Salmonella and Antimicrobial Resistance in Wild Rodents—True or False Threat?
Abstract
1. Introduction
2. Results
2.1. Isolation Rates and Antimicrobial Resistance
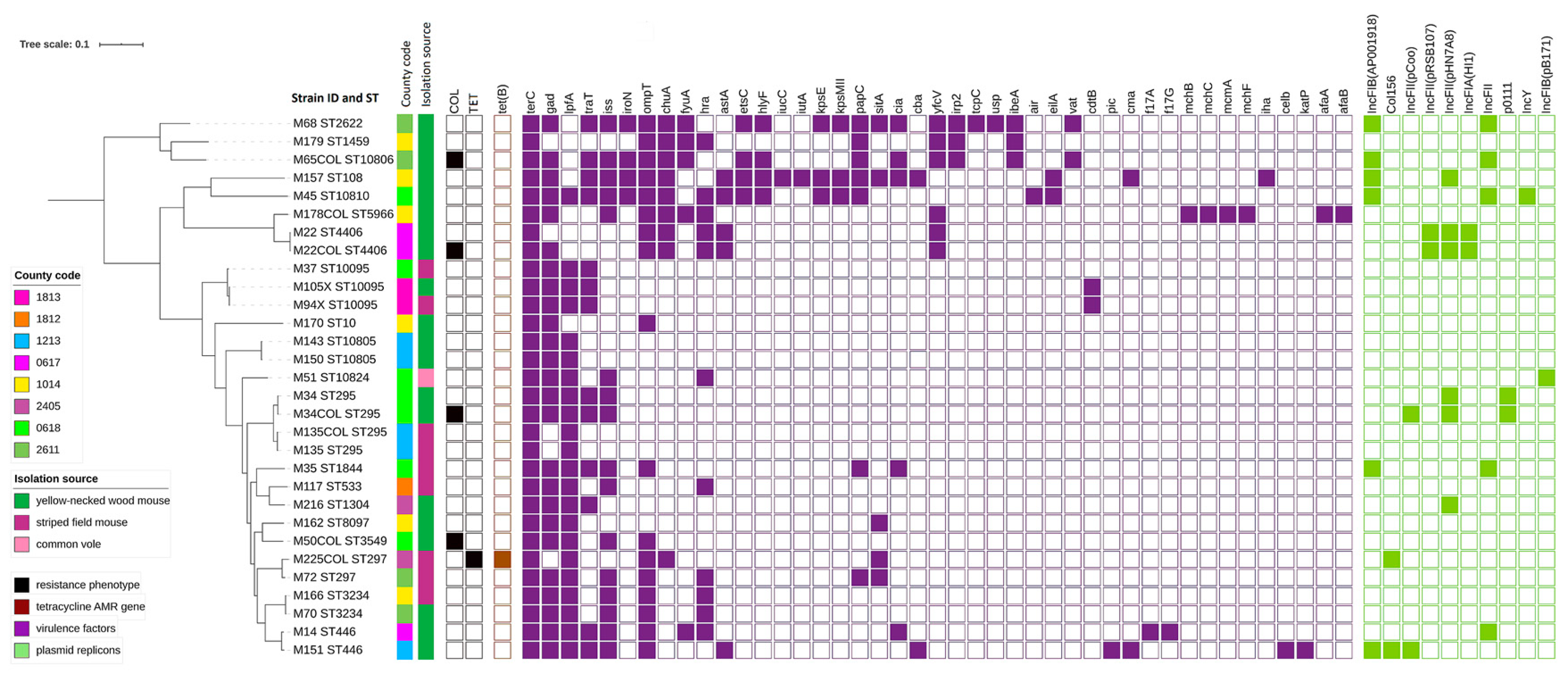
2.2. AMR Sequencing Results
2.3. Plasmids Replicons
2.4. Virulence Genes
2.5. Phylogenetic Analyses
3. Discussion
4. Materials and Methods
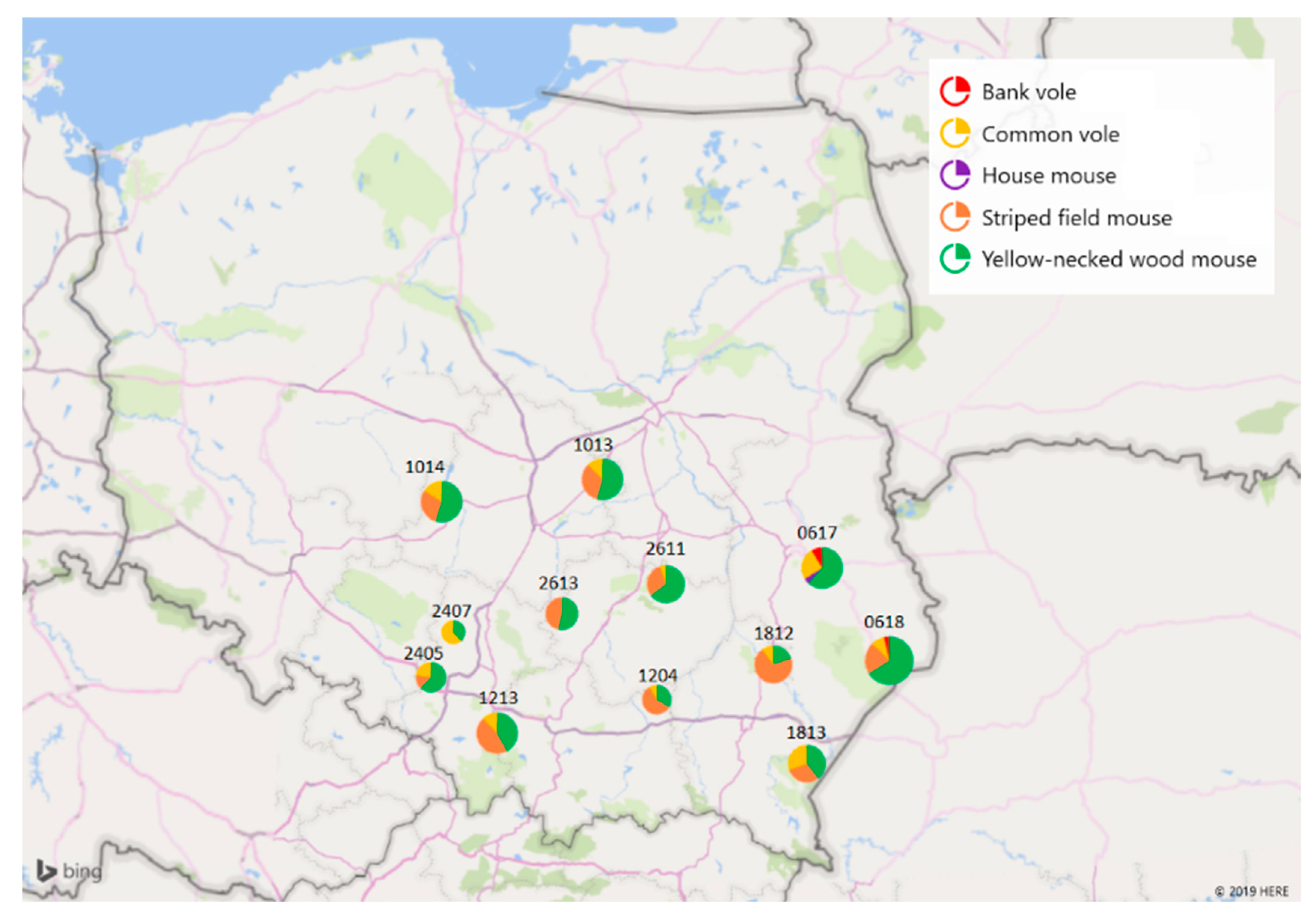
4.1. Sample Collection
4.2. Bacterial Isolation and Identification
4.3. Antimicrobial Resistance Testing
4.4. Whole-Genome Sequencing
4.5. Bioinformatic Processing and Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cassini, A.; Hogberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. The burden of amr collaborative group. attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the eu and the european economic area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States. 2019; U.S. Department: Atlanta, GA, USA, 2019.
- European Food Safety Authority. European centre for disease prevention and control. The European Union one health 2018 zoonoses report. EFSA J. 2019, 17, e05926. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. National Enteric Disease Surveillance: Salmonella Annual Report, 2016; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2018.
