1. Introduction
The increasing global demand for energy, caused by rapid development of technology and population growth, has led to significant interest in the development of efficient energy storage systems [
1,
2,
3]. Among various technologies, supercapacitors (SCs) have emerged as promising candidates to bridge the gap between traditional capacitors and batteries due to their high power density, excellent cycling stability, and fast charge–discharge capability [
4,
5,
6,
7]. Electrical double-layer capacitors (EDLCs) and pseudocapacitors (PCs) are two major classifications of SCs based on their energy storage mechanisms. EDLCs store energy through electrostatic charge accumulation at the electrode–electrolyte interface, whereas the faradaic redox reactions are mostly responsible for energy storage in PCs. As a result, PCs are associated with better energy storage capacities compared to EDLCs [
8,
9,
10,
11,
12].
With the rapid development of wearable and portable electronic devices, the need for miniaturized, high-performance energy storage units has become increasingly critical [
13]. In this regard, micro-supercapacitors (MSCs) are not only viewed as advanced energy storage devices but also as functional actuators in their own right. Their high-power density, ultrafast response, and stable cycling allow them to deliver immediate electrochemical and electromechanical outputs [
14,
15]. This actuator-like behavior is central for the next generation of compact and intelligent systems such as smartwatches, biomedical implants, flexible sensors, and micro-robots, where MSCs do not merely supply energy to actuators but can themselves embody the actuator function [
16,
17].
In practical applications such as smart wearables, biomedical sensors, and autonomous microsystems, MSCs provide the instantaneous energy bursts that directly drive functional motion, feedback loops, or signal actuation. This duality—operating simultaneously as energy reservoirs and actuator components—positions MSCs as indispensable building blocks for next-generation energy-autonomous platforms [
18]. While MSCs provide rapid and efficient actuation, these same advantages also accelerate structural and electrochemical stresses that threaten long-term reliability [
19]. Under continuous high-rate cycling, MSCs suffer from capacitance fading, resistance growth, and loss of electrochemical reversibility, which directly weaken actuation efficiency. Morphological instability, including pore blockage, surface roughening, and structural fatigue, reduces active sites and disrupts ion transport pathways [
20]. In parallel, interfacial degradation and asymmetric behavior of the anode and cathode exacerbate performance decline—cathodic reactions often display poor reversibility, while anodic processes are prone to side reactions and material loss. Together, these mechanisms shorten device lifespan and undermine the predictable operation required for reliable actuator performance [
21,
22].
Among available electrode materials, nickel hydroxide (Ni (OH)
2) has emerged as one of the most promising candidates for micro-supercapacitor applications, owing to its high theoretical capacitance, reversible redox activity, and compatibility with aqueous electrolytes. Its performance depends strongly on phase composition: while the α-phase offers high electrochemical activity due to larger interlayer spacing and facile transition pathways, the β-phase provides superior structural stability [
23,
24,
25]. Mixed α/β systems can therefore combine these advantages to yield higher capacitance and improved durability. However, Ni (OH)
2 still suffers from poor intrinsic conductivity and structural degradation under high-rate cycling, limiting its long-term reliability [
26,
27]. To address these weaknesses, Ni (OH)
2 is often integrated with nickel foam (NF), a three-dimensional conductive scaffold with high surface area and mechanical robustness [
28,
29,
30,
31,
32]. NF not only enhances charge transport and mechanical stability but also provides a durable platform for morphology control during activation. This Ni (OH)
2/NF synergy enables fabrication of MSCs that unite high energy responsiveness with improved structural integrity, reinforcing their potential as long-lasting, energy-autonomous actuators [
33,
34,
35,
36,
37].
In real-world deployments, maintaining the long-term reliability of MSCs is critical for ensuring stable actuation and power delivery in applications ranging from portable electronics to autonomous microsystems. However, gradual degradation—arising from electrode fatigue, electrolyte decomposition, or structural changes—often progresses unnoticed until significant performance loss occurs [
18]. To overcome this limitation, fast Fourier transform continuous cyclic voltammetry (FFTCCV) provides a powerful in situ diagnostic approach, enabling real-time tracking of capacitance and charge dynamics under different operating conditions [
38,
39]. By revealing subtle shifts in electrochemical behavior and distinguishing stability differences between electrodes, FFTCCV allows early identification of degradation pathways before failure becomes critical. Its compatibility with miniaturization further enables integration into compact systems, where an embedded MSC fabricated from the same materials as the main device can act as a reference actuator, continuously mirroring operating conditions and providing a reliable state-of-health signal.
This work demonstrates, for the first time, the use of FFTCCV for the in situ activation, morphology control, and real-time monitoring of micro-supercapacitors. By tuning the sweep rate, FFTCCV enabled controlled adjustment of α- and β-Ni (OH)2 phase composition and electrode morphology on NF, leading to optimized ion transport pathways and enhanced areal capacitance (546.5 mF·cm−2 at 5 mA·cm−2). This combined phase engineering and morphology tailoring improved structural stability and extended the operational lifespan of the devices. At the same time, continuous tracking of differential charge (ΔQ) and capacitance evolution provided time-resolved insight into degradation dynamics, enabling early recognition of performance loss. These integrated capabilities are particularly important for MSCs functioning as actuator components, where stability and reliability govern efficiency. By uniting activation, phase control, and predictive monitoring in a single approach, FFTCCV establishes a pathway toward high-performance, durable, and energy-autonomous actuators. Unlike prior FFT-voltammetry used mainly for post-analysis, we deploy FFTCCV as both a noise-reduction/signal-processing framework and a phase-engineering, morphology- and activation-control tool; scan-rate programming during activation actively drives crystallographic and morphological evolution of Ni (OH)2 on Ni foam, beyond prior FFT-only post-processing or diagnostics.
3. Results and Discussion
3.1. FFTCCV Electrochemical Measuments
FFTCCV proved to be a decisive tool for both activation and real-time monitoring of NF electrodes in the electrochemical cell. Unlike conventional CV, FFTCCV applies a Fourier algorithm to transform the current response into the frequency domain, thereby separating electrochemical signals from background noise and improving the signal-to-noise ratio by more than 60-fold. This enhanced resolution enables precise detection of subtle electrochemical variations—such as phase transitions, interfacial instabilities, or charge redistribution—that are often masked in classic CV. The method also enables continuous quantification of differential charge (ΔQ), providing time-resolved information on electrode activation and degradation. The detailed principles and mathematical framework of FFTCCV, including ΔQ calculation, have been reported in our previous studies [
40,
41,
42]. These combined features make FFTCCV particularly valuable for evaluating the long-term reliability of micro-supercapacitors, where seemingly small fluctuations can accumulate into significant performance losses during extended cycling.
Figure 1a,b illustrate the electrochemical behavior of NF in 2 M KOH during activation over 40,000 s with FFTCCV curves at 0.7 V·s
−1 and 0.007 V·s
−1, respectively. Analysis of these 3D curves provides detailed information on the current at specific times or potentials where the most significant changes occur. At high sweep rate (0.7 V·s
−1), the CV shape was largely preserved after prolonged cycling, indicating structural robustness of the activated film. At a slow sweep rate (0.007 V·s
−1), however, progressive changes in the voltammograms revealed significant surface restructuring. This demonstrates that FFTCCV not only enables activation but also provides a means of tailoring sweep rate to engineer electrode stability—balancing fast responsiveness with long-term durability for actuator performance.
