zhang, B.; Kuang, M.; Xiong, J.; Zhang, S.Y. Investigation of Flow Characteristics in Valveless Piezoelectric Pumps with Airfoil Baffles at Varying Angles of Attack. Appl. Sci. 2025, 15, 445. [Google Scholar] [CrossRef]
- Dong, J.S.; Cao, Y.; Chen, Q.Q.; Wu, Y.; Liu, R.G.; Liu, W.S. Performance of single piezoelectric vibrator micropump with check valve. J. Intell. Mater. Syst. Struct. 2020, 31, 117–126. [Google Scholar] [CrossRef]
- Rusli, M.Q.A.; Chee, P.S.; Arsat, R.; Lau, K.X.; Leow, P.L. Electromagnetic actuation dual-chamber bidirectional flow micropump. Sens. Actuators A Phys. 2018, 282, 17–27. [Google Scholar] [CrossRef]
- Chen, S.; Xie, Y.L.; Fang, X.; Zou, Z.M.; Lu, J.; Zhang, Z.H. A piezoelectric pump with composite vibrator for bubble resistance. Sens. Actuators A Phys. 2024, 379, 115972. [Google Scholar] [CrossRef]
- Cao, D.; Ji, S.; Yang, Z. Design and analysis of a wheel valve piezoelectric micropump with high performance. Sens. Actuators A Phys. 2024, 377, 115624. [Google Scholar] [CrossRef]
- Pan, Q.; Jiang, H.; Huang, Z.; Huang, B.; Li, R.; Wana, B. Development of a piezoelectric pump with ball valve structure. J. Intell. Mater. Syst. Struct. 2021, 32, 2289–2299. [Google Scholar] [CrossRef]
- Ye, Y.; Chen, J.; Pan, Q.S.; Feng, Z.H. Suppressing the generation of cavitation by increasing the number of inlet check valves in piezoelectric pumps. Sens. Actuators A Phys. 2019, 293, 56–61. [Google Scholar] [CrossRef]
- Wang, J.L.; Zhang, F.; Gui, Z.Z.; Wen, Y.X.; Zeng, Y.H.; Xie, T. Design and Analysis of a Cardioid Flow Tube Valveless Piezoelectric Pump for Medical Applications. Sensors 2024, 24, 122. [Google Scholar] [CrossRef]
- Dereshgi, H.A.; Yıldız, M.; Parlak, N. Performance comparison of novel single and bi-diaphragm PZT based valveless micropumps. J. Appl. Fluid Mech. 2020, 13, 401–412. [Google Scholar] [CrossRef]
- Stephen, P.S. Low Viscosity Magnetic Fluid Obtained by the Colloidal Suspension of Magnetic Particles. U.S. Patent US-PATENT-3,215,572, 1965. [Google Scholar]
- Yang, W.R.; Zhang, Y.M.; Yang, X.R.; Sun, C.X.; Chen, Y. Systematic analysis of ferrofluidic: A visualization review, advances engineering applications, and challenges. J. Nanopart. Res. 2022, 24, 102. [Google Scholar] [CrossRef]
- Li, W.; Li, Z.; Han, W.; Li, D.; Yan, S.; Zhou, J. Study of the flow characteristics of pumped media in the confined morphology of a ferrofluidic pump with annular microscale constraints. J. Fluids Eng. 2025, 147, 021201. [Google Scholar] [CrossRef]
- Pamme, N. Magnetism and microfluidics. Lab Chip 2006, 6, 24–38. [Google Scholar] [CrossRef]
- Kim, E.G.; Oh, J.; Choi, B. A study on the development of a continuous peristaltic micropump using magnetic fluids. Sens. Actuators A Phys. 2006, 128, 43–51. [Google Scholar] [CrossRef]
- Yunas, J.; Mulyanti, B.; Hamidah, I.; Mohd Said, M.; Pawinanto, R.E.; Wan Ali, W.A.F. Polymer-based MEMS electromagnetic actuator for biomedical application: A review. Polymers 2020, 12, 1184. [Google Scholar] [CrossRef] [PubMed]
