Stapes Prostheses in Otosclerosis Surgery: Materials, Design Innovations, and Future Perspectives
Abstract
1. Introduction
1.1. Background and Pathophysiology
1.2. Clinical Presentation and Diagnosis
1.3. Evolution of Otosclerosis Management
2. Stapes Prostheses
2.1. From Passive Pistons to “Innovative Actuators”
2.2. State-of-the-Art Developments
3. Surgical Treatment
3.1. Surgical Technique
3.2. Advances in Stapes Surgery Technique
- Manual-crimping prostheses, where the surgeon physically crimps the piston loop around the long process of the incus.
- Non-crimping or self-crimping prostheses, which use advanced coupling mechanisms or SMA to achieve fixation without manual deformation [21].
4. Endoscopic Stapedotomy
5. Discussion
5.1. Prosthesis Materials and Acoustic Performance
5.2. Fixation Techniques and Coupling Efficacy
5.3. Influence of Surgical Technique on Outcomes
5.4. Actuator Design Considerations: Coupling, Dynamics, and Durability
5.5. Clinical Ergonomics and Patient Safety
5.6. Future Directions and Innovations
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABG | Air–Bone Gap |
| CT | Computed Tomography |
| EES | Endoscopic Ear Surgery |
| HR-CT | High-Resolution Computed Tomography |
| MEMS | Micro-Electro-Mechanical Systems |
| SMA | Shape-Memory Alloys |
References
- Markou, K.; Goudakos, J. An Overview of the Etiology of Otosclerosis. Eur. Arch. Oto-Rhino-Laryngol. 2009, 266, 25–35. [Google Scholar] [CrossRef]
- Menger, D.J.; Tange, R.A. The Aetiology of Otosclerosis: A Review of the Literature. Clin. Otolaryngol. Allied Sci. 2003, 28, 112–120. [Google Scholar] [CrossRef]
- Schrauwen, I.; Van Camp, G. The Etiology of Otosclerosis: A Combination of Genes and Environment. Laryngoscope 2009, 120, 1195–1202. [Google Scholar] [CrossRef]
- Stankovic, K.M.; McKenna, M.J. Current Research in Otosclerosis. Curr. Opin. Otolaryngol. Head Neck Surg. 2006, 14, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Declau, F.; Van Spaendonck, M.; Timmermans, J.P.; Michaels, L.; Liang, J.; Qiu, J.P.; Van De Heyning, P. Prevalence of Histologic Otosclerosis: An Unbiased Temporal Bone Study in Caucasians. Adv. Otorhinolaryngol. 2007, 65, 6–16. [Google Scholar] [CrossRef]
- Batson, L.; Rizzolo, D. Otosclerosis: An Update on Diagnosis and Treatment. J. Am. Acad. Physician Assist. 2017, 30, 17–22. [Google Scholar] [CrossRef]
- Mann, W.J.; Amedee, R.G.; Fuerst, G.; Tabb, H.G. Hearing Loss as a Complication of Stapes Surgery. Otolaryngol. Head Neck Surg. 1998, 115, 324–328. [Google Scholar] [CrossRef]
- Bauchet St. Martin, M.; Rubinstein, E.N.; Hirsch, B.E. High-Frequency Sensorineural Hearing Loss after Stapedectomy. Otol. Neurotol. 2008, 29, 447–452. [Google Scholar] [CrossRef]
- Vincent, R.; Sperling, N.M.; Oates, J.; Jindal, M. Surgical Findings and Long-Term Hearing Results in 3050 Stapedotomies for Primary Otosclerosis: A Prospective Study with the Otology-Neurotology Database. Otol. Neurotol. 2006, 27, S25–S47. [Google Scholar] [CrossRef] [PubMed]
- Skarżyński, P.H.; Dziendziel, B.; Gos, E.; Włodarczyk, E.; Miaśkiewicz, B.; Rajchel, J.J.; Skarżyński, H. Prevalence and Severity of Tinnitus in Otosclerosis: Preliminary Findings from Validated Questionnaires. J. Int. Adv. Otol. 2019, 15, 277. [Google Scholar] [CrossRef] [PubMed]
- Just, T.; Guder, E.; Pau, H.W. Effect of the Stapedotomy Technique on Early Post-Operative Hearing Results—Preliminary Results. Auris Nasus Larynx 2012, 39, 383–386. [Google Scholar] [CrossRef]