From the exported CVs in 3D plots, the voltammograms of NF in 2 M KOH at 0, 20,000, and 40,000 s under sweep rates of 0.7 and 0.007 V·s
−1. For both cases, the cathodic charge evolution reveals distinct activation phases, including rapid nucleation, progressive surface coverage, and eventual stabilization [
43]. After 40,000 scans, the CV shape of NF electrodes changed markedly at low sweep rate, confirming surface structural evolution. Conversely, at higher sweep rate, the voltammogram shape was almost preserved, highlighting the stability of the formed film. Such control over activation kinetics is particularly relevant for actuator-like applications, where repeated charge–discharge cycles demand both efficiency and resilience.
To complement the 3D representation, the exported 2D CVs are shown in
Figure 1c,d at 0, 20,000, and 40,000 s for sweep rates of 0.7 and 0.007 V·s
−1. These line graphs clearly show how the cathodic charge evolves through distinct activation phases: (i) rapid nucleation in the initial cycles, (ii) progressive surface coverage, and (iii) eventual stabilization. After 40,000 scans, the CV shape of NF electrodes changed markedly at the low sweep rate, confirming surface structural evolution, whereas at the higher sweep rate the CV shape was nearly preserved, consistent with a stable hydroxide film.
As shown in
Figure 1, the presence of two cathodic peaks in the initial sweeps confirms the coexistence of α-Ni (OH)
2 and β-Ni (OH)
2 polymorphs. The cathodic peak at ~0.27 V corresponds to the α-Ni (OH)
2/β-NiOOH redox couple, while the shoulder near 0.37 V is associated with the transition from γ-NiOOH or β-NiOOH to β-Ni (OH)
2 Notably, both the anodic and cathodic peaks shift progressively over time, with the anodic peaks moving toward more positive potentials and the cathodic peaks toward more negative potentials. This asymmetry can be attributed to the non-stoichiometric and dynamic nature of nickel hydroxide during repeated cycling. Importantly, while several previous studies have reported simultaneous positive shifts for both anodic and cathodic peaks, our FFTCCV-based in situ monitoring reveals that this interpretation overlooks the subtle divergence in electrode behavior. By continuously tracking differential charge and peak evolution, FFTCCV exposes kinetic asymmetry between the oxidation and reduction processes information that conventional CV fails to resolve [
44,
45]. In our FFTCCV study, however, the cathodic peak appeared at more positive potentials due to preferential growth of the β-phase, which dominates at higher potentials compared to the α-phase. This β-phase enrichment enhances structural stability, thereby ensuring reliable cycling and extending actuator lifespan.
Total Differential Charge Exchange
The FFTCCV technique enables precise monitoring of differential charge exchange (ΔQ) through numerical integration of current–potential profiles (as detailed in our previous works [
42]), revealing scan-rate-dependent behavior during NF activation in a 2 M KOH electrolyte.
Figure 2 shows the increase in ΔQ in the cathodic region for NF electrochemical activation over 40,000 s at two sweep rates (0.007 and 0.7 V s
−1). In both cases, the charge increases over time, indicating progressive involvement of hydroxide layers in the redox process and the associated enhancement in capacity and efficiency.
At 0.7 V s−1, the steeper ΔQ increase suggests that rapid cycling favors faster nucleation events and limits the extent of structural rearrangement within each cycle. This condition promotes the preservation of hydrated and electrochemically accessible hydroxide structures, which stabilizes charge transfer and influences the relative balance of α- and β-Ni (OH)2 phases. In contrast, the 0.007 V s−1 sweep rate produces a more gradual ΔQ rise, indicating slower hydroxide layer engagement and allowing extended structural reorganization over time. These distinct kinetic regimes highlight sweep rate as a tuning parameter for phase composition and morphology, enabling electrochemical pathways to be linked directly to structural stability.
This ΔQ analysis establishes FFTCCV as a powerful diagnostic tool for electrochemical activation, capable of optimizing phase composition in real time while also identifying fluctuations that signal emerging degradation. By correlating kinetic parameters with structural features (XRD/SEM) and performance metrics (GCD/EIS), FFTCCV provides a comprehensive framework for tailoring NF-based electrodes to meet both high-performance energy storage demands, and the reliability required for integration in multifunctional devices.
These distinct kinetic regimes demonstrate that sweep rate in FFTCCV acts as a precise control parameter for phase composition and morphological development. More importantly, continuous tracking of ΔQ provides a direct indication of electrode health, offering early insight into performance stability. This dual role—simultaneously guiding activation conditions and monitoring degradation—aligns with the requirements of devices that must maintain predictable operation under repeated cycling.
3.2. Asymmetric Charge Dynamics and Their Impact on Device Reliability
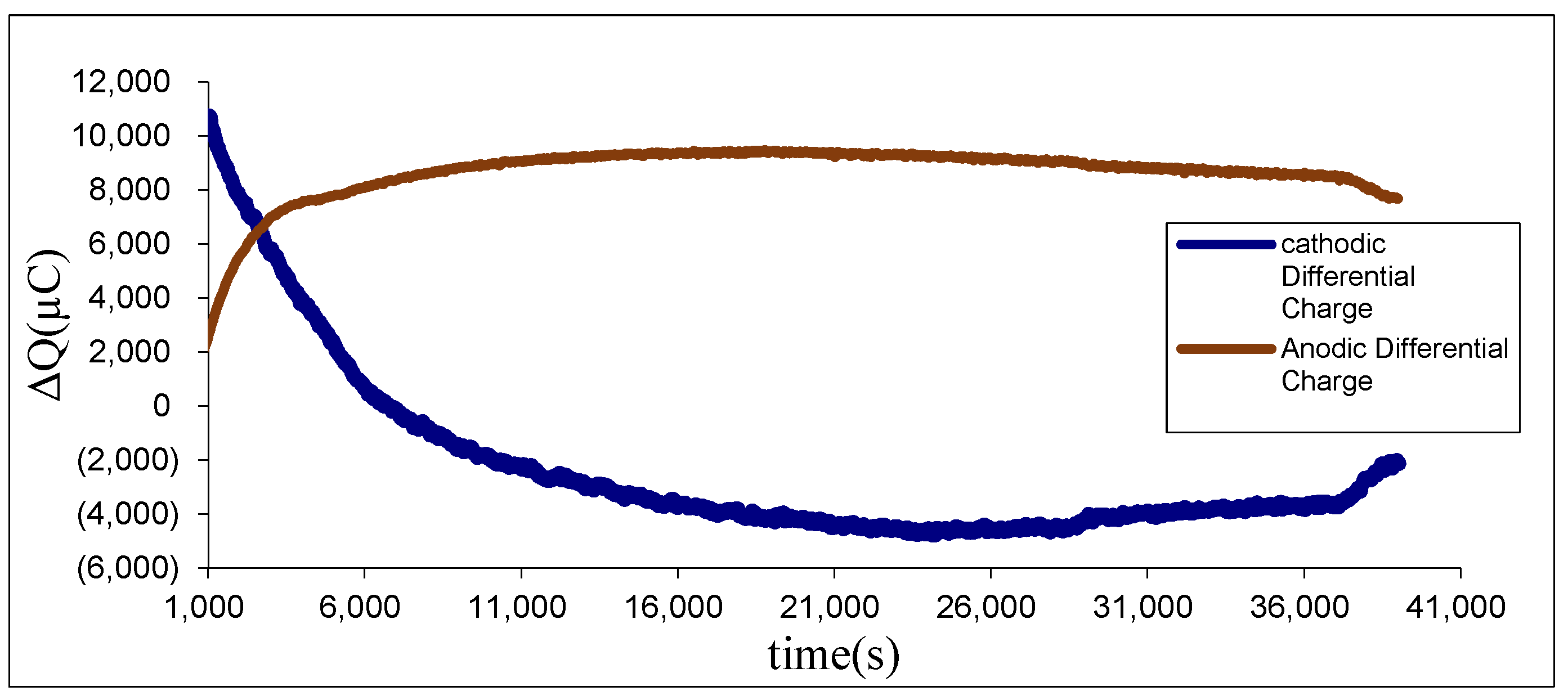
Beyond total charge monitoring, FFTCCV enables independent evaluation of anodic and cathodic contributions (
Figure 3), offering a deeper perspective on electrode-specific dynamics. Ideally, anodic and cathodic ΔQ should be balanced, reflecting symmetric charge storage and release. In practice, however, clear discrepancies emerge due to kinetic asymmetry, irreversible phase transitions, electrolyte decomposition, or progressive loss of electroactive material. As shown in
Figure 3, the anodic ΔQ increases rapidly in the early activation cycles, reaching ~9000–10,000 µC before gradually stabilizing. By contrast, the cathodic ΔQ exhibits a sharp maximum at the beginning but declines steadily throughout the 40,000 s activation, eventually becoming negative before showing slight recovery near the end. This distinct divergence provides direct evidence that cathodic processes are the dominant source of degradation, likely due to limited reversibility of reduction reactions, preferential loss of active sites, and structural instabilities in the reduced phase.