- Rathore, A.S. Joule heating and determination of temperature in capillary electrophoresis and capillary electrochromatography columns. J. Chromatogr. A 2004, 1037, 431–443. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.G.; Li, D.C.; Chen, F.; Zhao, Q.; Qing, J. A Double-Rotating ferrofluidic Vane Micropump with an Embedded Fixed Magnet. Actuators 2024, 13, 308. [Google Scholar] [CrossRef]
- Hatch, A.; Kamholz, A.E.; Holman, G.; Yager, P.; Bohringer, K.F. A ferrofluidicic magnetic micropump. J. Microelectromech. systems. 2001, 10, 215–221. [Google Scholar] [CrossRef]
- Wang, H.; Gao, Z.; Meng, Y.; Li, Y.; Zhang, Q. Performance of the ferrofluidic-spiral labyrinth combined seal for sealing liquid. Int. J. Appl. Electromagn. Mech. 2023, 72, 87–101. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, X.; Dou, X.; Han, L. Experimental study of symmetrical ferrofluidic seals with single magnetic source and small clearance. Tribol. Int. 2024, 193, 109467. [Google Scholar] [CrossRef]
- Liu, B.D.; Zhang, Z.; Yang, J.; Yang, J.; Li, D. A rotary ferrofluidicic vane micropump with C shape baffle. Sens. Actuators B Chem. 2018, 263, 452–458. [Google Scholar] [CrossRef]
- Zhu, G.; Li, Z.Q.; Li, W.; Liu, S.X.; Wang, Z.C.; Cheng, C. Design and Dynamic Characteristics Research of Fishmouth Shaped Baffle ferrofluidic Micro-Pump. Iran. J. Sci. Technol. Trans. Mech. Eng. 2025, 49, 1205–1214. [Google Scholar] [CrossRef]
- Tang, Z.; Shao, X.; Huang, J.; Yao, J.; Ding, G. Manipulate microfluid with an integrated butterfly valve for micropump application. Sens. Actuators A Phys. 2020, 306, 111965. [Google Scholar] [CrossRef]
- Yu, A.; Wang, Y.F.; Lv, S.J.; Tang, Q.H. Numerical analysis of the cavity vorticity transport and entropy production in a micropump. Int. Commun. Heat Mass Transf. 2024, 159, 108144. [Google Scholar] [CrossRef]
- Shaker, S.; Hajjawi, M.; Khan, A.; Kilani, M. Effect of inlet and outlet angles on the flow performance of the ferrofluidicic magnetic micropump. Cogent Eng. 2023, 10, 2158611. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Wu, Z. Micromixers—A review. J. Micromech. Microeng. 2004, 15, R1. [Google Scholar] [CrossRef]
- Unger, M.A.; Chou, H.P.; Thorsen, T.; Scherer, A.; Quake, S.R. Monolithic microfabricated valves and pumps by multilayer soft lithography. Science 2000, 288, 113–116. [Google Scholar] [CrossRef]
- Hartshorne, H.; Backhouse, C.J.; Lee, W.E. ferrofluidic-based microchip pump and valve. Sens. Actuators B Chem. 2004, 99, 592–600. [Google Scholar] [CrossRef]
- Li, W.; Li, Z.; Han, W.; Wang, Y.; Zhao, J.; Zhou, J. Morphologic transformation of ferrofluidic during micropump driving under field control. Ann. N. Y. Acad. Sci. 2025, 1543, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Doganay, S.; Cetin, L.; Ezan, M.A.; Turgut, A.A. rotating permanent magnetic actuator for micropump devices with magnetic nanofluids. J. Micromech. Microeng. 2020, 30, 075012. [Google Scholar] [CrossRef]