- Bagger-Sjöbäck, D.; Strömbäck, K.; Hultcrantz, M.; Papatziamos, G.; Smeds, H.; Danckwardt-Lillieström, N.; Tideholm, B.; Johansson, A.; Hellström, S.; Hakizimana, P.; et al. High-Frequency Hearing, Tinnitus, and Patient Satisfaction with Stapedotomy: A Randomized Prospective Study. Sci. Rep. 2015, 5, 13341. [Google Scholar] [CrossRef]
- Purohit, B.; Hermans, R.; Op de beeck, K. Imaging in Otosclerosis: A Pictorial Review. Insights Imaging 2014, 5, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Hannula, S.; Bloigu, R.; Majamaa, K.; Sorri, M.; Mäki-Torkko, E. Ear Diseases and Other Risk Factors for Hearing Impairment among Adults: An Epidemiological Study. Int. J. Audiol. 2012, 51, 833–840. [Google Scholar] [CrossRef]
- George, T.; Fakoya, A.O.; Bordoni, B. Anatomy, Head and Neck, Ear Ossicles; StatPearls: Petersburg, Russia, 2024. [Google Scholar]
- Sakano, H.; Harris, J.P. Revision Stapes Surgery. Curr. Otorhinolaryngol. Rep. 2022, 10, 40. [Google Scholar] [CrossRef]
- Zafar, N.; Hohman, M.H.; Khan, M.A. Otosclerosis; StatPearls: Petersburg, Russia, 2024. [Google Scholar]
- Foster, M.F.; Backous, D.D. Clinical Evaluation of the Patient with Otosclerosis. Otolaryngol. Clin. N. Am. 2018, 51, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Claussen, A.D.; Gantz, B.J. Cochlear Implantation in Advanced Otosclerosis: Pitfalls and Successes. Curr. Otorhinolaryngol. Rep. 2022, 10, 49–57. [Google Scholar] [CrossRef]
- Quaranta, N.; Pontillo, V.; Dispenza, F. Advanced Otosclerosis. In Sensorineural Hearing Loss Pathophysiology, Diagnosis and Treatment; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2019; pp. 189–206. [Google Scholar] [CrossRef]
- Sevy, A.; Arriaga, M. The Stapes Prosthesis: Past, Present, and Future. Otolaryngol. Clin. N. Am. 2018, 51, 393–404. [Google Scholar] [CrossRef]
- Rotteveel, L.J.C.; Proops, D.W.; Ramsden, R.T.; Saeed, S.R.; Van Olphen, A.F.; Mylanus, E.A.M. Cochlear Implantation in 53 Patients with Otosclerosis: Demographics, Computed Tomographic Scanning, Surgery, and Complications. Otol. Neurotol. 2004, 25, 943–952. [Google Scholar] [CrossRef]
- Emami, H.; Amirzargar, B.; Nemati, Y.; Rahimi, N. Endoscopic Versus Microscopic Stapedotomy: A Randomized Clinical Trial. Laryngoscope 2024, 134, 2395–2400. [Google Scholar] [CrossRef]
- Laske, R.D.; Röösli, C.; Chatzimichalis, M.V.; Sim, J.H.; Huber, A.M. The Influence of Prosthesis Diameter in Stapes Surgery: A Meta-Analysis and Systematic Review of the Literature. Otol. Neurotol. 2011, 32, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Nakkabi, I. Endoscopic Reverse Stapedotomy for Otosclerosis: A Technical Video Report. Cureus 2025, 17, e89914. [Google Scholar] [CrossRef] [PubMed]
- Shea, J.J. Thirty Years of Stapes Surgery. J. Laryngol. Otol. 1988, 102, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Heywood, R.L.; Quick, M.E.; Atlas, M.D. Long-Term Audiometric and Clinical Outcomes Following Stapedectomy with the Shape Memory Nitinol Stapes Prosthesis. Otol. Neurotol. 2019, 40, 164–170. [Google Scholar] [CrossRef]
- Lavy, J.; Khalil, S. Five-Year Hearing Results with the Shape Memory Nitinol Stapes Prosthesis. Laryngoscope 2014, 124, 2591–2593. [Google Scholar] [CrossRef]
- Hornung, J.A.; Brase, C.; Zenk, J.; Iro, H. Results Obtained with a New Superelastic Nitinol Stapes Prosthesis in Stapes Surgery. Otol. Neurotol. 2011, 32, 1415–1421. [Google Scholar] [CrossRef]
- Gerlinger, I.; Bakó, P.; Piski, Z.; Révész, P.; Ráth, G.; Karosi, T.; Lujber, L. KTP Laser Stapedotomy with a Self-Crimping, Thermal Shape Memory Nitinol Piston: Follow-up Study Reporting Intermediate-Term Hearing. Eur. Arch. Oto-Rhino-Laryngol. 2014, 271, 3171–3177. [Google Scholar] [CrossRef]