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global trends in emerging infectious diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef]
- Skarżyńska, M.; Hoszowski, A.; Zając, M.; Lalak, A.; Samcik, I.; Kwit, R.; Wasyl, D. Distribution of Salmonella serovars along the food chain in Poland, 2010–2015. J. Vet. Res. 2017, 69, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Lalak, A.; Wasyl, D.; Zając, M.; Skarżyńska, M.; Hoszowski, A.; Samcik, I.; Woźniakowski, G.; Szulowski, K. Mechanisms of cephalosporin resistance in indicator Escherichia coli isolated from food animals. Vet. Microbiol. 2016, 194, 69–73. [Google Scholar] [CrossRef]
- European Food Safety Authority, European Centre for Disease Prevention and Control. The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2017. EFSA J. 2019, 1–278. [CrossRef]
- Wasyl, D.; Zając, M.; Lalak, A.; Skarżyńska, M.; Samcik, I.; Kwit, R.; Jabłoński, A.; Bocian, Ł.; Woźniakowski, G.; Hoszowski, A.; et al. Antimicrobial Resistance in Escherichia coli Isolated from Wild Animals in Poland. Microb. Drug Resist. 2018, 24, 807–815. [Google Scholar] [CrossRef]
- Zając, M.; Wasyl, D.; Różycki, M.; Bilska-Zając, E.; Fafiński, Z.; Iwaniak, W.; Krajewska, M.; Hoszowski, A.; Konieczna, O.; Fafińska, P.; et al. Free-living snakes as a source and possible vector of Salmonella spp. and parasites. Eur. J. Wild. Res. 2016, 62, 161–166. [Google Scholar] [CrossRef]
- Nowakiewicz, A.; Zięba, P.; Gnat, S.; Trościańczyk, A.; Osińska, M.; Łagowski, D.; Kosior Korzecka, U.; Puzio, I. Bats as a reservoir of resistant Escherichia coli: A methodical view. Can we fully estimate the scale of resistance in the reservoirs of free-living animals? Res. Vet. Sci. 2020, 128, 49–58. [Google Scholar] [CrossRef]
- Arnold, K.E.; Williams, N.J.; Bennett, M. ‘Disperse abroad in the land’: The role of wildlife in the dissemination of antimicrobial resistance. Biol. Lett. 2016, 12. [Google Scholar] [CrossRef]
- Huijbers, P.M.C.; Flach, C.F.; Larsson, D.G.J. A conceptual framework for the environmental surveillance of antibiotics and antibiotic resistance. Environ. Int. 2019, 130, 104880. [Google Scholar] [CrossRef] [PubMed]
- Kruse, H.; Kirkemo, A.-M.; Handeland, K. Wildlife as source of zoonotic infections. Emerg. Infect. Dis. 2004, 10, 2067–2072. [Google Scholar] [CrossRef] [PubMed]
- Meerburg, B.G.; Singleton, G.R.; Kijlstra, A. Rodent-borne diseases and their risks for public health. Crit. Rev. Microbiol. 2009, 35, 221–270. [Google Scholar] [CrossRef] [PubMed]
- Healing, T.D.; Kaplan, C.; Prior, A. A note on some Enterobacteriaceae from the faeces of small wild British mammals. J. Hyg. Lond. 1980, 85, 343–345. [Google Scholar] [CrossRef]
- Pocock, M.J.; Searle, J.B.; Betts, W.B.; White, P.C. Patterns of infection by Salmonella and Yersinia spp. in commensal house mouse (Mus musculus domesticus) populations. J. Appl. Microbiol. 2001, 90, 755–760. [Google Scholar] [CrossRef]
- Kozak, G.K.; Boerlin, P.; Janecko, N.; Reid-Smith, R.J.; Jardine, C. Antimicrobial resistance in Escherichia coli isolates from swine and wild small mammals in the proximity of swine farms and in natural environments in Ontario, Canada. Appl. Environ. Microbiol. 2009, 75, 559–566. [Google Scholar] [CrossRef]