- Chang, Y.J.; Hu, C.Y.; Lin, H.S. A microchannel immunoassay chip with ferrofluidic actuation to enhance the biochemical reaction. Sens. Actuators B Chem. 2013, 182, 584–591. [Google Scholar] [CrossRef]
- Fu, L.M.; Fang, W.C.; Hong, T.F.; Lee, C.Y. A magnetic micropump based on ferrofluidicic actuation. Int. J. Autom. Smart Technol. 2014, 4, 77–82. [Google Scholar] [CrossRef]
- Ashouri, M.; Shafii, M.B.; Moosavi, A.; Hezave, H.A. A novel revolving piston minipump. Sens. Actuators B Chem. 2015, 218, 237–244. [Google Scholar] [CrossRef]
- Ashouri, M.; Shafii, M.B.; Moosavi, A. Theoretical and experimental studies of a magnetically actuated valveless micropump. J. Micromech. Microeng. 2016, 27, 015016. [Google Scholar] [CrossRef]
- Lee, C.Y.; Leong, J.C.; Wang, Y.N.; Fu, L.M. A ferrofluidicic magnetic micropump for variable-flow-rate applications. Jpn. J. Appl. Phys. 2012, 51, 047201. [Google Scholar] [CrossRef]
- Ando, B.; Ascia, A.; Baglio, S.; Pitrone, N. Development of novel ferrofluidicic pumps. In Proceedings of the 2006 International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006; pp. 2828–2831. [Google Scholar]
- Yamahata, C.; Chastellain, M.; Parashar, V.K.; Petri, A.; Hofmann, H.; Gijs, M.A. Plastic micropump with ferrofluidicic actuation. J. Microelectromech. Syst. 2005, 14, 96–102. [Google Scholar] [CrossRef]
- Kim, H.; Ali, A.; Kang, Y.; Lim, B.; Kim, C. Surface-Driven Particle Dynamics: Sequential Synchronization of Colloidal Flow Attempted in a Static Fluidic Environment. ACS Appl. Mater. Interfaces 2025, 17, 12772–12781. [Google Scholar] [CrossRef] [PubMed]
- Molinero-Fernández, Á.; Moreno-Guzmán, M.; López, M.Á.; Escarpa, A. Magnetic Bead-Based Electrochemical Immunoassays On-Drop and On-Chip for Procalcitonin Determination: Disposable Tools for Clinical Sepsis Diagnosis. Biosensors 2020, 10, 66. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zu, X.Y.; Du, Z.; Hu, Z.G. Research on magnetic bead motion characteristics based on magnetic beads preset technology. Sci. Rep. 2021, 11, 19995. [Google Scholar] [CrossRef]
- Zhou, M.; Su, H.; Wang, B.; Wan, C.; Du, W.; Chen, P. A magnet-actuated microfluidic array chip for high-throughput pretreatment and amplification and detection of multiple pathogens. Analyst 2022, 147, 2433–2441. [Google Scholar] [CrossRef]
- Giacometti, M.; Milesi, F.; Coppadoro, P.L.; Rizzo, A.; Fagiani, F.; Rinaldi, C. A Lab-On-chip Tool for Rapid, Quantitative, and Stage-selective Diagnosis of Malaria. Adv. Sci. 2021, 8, 2004101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.H.; Wang, Y.H.; Zhou, W.H.; Lei, Y.M.; Lu, J.S.; Yin, W.J. A Magnetically Driven Tandem Chip Enables Rapid Isolation and Multiplexed Profiling of Extracellular Vesicles. Angew. Chem. Int. Ed. Engl. 2023, 62, e202315113. [Google Scholar] [CrossRef]
- Golozar, M.; Chu, W.K.; Casto, L.D.; McCauley, J.; Butterworth, A.L.; Mathies, R.A. Fabrication of high-quality glass microfluidic devices for bioanalytical and space flight applications. MethodsX 2020, 7, 101043. [Google Scholar] [CrossRef]