Quantitative analysis further highlights this imbalance. The anodic charge (Qₐ) decreased from ~3.2 to 2.4 mC and the cathodic charge (Qc) from ~3.0 to 2.3 mC over ~5.8 × 103 cycles (10,000 s at 0.7 V·s−1), corresponding to decay rates of ~0.034 and ~0.030 µC per cycle, respectively. The slope ratio of ~1.1 indicates only minor kinetic asymmetry, while the Qₐ/Qc balance remained close to unity. This demonstrates that FFTCCV not only tracks overall ΔQ evolution but also quantifies subtle differences between anodic and cathodic processes, offering resolution beyond standard CV.
For microscale energy-storage units serving as functional elements in electromechanical systems, such asymmetry has critical implications. Stable anodic behavior ensures consistent charge delivery, but cathodic instability introduces imbalance, leading to reduced energy efficiency, fluctuating response signals, and shortened service life. By clearly decoupling anodic and cathodic charge contributions, FFTCCV not only diagnoses degradation pathways, but also provides design guidance—whether by engineering cathode morphology, optimizing electrolyte interactions, or stabilizing interfaces—to achieve more reliable and predictable device performance.
3.3. Calssic Cyclic Voltammetric Measurements
The electrochemical characteristics of the electrode prepared through electrochemical activation, by FFTCCV, were tested by classic CV.
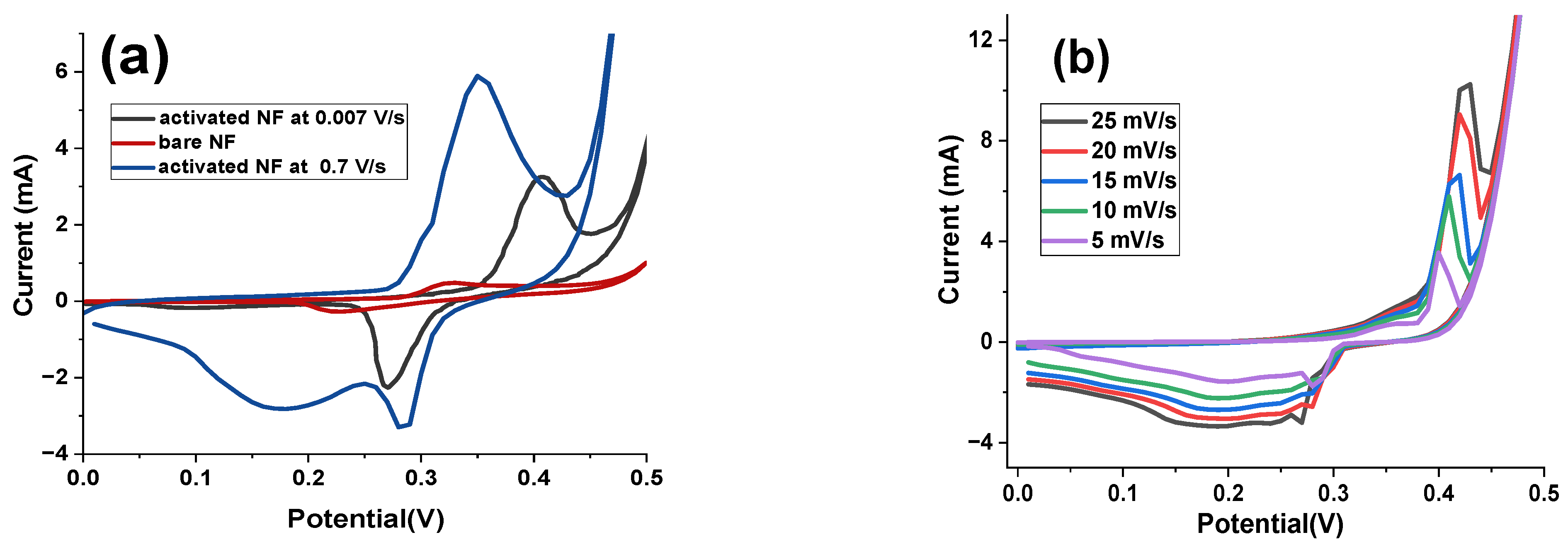
Figure 4a displays the CV plots of the prepared nickel hydroxide and bare NF electrodes at the scan rate of 2 mV s
–1. As obviously seen from
Figure 4a, the nickel hydroxide sample showed much more current response in comparison to NF support. Similarly, in
Figure 4a, the CV curve consisting of two couples of Faradaic redox peaks i.e., P
1/P
2 and P
3/P
4, as indicated in
Figure 4a.
The water oxidation process has started earlier in the case of the as-prepared nickel hydroxide electrode demonstrating the presence of higher values of active sites as revealed by the wall at about 0.55 V vs. Ag/AgCl. The presence of faradaic redox peaks reveals the pseudocapacitive benign of charge storage through this electrode material. Another issue which could be pointed out from
Figure 4b is that with increasing the potential sweep rate, the current density also enhanced and the oxidation and reduction redox peaks moved to a more positive and negative potentials, correspondingly. The reason for such an observation is the internal resistance of electrode and polarization of electrode as well as quasi-reversible benign of redox reactions occurred at the interface of electrode/electrolyte for nickel-based active materials [
31].