- Quaranta, N.; Pontillo, V.; Dispenza, F. Advanced Otosclerosis: Stapes Surgery or Cochlear Implantation? Otolaryngol. Clin. N. Am. 2018, 51, 189–206. [Google Scholar] [CrossRef]
- Kraus, E.M.; Russell, G.B.; Allen, S.J.; Pearson, S.A. Long-Term Hearing Results of Endoskeletal Ossicular Reconstruction in Chronic Ears Using Titanium Prostheses Having a Helical Coil: Part 1—Kraus K-Helix Crown, Incus to Stapes. Otol. Neurotol. 2022, 43, 1056. [Google Scholar] [CrossRef]
- Urquiza, R.; López, J.; Gonzalez-Herrera, A.; Povedano, V.; Ciges, M. Tympanic-Ossicular Prostheses and MEMS Technology: Whats and Whys. Acta Otolaryngol. 2009, 129, 411–415. [Google Scholar] [CrossRef]
- Judd, R.T.; Gluth, M.B.; Gurgel, R.K.; Dornhoffer, J.L.; Carlson, M.L.; Isaacson, B.; Kuthubutheen, J.; Hui, N.J.; Quick, M.; Anderson, R.D.; et al. Impact of Modifiable Surgical Factors on Ossiculoplasty Outcomes After Controlling for Ear Environment Risk: A Multi-Institutional Study. Otol. Neurotol. 2025. [Google Scholar] [CrossRef]
- Molinari, G.; Emiliani, N.; Cercenelli, L.; Bortolani, B.; D’Azzeo, R.; Burato, A.; Presutti, L.; Molteni, G.; Marcelli, E. A Novel 3D Printed Multi-Material Simulator for Endoscopic Stapes Surgery: The “3D Stapes Trainer”. Laryngoscope 2025, 135, 3356–3363. [Google Scholar] [CrossRef]
- Prasad, K.C.; Karunasagar, A.; Anjali, P.K. Stapes Surgery Teaching Tool: A Simple and Stable Technique. Indian J. Otolaryngol. Head Neck Surg. 2018, 70, 450. [Google Scholar] [CrossRef]
- Tănase, N.V.; Hainăroșie, R.; Brîndușe, L.A.; Cobilinschi, C.; Dutu, M.; Corneci, D.; Zainea, V. Study of Two Sedative Protocols for Drug-Induced Sleep Endoscopy: Propofol versus Propofol-Remifentanil Combination, Delivered in Target-Controlled Infusion Mode. Medicina 2024, 60, 1123. [Google Scholar] [CrossRef]
- Tănase, N.V.; Hainăroșie, R.; Brîndușe, L.A.; Corneci, D.; Voiosu, C.; Rusescu, A.; Cobilinschi, C.; Stanciu Găvan, C.; Zainea, V. A Clinical Comparative Study of Schnider and Eleveld Pharmacokinetic–Pharmacodynamic Models for Propofol Target-Controlled Infusion Sedation in Drug-Induced Sleep Endoscopy. Biomedicines 2025, 13, 822. [Google Scholar] [CrossRef] [PubMed]
- Porowski, M.; Skarżyński, H.; Skarżyński, P.H. Stapedotomy in Congenital Stapes Ankylosis with Mobile Footplate: A Case Report. Am. J. Case Rep. 2022, 23, e936466-1. [Google Scholar] [CrossRef]
- Faramarzi, M.; Roosta, S.; Daneshian, N. Comparison between Fluoroplastic and Platinum/Titanium Piston in Stapedotomy: A Prospective, Randomized Clinical Study. J. Int. Adv. Otol. 2020, 16, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Gjurić, M.; Rukavina, L. Evolution of Stapedectomy Prostheses over Time. Adv. Otorhinolaryngol. 2007, 65, 174–178. [Google Scholar] [CrossRef]
- Toscano, M.L.; Matz, O.; Hohman, M.H.; Shermetaro, C. Stapes Surgery for Otosclerosis. In Ent an Introduction and Practical Guide, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2025; pp. 100–103. [Google Scholar] [CrossRef]
- Choudhury, N.; Kumar, G.; Krishnan, M.; Gatland, D.J. Atypical Incus Necrosis: A Case Report and Literature Review. J. Laryngol. Otol. 2008, 122, 1124–1126. [Google Scholar] [CrossRef] [PubMed]
- Gerlinger, I.; Tóth, M.; Lujber, L.; Szanyi, I.; Móricz, P.; Somogyvári, K.; Németh, A.; Ráth, G.; Pytel, J.; Mann, W. Necrosis of the Long Process of the Incus Following Stapes Surgery: New Anatomical Observations. Laryngoscope 2009, 119, 721–726. [Google Scholar] [CrossRef]