- Zeng, L.; Hu, S.; Chen, X.; Zhang, P.; Gu, G.; Wang, Y. Extraction of small extracellular vesicles by label-free and biocompatible on-chip magnetic separation. Lab Chip 2022, 22, 2476–2488. [Google Scholar] [CrossRef]
- İçöz, K.; Akar, Ü.; Ünal, E. Microfluidic Chip based direct triple antibody immunoassay for monitoring patient comparative response to leukemia treatment. Biomed. Microdevices 2020, 22, 48. [Google Scholar] [CrossRef]
- Chen, H.; Chen, C.; Zhou, Y.; Yang, C.; Song, G.; Hou, F. Evaluation and Optimization of a Cross-Rib Micro-Channel Heat Sink. Micromachines 2022, 13, 132. [Google Scholar] [CrossRef]
- Waqas, H.; Khan, S.A.; Farooq, U.; Muhammad, T.; Alshehri, A.; Yasmin, S. Thermal transport analysis of six circular microchannel heat sink using nanofluid. Sci. Rep. 2022, 12, 8035. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Su, H.; Liu, H.; Zhang, Y.; Qiu, N.; Gao, B. Leakage and rotordynamic performance of a semi-Y labyrinth seal structure for centrifugal pump based on multi-frequency whirl method. J. Eng. Gas Turbines Power 2025, 147, 101022. [Google Scholar] [CrossRef]
- Li, W.X.; Li, Z.; Han, W.; Li, R.; Zhang, Y. Mechanism of bubble generation in ferrofluidic micro-pumps and key parameters influencing performance. Powder Technol. 2025, 467, 121562. [Google Scholar] [CrossRef]
- Lin, Y.H.; Piñan Basualdo, F.N.; Kalpathy Venkiteswaran, V.; Misra, S. Untethered soft magnetic pump for microfluidics-based Marangoni surfer. Sci. Rep. 2024, 14, 20280. [Google Scholar] [CrossRef]
- Han, J.; Yeom, J.; Mensing, G.; Flachsbart, B.; Shannon, M.A. Characteristics of electrostatic gas micro-pump with integrated polyimide passive valves. J. Micromech. Microeng. 2012, 22, 095007. [Google Scholar] [CrossRef]







| Type | Advantages | Disadvantages | Applications |
|---|---|---|---|
| Piezoelectric |
|
|
|
| Electromagnetic |
|
|
|
| Thermopneumatic |
|
|
|
| Phase change |
|
|
|
| Shape memory alloy |
|
|
|
| Electroactive polymer |
|
|
|
| Ref. | Year | Type | Length (μm) | Width 1 (μm) | Width 2 (μm) | Height (μm) | Angle (°) | Material |
|---|---|---|---|---|---|---|---|---|
| [120] | 2015 | PE | 3000 | No | 75 | No | 8 | Si |
| [121] | 2023 | PE | 700 | 300 | 100 | 500 | No | PDMS |
| [122] | 2020 | PE | 1093 | 67 | 40 | No | 7 | Si |
| [116] | 2019 | PE(PZT-5A) | 1300 | 130 | 70 | 100 | 10 | No |
| [123] | 2018 | EM(Cu) | 1100 | 125 | 50 | 500 | 10 | PDMS |
| [124] | 2020 | ES(Cu) | 2300 | 500 | 100 | No | 16 | PDMS |
| [125] | 2018 | EM | 1000 | No | No | No | 15.64 | PDMS |
| [126] | 2020 | PE(PZT) | No | 2000 | 200 | 100 | No | PMMA |
| [127] | 2014 | FMP | 7484 | 2796 | 1054 | 1000 | 13.2 | PMMA |
| [128] | 2015 | FMP | 4963 | 1000 | 200 | 450 | 9.5 | PMMA |
| [129] | 2012 | FMP | 7484 | 2796 | 1054 | 1000 | 13.2 | PMMA |
| [88] | 2022 | PM | No | No | No | No | 9.5 | PMMA |
| [130] | 2024 | EM | 1553 | 1080 | 600 | No | 17.6 | PDMS |