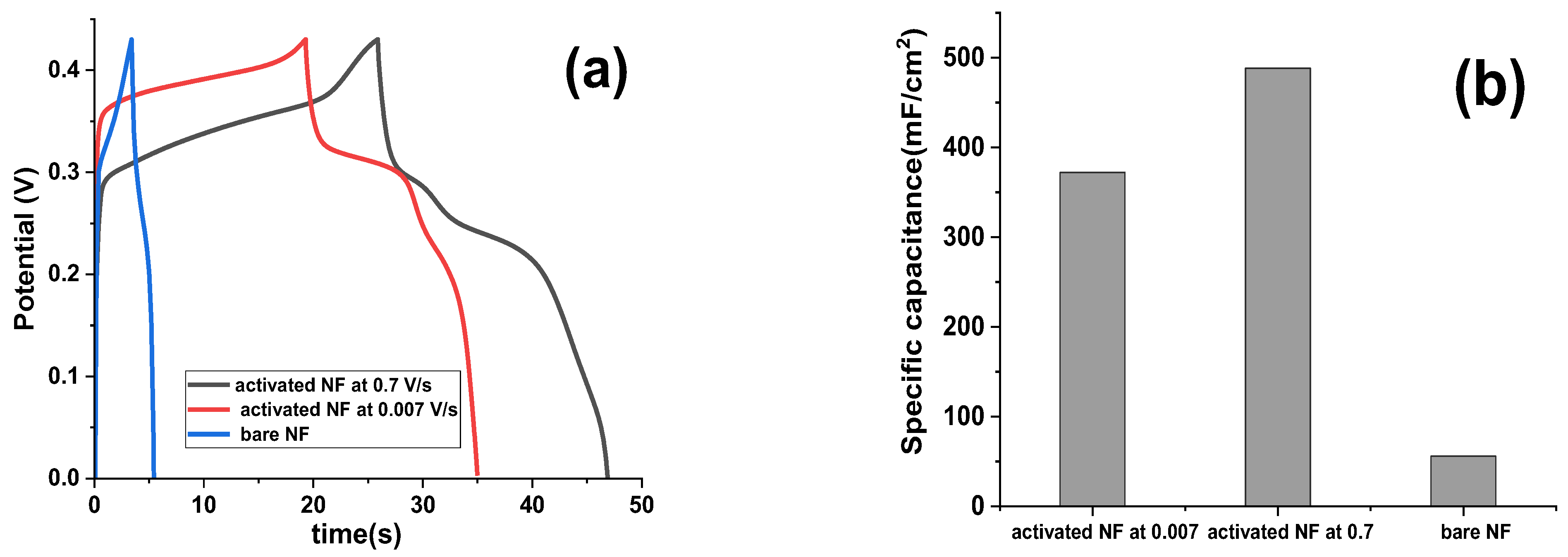
To study the impact of electrochemical activation, by FFTCCV, at various scan rates on the performance of NF, galvanostatic charge–discharge (GCD) curves were analyzed for both bare and activated NF electrodes. Activation was performed using FFTCCV at high (0.7 V/s) and low (0.007 V/s) scan rates, with results presented in
Figure 5. At a scan rate of 0.7 V/s, the α-Ni(OH)
2 phase exhibited a more pronounced GCD plateau compared to the β-Ni(OH)
2 phase (
Figure 5a), corresponding to a specific capacitance of 488 mF/cm
2 (
Figure 5b). This enhanced electrochemical performance is attributed to the superior pseudocapacitive properties of the α-phase, which remains stable under rapid cycling conditions. The restricted conversion from α- to β-Ni(OH)
2 at higher scan rates is likely due to limited ion diffusion pathways, hindering the phase transition. Conversely, at 0.007 V/s, the β-Ni(OH)
2 phase dominated, displaying a more prominent plateau and a lower specific capacitance of 372 mF/cm
2 (
Figure 5a,b). This shift is facilitated by extended ion diffusion times at lower scan rates, promoting β-phase formation.
3.4. Structural and Morphological Characterizations
3.4.1. XRD Analysis: Phase Evolution Under Varied FFTCCV Scan Rates
As shown in
Figure 6, X-ray diffraction (XRD) patterns of FFTCCV-activated NF in 2 M KOH (up to 40,000 s) exhibit distinct phase evolution depending on the applied scan rate. At the optimal scan rate of 0.7 V/s (
Figure 6a), the XRD pattern is dominated by characteristic reflections of the α-Ni(OH)
2 phase at 10.65° (003), 22.73° (006), and 34.24° (012) (JCPDS No. 38-0175), indicative of a rhombohedral structure with a large interlayer spacing (7.6 Å) favorable for rapid ion transport [
25,
26]. Minor peaks at 18.89° (001) and 30.23° (100) correspond to β-Ni(OH)
2, suggesting partial stabilization of the thermodynamically favored phase, consistent with kinetic phase control under high-rate polarization [
22]. Low-angle XRD analysis (
Figure 6c) further confirms the dominance of the α-phase, as evidenced by the pronounced low-angle peaks and minimal β-phase contributions.
In contrast, at a low scan rate of 0.007 V/s (
Figure 6b), the XRD pattern is characterized by intense reflections at 20.62° (001), 30.45° (100), 57.65° (110), and 69.88° (103) (JCPDS No. 14-0117), corresponding to the hexagonal β-Ni(OH)
2 phase, which possesses a smaller interlayer spacing (~4.6 Å) and greater thermodynamic stability under extended polarization [
22,
26]. Weak residual signatures of the α-phase at 10.23° (003) and 33.86° (012) are present in
Figure 6d, but their low intensity confirms β-phase predominance. These results demonstrate the ability of FFTCCV to modulate phase composition by adjusting scan rate, in agreement with previous reports on hydrated nickel hydroxide systems.
Mechanistically, phase evolution under FFTCCV can be understood by considering that rapid polarization at high scan rates (0.7 V/s) favors nucleation and kinetic stabilization of the α-Ni(OH)2 phase (with incorporated structural water), whereas prolonged activation at low scan rates (0.007 V/s) allows for thermodynamic conversion to the more stable β-Ni(OH)2 phase. The phase selectivity achieved via FFTCCV is particularly advantageous for optimizing the electrochemical properties of NF electrodes, as the α-phase provides enhanced ion accessibility and capacitance, while the β-phase imparts improved cycling stability. The observed XRD results are highly reproducible across multiple independent experiments, with no evidence of additional crystalline impurities within the detection limits of the instrument. These findings underscore the unique advantage of FFTCCV in enabling precise, scan-rate-dependent control over nickel hydroxide phase composition, offering a rational strategy for tailoring NF-based electrodes for high-performance supercapacitor applications.
3.4.2. Morphological Evolution of NF Under FFTCCV Activation
The surface morphology and elemental composition of NF electrodes were systematically characterized before and after FFT-CCV activation in 2 M KOH at sweep rates of 0.7 V·s
−1 and 0.007 V·s
−1 using FE-SEM, elemental mapping, and EDS analysis, highlighting the advantages of FFTCCV in tailoring NF morphology for enhanced supercapacitor performance.