- Zenner, H.P.; Freitag, H.G.; Linti, C.; Steinhardt, U.; Jorge, J.R.; Preyer, S.; Mauz, P.S.; Sürth, M.; Planck, H.; Baumann, I.; et al. Acoustomechanical Properties of Open TTP® Titanium Middle Ear Prostheses. Hear. Res. 2004, 192, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Zirkler, J.; Rahne, T.; Plontke, S.K. Stapeschirurgie Bei Otosklerose Mit Einer Neuen Titanprothese Mit Superelastischem Nitinol-Clip: Erste Erfahrungen. HNO 2016, 64, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Wycherly, B.J.; Berkowitz, F.; Noone, A.-M.; Kim, H.J. Computed Tomography and Otosclerosis: A Practical Method to Correlate the Sites Affected to Hearing Loss. Ann. Otol. Rhinol. Laryngol. 2010, 119, 789–794. [Google Scholar] [CrossRef]
- Celik, T.; Erdur, O.; Gul, O.; Firat Koca, C.; Colpan, B. Comparison of Endoscopic and Microscopic Methods in Stapedotomy: A Retrospective Analysis. Eur. Arch. Otorhinolaryngol. 2023, 280, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Moneir, W.; Abd El-Fattah, A.M.; Mahmoud, E.; Elshaer, M. Endoscopic Stapedotomy: Merits and Demerits. J. Otol. 2018, 13, 97–100. [Google Scholar] [CrossRef]
- Kuo, C.W.; Wu, H.M. Fully Endoscopic Laser Stapedotomy: Is It Comparable with Microscopic Surgery? Acta Otolaryngol. 2018, 138, 871–876. [Google Scholar] [CrossRef]
- Hildmann, H.; Sudhoff, H.; Bernal-Sprekelsen, M. Middle Ear Surgery; Springer Nature: London, UK, 2006; p. 195. [Google Scholar]
- Bartel, R.; Sanz, J.J.; Clemente, I.; Simonetti, G.; Viscacillas, G.; Palomino, L.; Asarta, I.; Lao, X. Endoscopic Stapes Surgery Outcomes and Complication Rates: A Systematic Review. Eur. Arch. Oto-Rhino-Laryngol. 2021, 278, 2673–2679. [Google Scholar] [CrossRef]
- Borghei, P.; Khorsandi-Ashtiani, M.T.; Heidari, R.; Saeidi, M.; Kouhi, A. Functional Outcomes of Stapes Surgery with Titanium and Teflon Prosthesis: Randomized Controlled Trial. J. Otolaryngol. Stud. 2019, 2, 101. [Google Scholar]
- Massey, B.L.; Kennedy, R.J.; Shelton, C. Stapedectomy Outcomes: Titanium versus Teflon Wire Prosthesis. Laryngoscope 2005, 115, 249–252. [Google Scholar] [CrossRef]
- Gargula, S.; Daval, M.; Lecoeuvre, A.; Ayache, D. Comparison of Dislocation Rates of Teflon and Titanium Stapes Prostheses: A Retrospective Survival Analysis on 855 Patients. J. Otolaryngol.—Head Neck Surg. 2023, 52, 52. [Google Scholar] [CrossRef]
- Cotulbea, S.; Marin, A.; Stefanescu, H. Stapedectomy and Stapedotomy. Functional Results after Insertion of Teflon, Wire-Teflon, and Titanium Stapes Pistons. Laryngo-Rhino-Otol. 2004, 83, 11_7. [Google Scholar] [CrossRef]
- Rajan, G.P.; Eikelboom, R.H.; Anandacoomaraswamy, K.S.; Atlas, M.D. In Vivo Performance of the Nitinol Shape-Memory Stapes Prosthesis during Hearing Restoration Surgery in Otosclerosis: A First Report. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 72, 305–309. [Google Scholar] [CrossRef]
- Huber, A.M.; Veraguth, D.; Schmid, S.; Roth, T.; Eiber, A. Tight Stapes Prosthesis Fixation Leads to Better Functional Results in Otosclerosis Surgery. Otol. Neurotol. 2008, 29, 893–899. [Google Scholar] [CrossRef]
- Vasiljević, M.; Dragović, K.; Bržan, P.P.; Rebol, J. Comparison Between Titanium and Thermally Activated Prostheses in Stapes Surgery. Appl. Sci. 2025, 15, 8211. [Google Scholar] [CrossRef]