| [131] | 2018 | PM | 1000 | 182.8 | 30 | 200 | 14 | No |
| [132] | 2023 | No | 1452 | 2774 | 1000 | 1500 | 62.8 | PMMA |
| Ref. | Year | Type | Diaphragm | Thickness | Material | Size | Valve | U | P | Q | f |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [120] | 2019 | PE(PZT) | PDMS | 250 | No | Φ12 | Diffuser | 50 | 0.2 | 20 | 100 |
| [121] | 2016 | PE(PZT) | Si | <20 | Si | 10 × 10 | \ | 80 | 3.1 | 0.36 | 2.52 k |
| [101] | 2015 | PM | PDMS | 200 | \ | 4 × 4 | Yes | No | 30 | 496 | 3 |
| [188] | 2025 | PE(PZT) | No | No | No | Φ38 | \ | 100 | 0.78 | 200.7 | 43 |
| [115] | 2024 | PE(PZT) | Copper | 200 | No | No | Umbrella | 110 | 42.3 | 767 | 400 |
| [189] | 2019 | PE | PMMA | No | No | Φ40, t0.8 | Umbrella | 60 | 0.82 | No | 300 |
| [131] | 2018 | EM(Cu) | PDMS | 100 | PDMS | Φ10 | Diffuser | No | 0.35 | 0.52 | 45 |
| [132] | 2020 | ES(Cu) | Iron-based MG | No | PDMS | Φ8 | Diffuser | 20 | No | 0.17 | 1 |
| [113] | 2018 | EM(Cu) | NdFeB | 5000 | Teflon | Φ2, H35 | Ball | No | 0.54 | 0.21 | 5 |
| [81] | 2021 | PE | No | 200 | stainless steel | Φ18 | Spring | 300 | No | No | 60 |
| [63] | 2022 | EM(NdFeB) | PDMS | 275 | PMMA | Φ6 | No | 4.8 | 0.5 | 0.86 | 50 |
| [128] | 2023 | PE | PDMS | No | PMMA | 78 × 12 | Yes | 150 | 1.7 | 700 | 716 |
| [190] | 2018 | EM | PDMS | No | PDMS | No | Diffuser | 1.4 | 1.2 | 76 μL | 9 |
| [157] | 2025 | PE(PZT) | No | No | resin | 22 × 22 × 5 | \ | 42 | No | 618 | 22.5 k |
| [191] | 2024 | PE(PZT) | PET | 50–200 | PMMA | Φ25, H3 | Umbrella | 300 | 18.7 | 41.4 | No |
| [192] | 2024 | PE(PZT) | No | No | PET | 10 × 10 × 1 | Cantilever | 200 | 100 | 99.6 | 790 |
| [193] | 2021 | PE(PZT) | PDMS | 200 | PMMA | 60 × 60 × 12 | Ball | 448 | 15.3 | 131.6 | 750 |
| [194] | 2019 | PE(PZT) | PI | 40 | PMMA | 100 × 20 × 15 | Cantilever | 300 | 15 | 0.1 | 60 |
| [129] | 2024 | PE(PZT) | PDMS | 600 | PDMS | Φ10, H0.5 | Diffuser | 9 | No | 5.69 | 25 |
| [195] | 2024 | PE(PZT) | No | \ | resin | 35 × 35 × 0.8 | \ | 100 | 19.74 | 20.12 | 120 |
| [196] | 2020 | PE(PZT) | Si | 100 | PMMA | Φ25, 4 | Diffuser | 45 | No | 9.1 | No |
| [130] | 2020 | PE(PZT) | PMMA | 200 | PDMS | Φ10 | Diffuser | 100 | 0.35 | 150 | 600 |
| Ref. | Year | Construction | Pressure | Velocity | Material | Layer | Size | Fabrication | Magnet | Field |
|---|---|---|---|---|---|---|---|---|---|---|
| [75] | 2024 | Circle | 1.35 | 1756.3 | PMMA | 2 | Φ10, H0.5 | No | PM, 1, Φ5 | 495 |
| [217] | 2020 | Hemicycle | No | 9.02 | PMMA | 2 | Φ50, 0.4 × 0.4 | CNC | PM, 2, Φ15, t5 | 160 |
| [79] | 2018 | Annular | No | 3.5 × 105 | Stratasys J750 | 2 | Φ80, H30, d7 | 3D Printing | EM, 10, 200 N, 1–6 A | No |
| [201] | 2006 | Annular | No | 3.8 | Si | 4 | No | Etching coating | PM, 2 | 95 |
| [218] | 2013 | Annular | No | No | PDMS | 3 | Φ15, H0.5 | Injection molding | PM, 1 | No |
| [219] | 2014 | Annular+ | No | 128 | PMMA | 2 | Φ21, d1 | CO2 laser | PM, 2 | No |
| [77] | 2004 | Circle | 2 | No | Si | 2 | 15 × 28 × 0.8 | MEMES | PM, 1, Φ3, t2 | 340 |