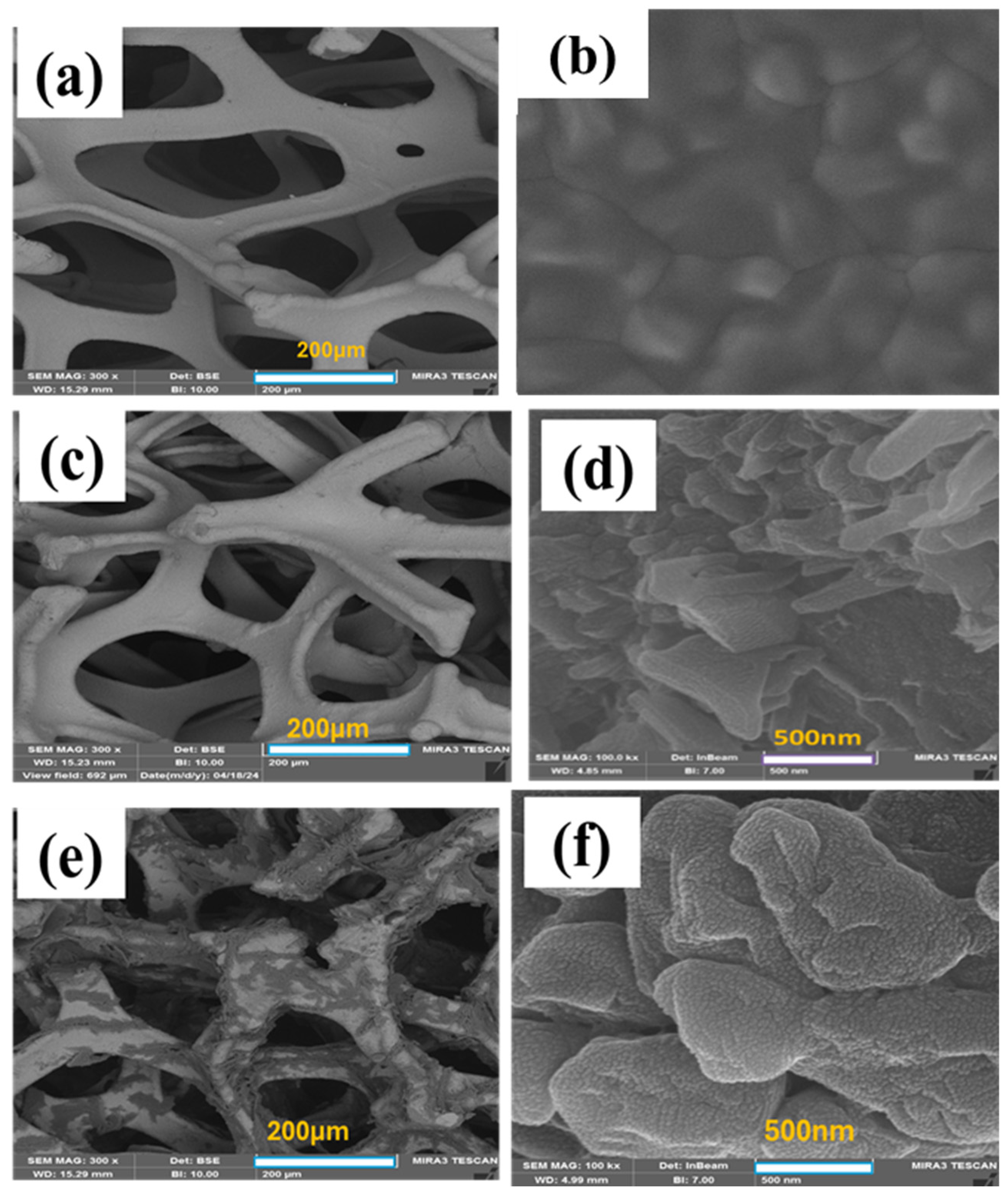
Figure 7 provides a unified comparison of FE-SEM surface morphologies with consistent scale bars across three conditions. Pristine NF (
Figure 7a,b) displays the characteristic three-dimensional (3D) macroporous architecture with pore diameters of 100–600 μm, facilitating electrolyte penetration and providing a robust scaffold for subsequent modification. At higher magnification, the bare surface shows smooth metallic ligaments with well-defined grain boundaries, typical of polycrystalline nickel. Upon FFTCCV activation at a low scan rate of 0.007 V·s
−1 for 40,000 s (
Figure 7c,d), the NF surface undergoes extensive nanostructural transformation. Densely packed hexagonal platelets, characteristic of β-Ni (OH)
2, coexist with irregular α-Ni (OH)
2 flake-like domains. This dual-phase morphology, consistent with XRD analysis, demonstrates slow polarization favors thermodynamically stable β-Ni (OH)
2 while retaining traces of the α-phase. By contrast, FFTCCV activation at a high scan rate of 0.7 V·s
−1 for 40,000 s (
Figure 7e,f) produces a conformal coating of ultrathin, wrinkled α-Ni (OH)
2 nanosheets, reflecting kinetic stabilization of the metastable α-phase. This morphology is markedly more uniform and continuous than that produced under low scan rates or by conventional CV, which often yield granular, inhomogeneous films. Occasional particulate domains are also observed, indicative of minor β-Ni (OH)
2 contributions and confirming the presence of a mixed-phase system. Together, the unified FE-SEM comparison underscores the sweep-rate dependence of FFTCCV morphology control: low rates promote β-phase platelets, while high rates favor α-phase nanosheets with higher surface area. This kinetic/thermodynamic interplay is particularly advantageous for supercapacitor electrodes, where α-rich films enhance capacitance and β-rich domains impart cycling stability.
Complementary EDS spectra (
Figure 8) further support the morphological observations. In pristine NF (
Figure 8a), only Ni and O are detected, with a Ni:O ratio of 97.98:2.02, consistent with a thin native oxide layer on the metallic surface. After FFTCCV activation at 0.007 V·s
−1 (
Figure 8b), oxygen content increases markedly to 15.22 at.%, confirming substantial hydroxide growth and the coexistence of α/β-Ni (OH)
2 domains observed in FE-SEM. At the higher scan rate of 0.7 V·s
−1 (
Figure 8c), oxygen content further rises to 32.01 at.% with a homogeneous Ni/O distribution, reflecting complete and uniform hydroxide coverage dominated by α-Ni (OH)
2 nanosheets. These compositional trends, aligned with the surface morphologies, verify FFTCCV as an effective activation and morphology-engineering strategy that couples nanoscale architecture with compositional evolution to optimize NF-based electrodes for high-performance supercapacitors.
Mechanistically, FFTCCV enables superior morphology control through three coupled effects: (i) continuous cycling promotes uniform nucleation and growth compared to localized deposition in conventional methods; (ii) real-time FFT monitoring dynamically optimizes activation conditions for stable phase development; and (iii) high scan rates reduce diffusion layer constraints, enabling kinetic stabilization of nanosheets. Together, these features yield high-surface-area, mixed-phase nanostructures that not only enhance capacitance but also support consistent charge–discharge reversibility and long-term stability. For integration into microscale energy systems, such stable and tunable morphology ensures predictable electrochemical behavior and repeatable functional response, bridging material engineering with device-level reliability. These results underscore FFTCCV as a powerful activation strategy, producing NF-based electrodes capable of sustaining both high performance and extended operational lifespan.