- Allen, S.E.; Boerlin, P.; Janecko, N.; Lumsden, J.S.; Barker, I.K.; Pearl, D.L.; Reid-Smith, R.J.; Jardine, C. Antimicrobial resistance in generic Escherichia coli isolates from wild small mammals living in swine farm, residential, landfill, and natural environments in southern Ontario, Canada. Appl. Environ. Microbiol. 2011, 77, 882–888. [Google Scholar] [CrossRef]
- Ribas, A.; Saijuntha, W.; Agatsuma, T.; Prantlová, V.; Poonlaphdecha, S. Rodents as a Source of Salmonella Contamination in Wet Markets in Thailand. Vector Borne Zoonotic Dis. 2016, 16, 537–540. [Google Scholar] [CrossRef]
- Williams, S.H.; Che, X.; Paulick, A.; Guo, C.; Lee, B.; Muller, D.; Uhlemann, A.-C.; Lowy, F.D.; Corrigan, R.M.; Lipkin, W.I. New York city house mice (mus musculus) as potential reservoirs for pathogenic bacteria and antimicrobial resistance determinants. mBio 2018, 9, e00624-18. [Google Scholar] [CrossRef]
- Guenther, S.; Grobbel, M.; Heidemanns, K.; Schlegel, M.; Ulrich, R.G.; Ewers, C.; Wieler, L.H. First insights into antimicrobial resistance among faecal Escherichia coli isolates from small wild mammals in rural areas. Sci. Total Environ. 2010, 408, 3519–3522. [Google Scholar] [CrossRef]
- Österblad, M.; Norrdahl, K.; Korpimäki, E.; Huovinen, P. How wild are wild mammals? Nature. 2001, 409, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Gilliver, M.A.; Bennett, M.; Begon, M.; Hazel, S.M.; Hart, C.A. Antibiotic resistance found in wild rodents. Nature 1999, 401, 233–234. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Critically Important Antimicrobials for Human Medicine, 6th Revision 2018 ed; World Health Organization: Geneva, The Switzerland, 2019; pp. 1–45. ISBN 978-92-4-151552-8. [Google Scholar]
- Swift, B.M.C.; Bennett, M.; Waller, K.; Dodd, C.; Murray, A.; Gomes, R.L.; Humphreys, B.; Hobman, J.L.; Jones, M.A.; Whitlock, S.; et al. Anthropogenic environmental drivers of antimicrobial resistance in wildlife. Sci. Total Environ. 2019, 649, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Ellington, M.J.; Ekelund, O.; Aarestrup, F.M.; Canton, R.; Doumith, M.; Giske, C.; Grundman, H.; Hasman, H.; Holden, M.T.G.; Hopkins, K.L.; et al. The role of whole genome sequencing in antimicrobial susceptibility testing of bacteria: Report from the EUCAST Subcommittee. Clin. Microbiol. Infect. 2017, 23, 2–22. [Google Scholar] [CrossRef]
- Sunde, M.; Urdahl, A.M.; Norström, M.; Madslien, K.; Danielsen, V.A.; Barstad, S.A.; Welde, H.; Schau Slettemeås, J.; das Neves, C.G. Report 6-2018: Antibiotic Resistance in Terrestrial Wild Mammal Species in Norway–Roe Deer and Wild Reindeer as Indicators Species; Norwegian Veterinary Institute: Oslo, Norway, 2018; pp. 1–16. [Google Scholar]
- Gomes, C.; Ruiz-Roldan, L.; Mateu, J.; Ochoa, T.J.; Ruiz, J. Azithromycin resistance levels and mechanisms in Escherichia coli. Sci Rep. 2019, 9, 6089. [Google Scholar] [CrossRef]
- Froehlich, B.; Parkhill, J.; Sanders, M.; Quail, M.A.; Scott, J.R. The pCoo plasmid of enterotoxigenic Escherichia coli is a mosaic cointegrate. J. Bacteriol. 2005, 187, 6509–6516. [Google Scholar] [CrossRef]
- Johnson, T.J.; Nolan, L.K. Pathogenomics of the virulence plasmids of Escherichia coli. Microbiol. Mol. Biol. Rev. 2009, 73, 750–774. [Google Scholar] [CrossRef]
- Sethuvel, D.P.M.; Perumalla, S.; Anandan, S.; Michael, J.S.; Ragupathi, N.K.D.; Gajendran, R.; Walia, K.; Veeraraghavan, B. Antimicrobial resistance, virulence & plasmid profiles among clinical isolates of Shigella serogroups. Indian, J. Med. Res. 2019, 149, 247–256. [Google Scholar] [CrossRef]