| [220] | 2015 | Liner+ | 0.99 | 934 | PMMA | 6 | Φ6, H1 | CO2 laser | PM, 1 | 510 |
| [221] | 2015 | Circle | 0.65 | 1310 | PMMA | 4 | Φ3.5, H2 | CO2 laser | PM, 1, 4 × 6 × 2 | 1200 |
| [208] | 2018 | Annular | 1.13 | 49.32 | PDMS | 2 | Φ25, H3.5 | Soft lithography | PM | 450 |
| [222] | 2012 | Annular | 0.66 | 93 | PMMA | 2 | Φ21, d4 | CO2 laser | PM | No |
| [215] | 2004 | Liner | 12 | No | Glass | 2 | 3 × 4 | Photo etching | PM, 2 | No |
| [205] | 2001 | Annular | 1.32 | 45.8 | Si | 4 | No | Photo etching | PM, 1, Φ6, t3 | 350 |
| [223] | 2006 | Liner | 0.2 | 1200 | Glass | \ | Φ44 | No | EM, 7 | No |
| [224] | 2005 | Liner | 2.5 | 30 | PMMA | 7 | 0.1 × 22 × 6 | Powder blasting | PM, 1 | 80 |
| Ref. | First Author | Year | Type | P (kpa) | Q (mL/min) |
|---|---|---|---|---|---|
| [75] | Wang, Y. | 2024 | Ferrofluidic Micropump | 1.35 | 1756.3 |
| [220] | Ashouri, M. | 2015 | Ferrofluidic Micropump | 0.994 | 934 |
| [221] | Ashouri, M. | 2015 | Ferrofluidic Micropump | 0.647 | 1310 |
| [202] | Liu, B.D. | 2018 | Ferrofluidic Micropump | 1.13 | 49.32 |
| [199] | Hatch, A. | 2001 | Ferrofluidic Micropump | 1.32 | 45.8 |
| [120] | Gidde, R.R. | 2019 | Piezoelectric Micropump (PZT-5A) | 0.2 | 20 |
| [134] | Aggarwal, S. | 2016 | Piezoelectric Micropump (PZT) | 3.1 | 0.36 |
| [188] | Huang, J. | 2025 | Piezoelectric Micropump (PZT-5A) | 0.784 | 200.7 |
| [63] | Qi, C. | 2022 | Electrostatic Micropump (NdFeB) | 0.5 | 0.86 |
| [208] | Liu, B.D. | 2018 | Electrostatic Micropump (Cu) | 0.539 | 0.21 |
| [190] | Rusli, M.Q.A. | 2018 | Electrostatic Micropump | 1.2 | 0.076 |
| [238] | Lin, Y. | 2015 | Pneumatic Micropump | 30 | 496 |
| [97] | Lin, J.L. | 2022 | Pneumatic Micropump | 68.95 | 12.48 |
| [138] | Ni, J. | 2012 | Pneumatic Micropump | 25 | 0.034 |
| [98] | Machauf, A. | 2005 | Electrostatic Micropump (Cu) | \ | 0.001 |
| [239] | Han, J. | 2012 | Electrostatic Micropump (Cu) | \ | 0.11 |
| [99] | Lee, K.S. | 2013 | Electrostatic Micropump (Cu) | \ | 40/250/150 |
| [160] | Ochoa, M. | 2012 | Thermopneumatic Micropump | 5.86 | 0.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Li, Z.; Han, B.; Guo, Q.; Qing, Z. Performance Comparison of Mechanical and Ferrofluidic Micropumps: Structural and Operational Perspectives. Actuators 2025, 14, 460. https://doi.org/10.3390/act14090460
Zhou X, Li Z, Han B, Guo Q, Qing Z. Performance Comparison of Mechanical and Ferrofluidic Micropumps: Structural and Operational Perspectives. Actuators. 2025; 14(9):460. https://doi.org/10.3390/act14090460
Chicago/Turabian StyleZhou, Xing, Zhenggui Li, Baozhu Han, Qinkui Guo, and Zhichao Qing. 2025. "Performance Comparison of Mechanical and Ferrofluidic Micropumps: Structural and Operational Perspectives" Actuators 14, no. 9: 460. https://doi.org/10.3390/act14090460
APA StyleZhou, X., Li, Z., Han, B., Guo, Q., & Qing, Z. (2025). Performance Comparison of Mechanical and Ferrofluidic Micropumps: Structural and Operational Perspectives. Actuators, 14(9), 460. https://doi.org/10.3390/act14090460