- Huber, A.M.; Ma, F.; Felix, H.; Linder, T. Stapes Prosthesis Attachment: The Effect of Crimping on Sound Transfer in Otosclerosis Surgery. Laryngoscope 2003, 113, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Lin, H.; Zhang, T.Y.; Tan, J. Laser versus Non-Laser Stapedotomy in Otosclerosis: A Systematic Review and Meta-Analysis. Auris Nasus Larynx 2014, 41, 337–342. [Google Scholar] [CrossRef]
- Shelton, C. Laser Stapedotomy. In Otologic Surgery; Elsevier: Amsterdam, The Netherlands, 2010; pp. 263–273. [Google Scholar] [CrossRef]
- Harvey, S.A. Stapedectomy: Laser versus Drill versus the Use of Pick Instruments. Oper. Tech. Otolaryngol.—Head Neck Surg. 2003, 14, 255–262. [Google Scholar] [CrossRef]
- Fang, L.; Xu, J.; Wang, W.; Huang, Y. Would Endoscopic Surgery Be the Gold Standard for Stapes Surgery in the Future? A Systematic Review and Meta-Analysis. Eur. Arch. Otorhinolaryngol. 2021, 278, 925–932. [Google Scholar] [CrossRef]
- Hudson, S.K.; Gurgel, R.K.; Shelton, C. Revision Stapedectomy with Bone Cement: Are Results Comparable to Those of Standard Techniques? Otol. Neurotol. 2014, 35, 1501–1503. [Google Scholar] [CrossRef] [PubMed]
- Lippy, W.H.; Burkey, J.M.; Schuring, A.G.; Berenholz, L.P. Comparison of Titanium and Robinson Stainless Steel Stapes Piston Prostheses. Otol. Neurotol. 2005, 26, 874–877. [Google Scholar] [CrossRef]
- Khatir, O.; Sidi Mohamed, F.; Albedah, A.; Hamada, A.; Pawłowski, Ł.; Sahli, A.; Abdelkader, B.; Boudjemaa, I.; Bouiadjra, B.B. Enhancing Middle Ear Implants: Study of Biocompatible Materials with Hydroxyapatite Coating. Mech. Adv. Mater. Struct. 2024, 32, 3793–3800. [Google Scholar] [CrossRef]
- Mangham, C.A. Nitinol-Teflon Stapes Prosthesis Improves Low-Frequency Hearing Results after Stapedotomy. Otol. Neurotol. 2010, 31, 1022–1026. [Google Scholar] [CrossRef] [PubMed]
- Roosli, C.; Schmid, P.; Huber, A.M. Biocompatibility of Nitinol Stapes Prosthesis. Otol. Neurotol. 2011, 32, 265–270. [Google Scholar] [CrossRef] [PubMed]

| Prosthesis | Material | Coupling Mechanism | Key Design Features | Advantages | Limitations/ Considerations |
|---|---|---|---|---|---|
 Robinson Bucket Robinson BucketHandle | Titanium | Manual placement; piston-type | Shaped like a bucket handle; one end attached to the incus, the other contacts the footplate | Stable positioning, improved energy transfer | Requires precise manual handling; potential incus trauma if malpositioned |
 Wengen Clip-on Wengen Clip-on | Titanium | Pre-crimped clip-on loop | Snaps directly onto incus without crimping | Reduces surgical time; minimizes manipulation of ossicles | Risk of loosening over time; dependent on loop tension |
 Szkarzinsky piston Szkarzinsky piston | Titanium | Manual placement | Thin shaft with a hook-shaped proximal end for attachment to the incus; cylindrical distal end for insertion into the oval window | Simple design, biocompatible materials, effective sound transmission | Requires precise placement; performance depends on correct sizing |
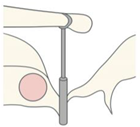 Eclipse Nitinol Piston Eclipse Nitinol Piston | Nitinol (SMA) | Heat-activated self-crimping | Shape memory alloy closes around incus when heated | Eliminates manual crimping; consistent coupling force | Requires heat activation; risk of thermal injury if poorly controlled |
 Eclipse Flat Ribbon Nitinol Piston Eclipse Flat Ribbon Nitinol Piston | Nitinol (SMA) | Heat-activated self-crimping | Wide ribbon loop distributes pressure | Gentle fixation; protects incus from focal necrosis | Length changes possible after activation; careful sizing needed |