3.5. Charge–Discharge Performance of FFT-CCV Activated NF
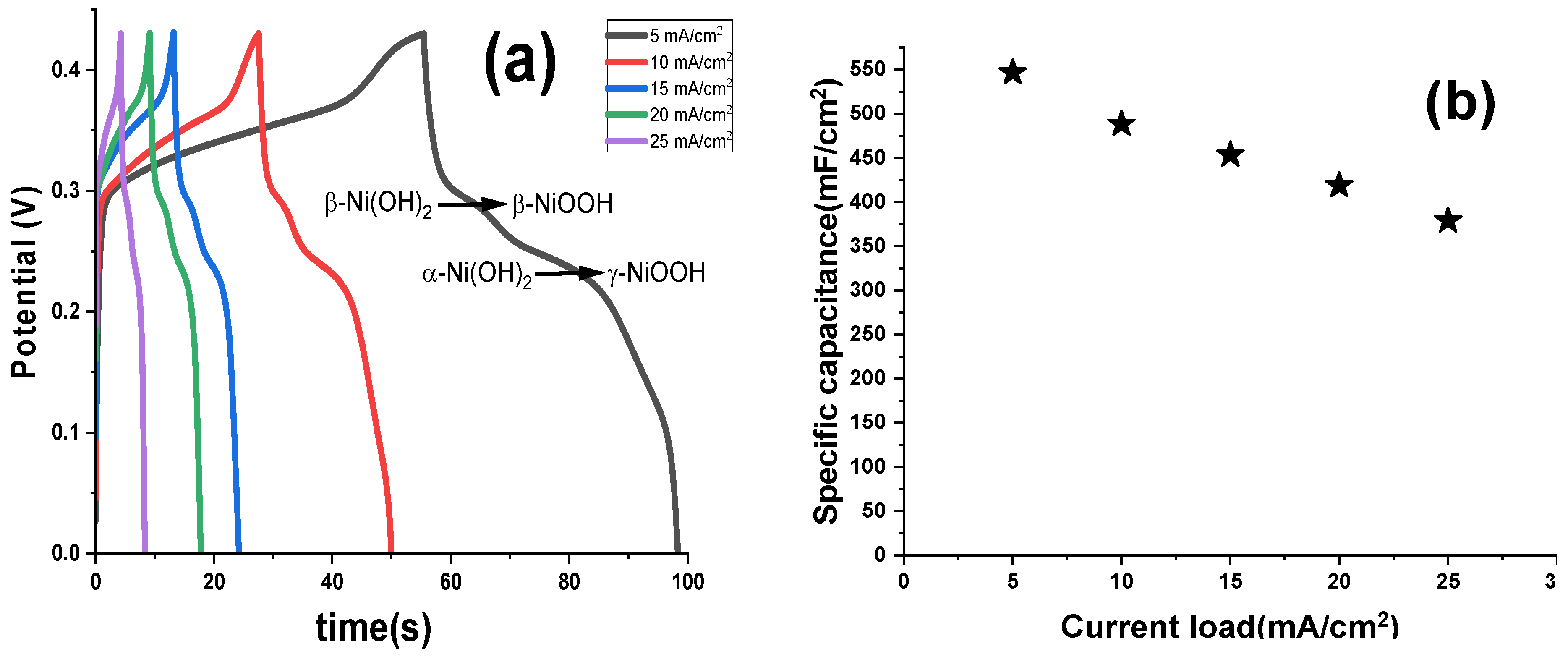
The GCD profiles of NF electrodes, activated via FFTCCV for 10,000 s in 2 M KOH at a scan rate of 0.7 V/s, were evaluated at current densities ranging from 5 to 25 mA/cm
2 (
Figure 9). The dominance of the α-Ni (OH)
2 phase under these activation conditions is evident from the pronounced GCD plateau at lower potentials, indicative of enhanced pseudocapacitive behavior. SC of 546.5, 488.37, 453.48, 418.60, and 378.53 mF/cm
2 were recorded at 5, 10, 15, 20, and 25 mA/cm
2, respectively. These values significantly outperform previously reported capacitances for similar systems (e.g., 15 mF/cm
2 [
46]), highlighting the superior electrochemical performance of the α-phase, particularly at lower current densities where efficient ion diffusion and surface utilization maximize electroactive site availability.
At higher current densities, a gradual decline in specific capacitance is observed, likely due to the increasing prominence of the β-Ni (OH)2 phase, which reduces the availability of electroactive sites. This phase transition is consistent with kinetic limitations under rapid charge–discharge conditions, where ion diffusion pathways are constrained. Despite this, the NF electrode retains 69.3% of its initial capacitance when the current density increases from 5 to 25 mA/cm2, demonstrating robust stability under elevated discharge loads. The activation protocol at 0.7 V/s for 10,000 s optimizes the phase composition, striking a balance that favors the electrochemically active α-Ni (OH)2 phase. This configuration enhances both energy storage capacity and charge–discharge rate performance, positioning FFTCCV as an effective strategy for tailoring NF electrodes for high-performance electrochemical applications.
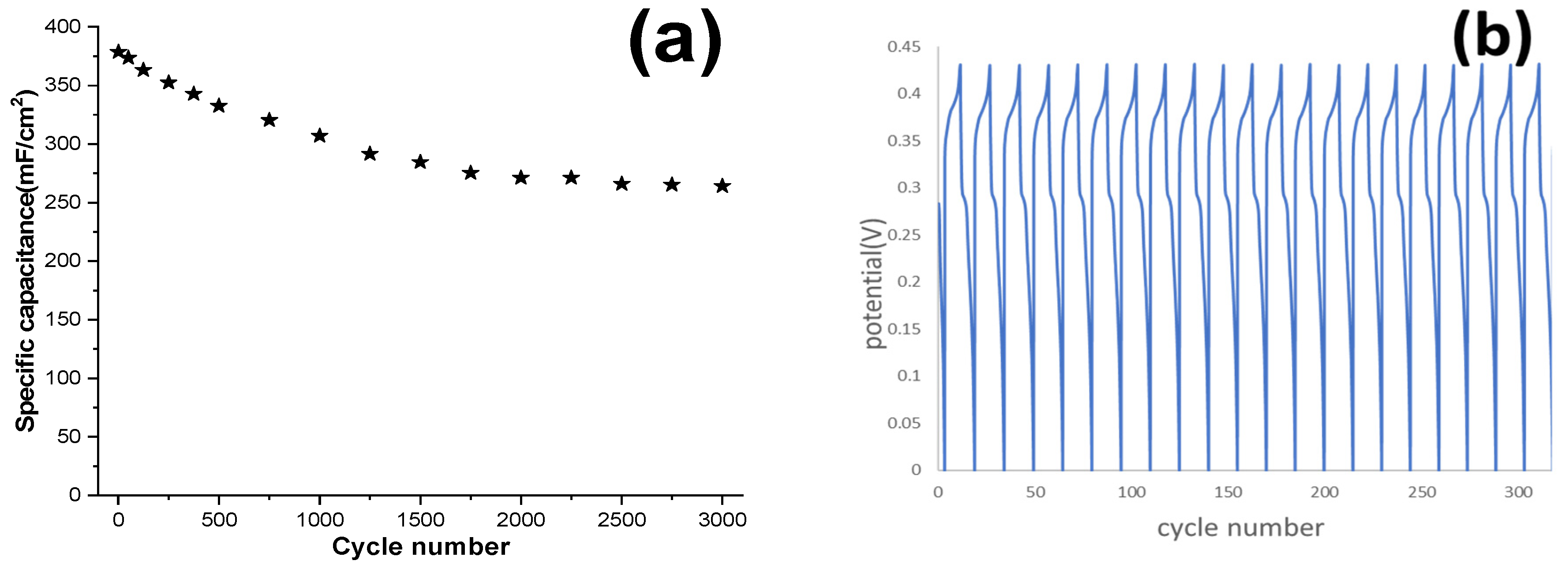
Long-term stability of NF electrodes, activated by FFT-CCV for 10,000 s, at a scan rate of 0.7 V/s, was evaluated through GCD cycling up to 3000 cycles at a current density of 20 mA/cm
2 (
Figure 10). The SC of the mixed α and β-Ni (OH)
2 phases decreased from 378.6 to 268 mF/cm
2, retaining approximately 70% of the initial capacitance after 3000 cycles at 25 mA/cm
2 (
Figure 10a). As shown in
Figure 10b, the first 15 GCD cycles at 25 mA/cm
2, further confirm the stability and reproducibility of the activated NF electrode. These results demonstrate that activation at 0.7 V/s facilitates a facile and effective method for preparing NF as a current collector in micro-supercapacitors.