 Megerian Replacement Megerian ReplacementProsthesis | Nitinol (SMA) | Heat-activated multi-arm fixation | Six tapered arms adapt to the contours of the incus, even if necrosed | Useful in revision cases; secure adaptation to irregular anatomy | Bulkier design; requires more space in oval window niche |
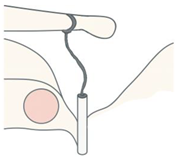 Eclipse Malleus Piston Eclipse Malleus Piston | Nitinol (SMA) | Heat-activated self-crimping | Offset axis (15°) for malleus-to-footplate alignment | Suitable for malleo-vestibulopexy or revision cases | Limited use in primary cases; higher technical demand |
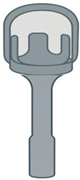 Bartels Bucket Handle Bartels Bucket Handle | Titanium | Manual placement; adjustable | Adjustable bucket diameter; stepped-down shaft; depth gauge | Adaptable to variable incus sizes; useful with overhanging facial nerve | Requires precise placement; increased complexity |
| Material | Mechanical Parameters |
|---|---|
| Fluoroplastic (Teflon) | Density ≈ 2.2 g/cm3; Young’s modulus ≈ 0.5 GPa; internal damping coefficient ≈ 0.03–0.05; transmission efficiency ~80–85% at 1–2 kHz. |
| Titanium | Density ≈ 4.5 g/cm3; Young’s modulus ≈ 110 GPa; acoustic impedance ~27 × 106 kg/m2s; damping coefficient < 0.01; transmission efficiency > 90% up to 4 kHz. |
| Nitinol (NiTi SMA) | Density ≈ 6.5 g/cm3; effective Young’s modulus ≈ 30–75 GPa (temperature-dependent); damping coefficient ≈ 0.02–0.03; mechanical coupling gain +2–3 dB vs. Teflon at >2 kHz. |
| Prosthesis Type (Material) | Properties & Design Advantages | Audiological Outcomes (ABG Closure or Improvement) | Notable Considerations |
|---|---|---|---|
| Fluoroplastic (Teflon) Piston with platinum/steel loop | Low-cost, inert, and lightweight polymer. Often coupled via a manually crimped wire loop. Long history of biocompatible use. | ~86% achieved post-operative ABG < 10 dB in one large series, comparable to titanium outcomes. Significant hearing gains were observed with the small-fenestra technique. | Requires manual crimping onto the incus. Slight risk of long-term attachment loosening—e.g., ~3.5% incus dislocation by 2 years reported. Very low extrusion or rejection rates. |
| Titanium Piston (loop or clip design) | High-strength, low-density metal with low acoustic/mechanical impedance. Can be made thinner for better visualization Available in manual loop and Soft CliP (spring-clip) versions that eliminate crimping. | Closure of ABG to <10 dB in ~71% of cases (vs. 86% for Teflon in one study) [54], though other trials show equivalent success (≤10 dB ABG in ~85–90%) [53]. Overall, hearing outcomes are statistically comparable to those of Teflon in meta-analyses [55]. | Secure incus coupling; one 855-patient study found 0% prosthesis dislocations in titanium vs. 3% in Teflon (p = 0.12) [55]. More expensive than fluoroplastics. Clip-on designs speed up surgery and avoid over-crimping, with improved speech discrimination noted [56]. |
| Nitinol “SMart” Piston (NiTi shape-memory) | A SMA that self-crimps when activated by heat (e.g., laser). Provides consistent 360° incus loop compression without manual force, ensuring tight, uniform coupling—excellent fatigue resistance and biocompatibility; moderate stiffness closer to bone [57]. | Achieved long-term ABG ≈ 10 dB that remained stable over >10 years [28]. One trial showed significantly better mean ABG (8.0 dB vs. 11.6 dB) and a higher rate of ABG ≤ 10 dB (71% vs. 43%) with Nitinol vs. conventional pistons. Tight fixation yielded ~2.5 dB improved sound transfer intraoperatively, especially at high frequencies [58]. | No manual crimp is needed, simplifying placement and reducing variability [14]. Requires heat activation; necessary care to avoid thermal injury (e.g., to the chorda tympani nerve). Contains nickel (allergy considerations are minimal in practice). Clinical studies indicate no increase in complications and equivalent overall hearing outcomes to manual techniques when used appropriately [59]. |