The SC (546.5 mF/cm
2 at 5 mA/cm
2) and capacitance retention of the α,β-Ni (OH)
2/NF electrode surpass or are comparable to previously reported Ni (OH)
2-based electrodes (
Table 1), such as NF with 231.6 mF/cm
2 at 5 mA/cm
2. The enhanced performance is attributed to the coexistence of α- and β-Ni (OH)
2 phases, which provide a synergistic effect, coupled with a shortened ion diffusion path length due to the porous NF structure.
3.6. EIS Measurement of FFT-CCV Activated NF
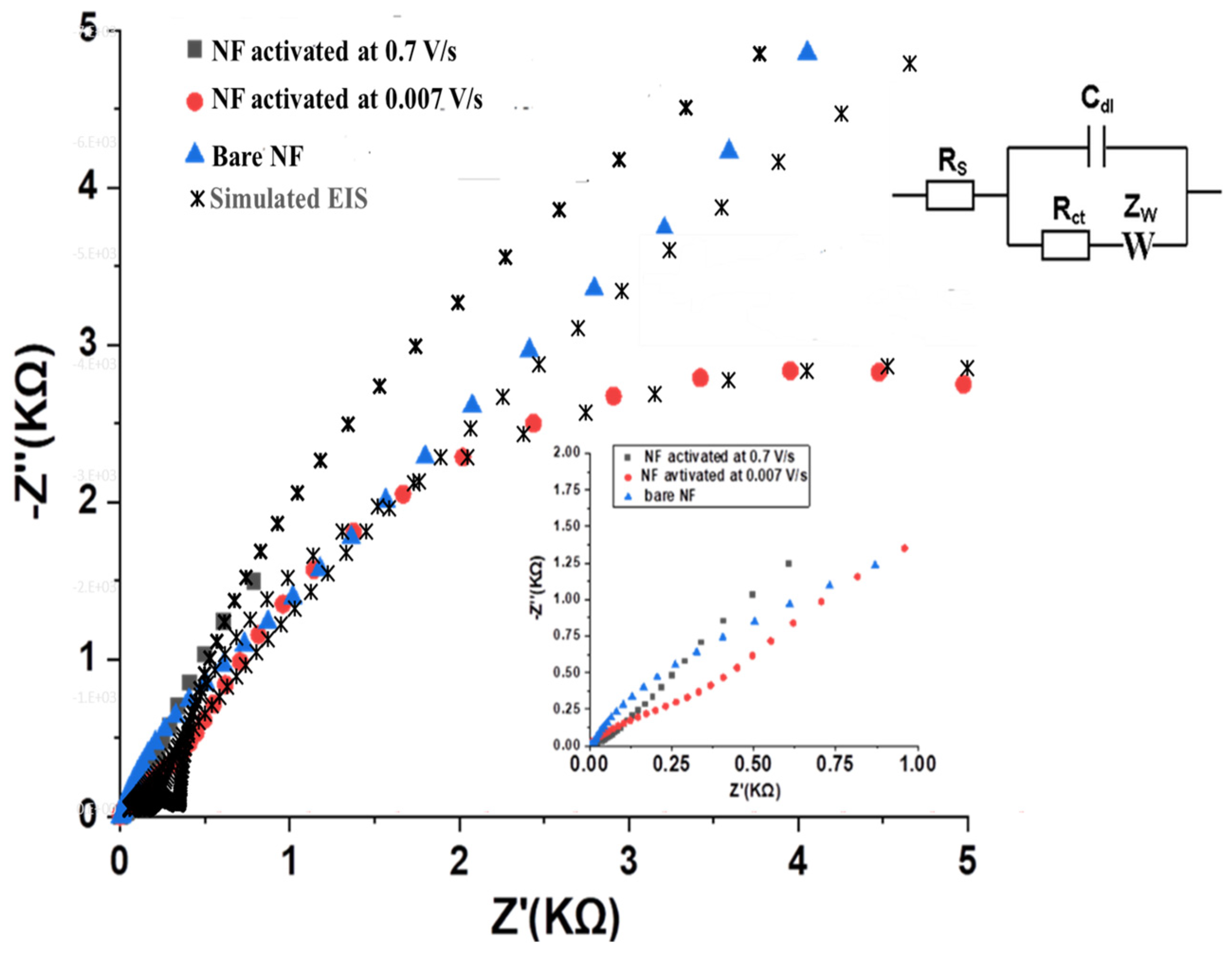
Electrochemical impedance spectroscopy (EIS) was conducted to investigate the interfacial properties, charge transfer kinetics, and ion diffusion characteristics of Ni foam electrodes before and after FFTCCV activation. Nyquist plots for bare NF and NF activated at 0.007 V·s
−1 and 0.7 V·s
−1 are shown in
Figure 11, where experimental data are plotted as symbols and equivalent-circuit fits as solid lines. Measurements were performed in 2 M KOH over 0.01 Hz–100 kHz.
The bare NF electrode exhibits a large semicircular arc at high frequencies, with the real axis intercept (Z′) extending to approximately 4.5 kΩ. This substantial semicircle diameter indicates a high charge transfer resistance (Rct), reflecting the limited electrochemical activity and poor surface wettability of the unmodified NF. The transition to a less steep line in the low-frequency region suggests non-ideal capacitive behavior and hindered ion transport.
In contrast, FFTCCV activation significantly reduces the impedance of the NF electrodes. The Nyquist plot for NF activated at 0.007 V/s (red circles) displays a smaller semicircle, with Z′ reaching around 2.5 kΩ, indicating a marked decrease in Rct and improved interfacial charge transfer relative to bare NF. However, the most pronounced improvement is observed for the NF electrode activated at 0.7 V/s (gray squares), which shows a single, small semicircle at high frequencies with a Z′ intercept of approximately 0.7 kΩ. This indicates minimal charge transfer resistance and highly efficient electron transfer at the electrode–electrolyte interface. At low frequencies, the slope of the impedance plot for the 0.7 V/s-activated NF approaches a vertical line, characteristic of ideal capacitive behavior and efficient ion diffusion. This is in stark contrast to both bare and low-scan-rate-activated NF, where the low-frequency lines are more inclined, reflecting less ideal capacitive response.
Equivalent circuit fitting (Randles model, inset
Figure 11) confirms these trends. The extracted parameters—summarized in the Supporting Information,
Table 2—are derived directly from the fitted equivalent-circuit data. Both Rs and Rct are strongly reduced after FFTCCV activation, especially at 0.7 V·s
−1, while the CPE exponent approaches unity, indicating more homogeneous charge distribution and efficient interfacial kinetics.
Collectively, these EIS results confirm that FFTCCV activation—especially at a sweep rate of 0.7 V/s—optimizes the interfacial and electrochemical properties of NF. The decrease in charge transfer resistance and emergence of nearly ideal capacitive behavior directly correlate with the improved areal capacitance and cycling stability observed in electrochemical measurements, highlighting the effectiveness of this activation strategy for high-performance micro-supercapacitor electrodes.