| Key Aspect | Engineering Principle/Description | Design Implications |
|---|---|---|
| Coupling Mechanism (Incus Attachment) | Proper attachment of the prosthesis to the incus is essential for effective force transmission. Most pistons use a loop or crimp band that is manually fixed around the long (lenticular) process of the incus, creating a semi-rigid mechanical coupling. Excessive crimping can concentrate stress and risk necrosis of the incus, while insufficient crimping reduces acoustic efficiency. An optimal interface maintains firm contact with slight compliance, avoiding complete rigidity that could impair motion or increase dislocation risk. Recent actuator-inspired designs, such as clip pistons and self-crimping NiTi loops, aim to standardize attachment force and improve coupling reliability. | Proper crimping ensures efficient transmission and mechanical stability. Controlled compliance reduces stress and enhances prosthesis longevity. |
| Piston–Footplate Interface | The distal piston tip (typically 0.4–0.8 mm in diameter) transmits vibrational energy to the cochlear fluids through the oval window. Efficient transfer requires a tight seal (using vein, fat, or fascia grafts) to prevent perilymph leakage. Piston diameter determines the balance between hydraulic pressure and volume displacement (Pascal’s law). Smaller pistons enhance low-frequency sensitivity but reduce high-frequency volume velocity; conversely, larger diameters improve high-frequency transmission but increase vestibular load. Finite-element and in vitro studies confirm that 0.4 mm pistons produce ~14 dB loss at high frequencies compared to the natural stapes footplate area (3.2 mm2). | Optimal piston size (0.4–0.6 mm) achieves a balance between frequency response and mechanical safety. Clinical evidence supports improved hearing with 0.6 mm pistons in some cases. |
| Actuator Behavior and Damping | As a passive actuator, the prosthesis must transmit vibrations efficiently across the auditory frequency range without adding significant mass or stiffness. Ideal designs contribute ~0.1–0.2 g additional mass and maintain resonance above the auditory band. Incorporating internal damping (e.g., PTFE segments or NiTi loops) can suppress unwanted resonant peaks and stabilize the transfer function. The overall energy pathway—incus motion → prosthesis vibration → perilymph displacement—remains consistent, but mechanical efficiency depends on precise alignment and angle of insertion. The piston should extend approximately 0.5 mm into the vestibule to ensure stable engagement without trauma. | Controlled damping and correct alignment enhance actuator stability and vibration fidelity. Proper piston length and orientation prevent energy loss and damage to the inner ear. |
| Summary Insight | Stapes prosthesis mechanics reflect actuator design trade-offs between stiffness, compliance, and damping. Secure coupling and well-calibrated flexibility optimize sound transmission while preventing structural fatigue or biological damage. The engineering goal is to emulate the natural stapes’ piston-like action, efficiently converting incus oscillation into perilymph fluid displacement. | Well-engineered compliance and damping improve both mechanical performance and clinical outcomes. |