3.7. Results Discussion: Towards Battery Health Monitoring
This section discusses the potential application of our system as a sensing device for SCs or batteries to monitor their SoH. While specific numerical data values may not be explicitly detailed here, relevant examples are provided in
Figure 2 and
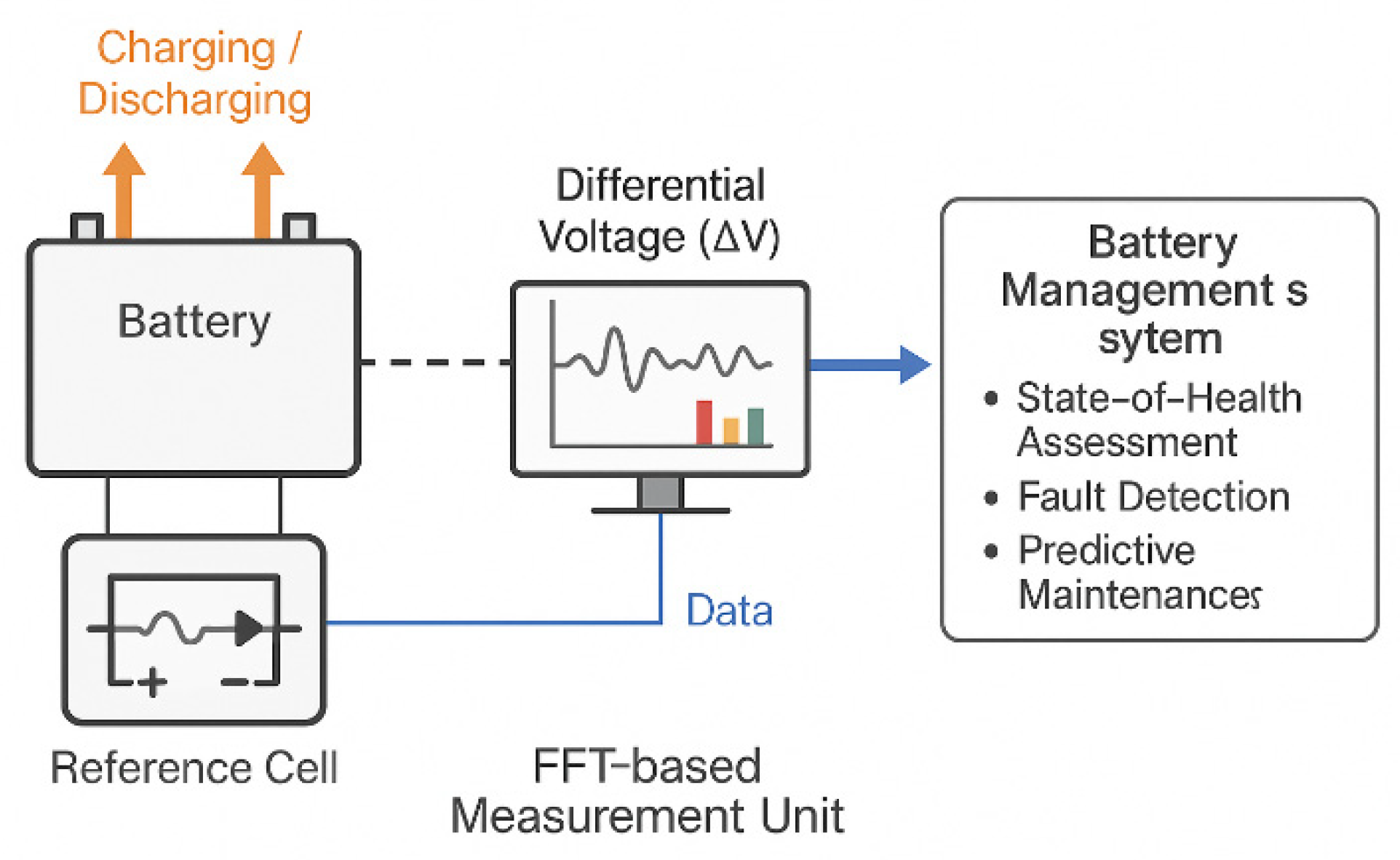
Figure 3, which illustrate the electrochemical signals obtained through FFTCCV. The methodology demonstrated in this work for activating (NF) via FFTCCV not only enhances the electrochemical performance of MSCs but also establishes a framework for embedded health monitoring in energy-autonomous devices. here, FFTCCV enabled precise, real-time tracking of ΔQ, capacitance, and phase composition during activation and cycling. This high temporal resolution offers unique insights into kinetic phase transitions, morphology evolution, and asymmetries between anodic and cathodic processes that signify material degradation. Such diagnostic precision is critical when MSCs serve as functional components in microsystems, where consistent and predictable behavior directly impacts device reliability and importantly, the principles demonstrated here can extend beyond MSCs to larger-scale electrochemical systems, such as lithium-ion batteries. Similar time-resolved electrochemical signatures could reveal early indicators of electrode fatigue, electrolyte decomposition, or active material loss long before measurable capacity fade occurs. A practical architecture is schematized in
Figure 12: a micro-scale reference cell (materials/electrolyte matched to the primary device) is perturbed at low amplitude; an FFT-based measurement unit extracts differential-voltage/ΔQ features and streams data to a battery management system (BMS) for SoH assessment, fault detection, and predictive maintenance. In MSC-based actuating systems, such an embedded sensor cell could report degradation in real time, enabling adaptive load management and fault prevention. In larger battery packs, BMS integration would allow continuous SoH tracking, early-fault detection, and predictive maintenance. Established techniques such as EIS provide effective baselines for detecting degradation and tracking aging-related parameters [
51,
52]. By linking advanced degradation simulations with time-resolved electrochemical data, it can enable early detection of battery degradation, ensuring optimal performance and longevity [
53].
4. Conclusions
This work demonstrates that FFTCCV is not only an advanced activation method for NF current collectors but also a real-time diagnostic platform that unites activation, morphology control, and health monitoring in a single framework. By precisely controlling scan rate and activation time, we engineered a mixed α/β-Ni (OH)2 nanostructure with optimized ion transport and surface reactivity. The continuous tracking of ΔQ evolution provided time-resolved insight into phase transformation and morphological changes, identifying optimal activation conditions (0.7 V/s, 10,000 s) that yielded high areal capacitance (546.5 mF/cm2) and robust cycling stability (75.3% retention after 3000 cycles). Beyond material optimization, this approach establishes a pathway for predictive health monitoring. A micro-scale supercapacitor, fabricated with identical materials and electrolyte as the primary unit, can function as an embedded reference cell, mirroring degradation behavior and providing continuous SoH signals. This circumvents limitations of direct monitoring of the main device, where high load currents and circuit integration obscure electrochemical data. Comparable in spirit to electrochemical noise analysis in corrosion science but with quantitative and phase-resolved precision, FFTCCV enables real-time degradation tracking, predictive maintenance scheduling, and adaptive control strategies. The application p FFTCCV not merely as a laboratory diagnostic but as a practical tool for next-generation smart power systems. By coupling in situ activation, morphology/phase engineering, and dynamic SoH monitoring, FFTCCV paves the way for energy-autonomous systems that are both high performing and self-aware. The integration of such capabilities into actuator-oriented MSCs, as well as broader electrochemical storage platforms, represents a transformative step toward smart, long-lived, and reliable devices. In ongoing work, we will integrate FFTCCV-programmed micro-supercapacitors into actuator and sensor prototypes and quantify application-relevant system metrics—areal energy/power, pulse delivery, round-trip efficiency, and load-driven lifetime—under realistic duty cycles.