| Innovation | Principle and Description | Current Status and Challenges | Clinical/Engineering Impact |
|---|---|---|---|
| MEMS-Based Piezoelectric Micro-Actuators | Integration of piezoelectric micro-actuators or sensors within middle-ear prostheses to actively modulate stiffness or displacement in response to sound input. These micro-electro-mechanical systems (MEMS) aim to enhance hearing through real-time vibration control dynamically. | Currently in experimental development for fully implantable hearing devices; not yet applicable to otosclerosis surgery. Challenges include miniaturization, power supply, biocompatibility, and long-term stability in the humid middle-ear environment. | Represents a potential future shift from passive to active actuation, enabling adaptive hearing enhancement, self-monitoring, and feedback-controlled motion. However, remains a complementary rather than replacement technology. |
| 3D-Printed Custom Prostheses | Additive manufacturing (e.g., titanium alloy powder-bed fusion) enables personalized stapes or ossicular prostheses based on HR-CT imaging. Each implant can be tailored to the patient’s anatomy (length, angulation, coupling geometry). | Laboratory feasibility has been demonstrated; however, regulatory validation and long-term biocompatibility data are still limited. Integration of porous or textured surfaces may promote tissue adhesion and biological fixation. | Enables patient-specific prosthesis design and eliminates the need for intraoperative trimming. Offers ergonomic and acoustic advantages through perfect fit and potential tissue integration. |
| Novel Prosthesis Designs | Innovative geometries beyond the classical piston model, including bucket-handle and malleus-anchoring (malleovestibular) prostheses. These create alternative mechanical pathways for energy transmission to the inner ear, specifically the cochlea. | Early prototypes tested in revision and complex cases; still under evaluation for consistent acoustic performance and surgical handling. Require precise positioning and may have higher technical demands. | Expands applicability to complex or revision surgeries (e.g., incus erosion, footplate damage) [34,68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gherasie, L.-M.; Zainea, V.; Hainarosie, R.; Rusescu, A.; Ionita, I.-G.; Alius, R.-O.; Voiosu, C. Stapes Prostheses in Otosclerosis Surgery: Materials, Design Innovations, and Future Perspectives. Actuators 2025, 14, 502. https://doi.org/10.3390/act14100502
Gherasie L-M, Zainea V, Hainarosie R, Rusescu A, Ionita I-G, Alius R-O, Voiosu C. Stapes Prostheses in Otosclerosis Surgery: Materials, Design Innovations, and Future Perspectives. Actuators. 2025; 14(10):502. https://doi.org/10.3390/act14100502
Chicago/Turabian StyleGherasie, Luana-Maria, Viorel Zainea, Razvan Hainarosie, Andreea Rusescu, Irina-Gabriela Ionita, Ruxandra-Oana Alius, and Catalina Voiosu. 2025. "Stapes Prostheses in Otosclerosis Surgery: Materials, Design Innovations, and Future Perspectives" Actuators 14, no. 10: 502. https://doi.org/10.3390/act14100502
APA StyleGherasie, L.-M., Zainea, V., Hainarosie, R., Rusescu, A., Ionita, I.-G., Alius, R.-O., & Voiosu, C. (2025). Stapes Prostheses in Otosclerosis Surgery: Materials, Design Innovations, and Future Perspectives. Actuators, 14(10), 502. https://doi.org/10.3390/act14100502