- Mbelle, N.M.; Feldman, C.; Osei Sekyere, J.; Maningi, N.E.; Modipane, L.; Essack, S.Y. The Resistome, Mobilome, Virulome and Phylogenomics of Multidrug-Resistant Escherichia coli Clinical Isolates from Pretoria, South Africa. Sci. Rep. UK 2019, 9, 16457. [Google Scholar] [CrossRef]
- Yasir, M.; Farman, M.; Shah, M.W.; Jiman-Fatani, A.A.; Othman, N.A.; Almasaudi, S.B.; Alawi, M.; Shakil, S.; Al-Abdullah, N.; Ismaeel, N.A.; et al. Genomic and antimicrobial resistance genes diversity in multidrug-resistant CTX-M-positive isolates of Escherichia coli at a health care facility in Jeddah. J. Infect. Public Health 2020, 13, 94–100. [Google Scholar] [CrossRef]
- Beceiro, A.; Tomas, M.; Bou, G. Antimicrobial resistance and virulence: A successful or deleterious association in the bacterial world? Clin. Microbiol. Rev. 2013, 26, 185–230. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; He, L.; Huang, S.-H. Identification of a surface protein on human brain microvascular endothelial cells as vimentin interacting with Escherichia coli invasion protein IbeA. Biochem. Biophys. Res. Commun. 2006, 351, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; He, L.; Wu, C.-H.; Cao, H.; Xie, Z.-H.; Ouyang, Y.; Wang, Y.; Jong, A.; Huang, S.-H. PSF is an IbeA-binding protein contributing to meningitic Escherichia coli K1 invasion of human brain microvascular endothelial cells. Med. Microbiol. Immunol. 2007, 196, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Scuron, M.D.; Boesze-Battaglia, K.; Dlakić, M.; Shenker, B.J. The cytolethal distending toxin contributes to microbial virulence and disease pathogenesis by acting as a tri-perditious toxin. Front. Cell Infect. Microbiol. 2016, 6, 168. [Google Scholar] [CrossRef] [PubMed]
- Bouzari, S.; Varghese, A. Cytolethal distending toxin (CLDT) production by enteropathogenic Escherichia coli (EPEC). FEMS Microbiol. Lett. 1990, 59, 193–198. [Google Scholar] [CrossRef]
- Sheikh, J.; Dudley, E.G.; Sui, B.; Tamboura, B.; Suleman, A.; Nataro, J.P. EilA, a HilA-like regulator in enteroaggregative Escherichia coli. Mol. Microbiol. 2006, 61, 338–350. [Google Scholar] [CrossRef]
- Kuhnert, P.; Boerlin, P.; Frey, J. Target genes for virulence assessment of Escherichia coli isolates from water, food and the environment. FEMS Microbiol. Rev. 2000, 24, 107–117. [Google Scholar] [CrossRef]
- Andrade, F.B.; Abreu, A.G.; Nunes, K.O.; Gomes, T.A.T.; Piazza, R.M.F.; Elias, W.P. Distribution of serine protease autotransporters of Enterobacteriaceae in typical and atypical enteroaggregative Escherichia coli. Infect. Genet. Evol. 2017, 50, 83–86. [Google Scholar] [CrossRef]
- Croxen, M.A.; Law, R.J.; Scholz, R.; Keeney, K.M.; Wlodarska, M.; Finlay, B.B. Recent advances in understanding enteric pathogenic Escherichia coli. Clin Microbiol. Rev. 2013, 26, 822–880. [Google Scholar] [CrossRef]
- Bertin, Y.; Girardeau, J.-P.; Darfeuille-Michaud, A.; Martin, C. Epidemiological study of pap genes among diarrheagenic or septicemic escherichia coli strains producing cs31a and f17 adhesins and characterization of pap31a fimbriae. J. Clin. Microbiol. 2000, 38, 1502. [Google Scholar] [CrossRef]
- Johnson, T.J.; Wannemuehler, Y.M.; Nolan, L.K. Evolution of the iss gene in Escherichia coli. Appl. Environ. Microbiol. 2008, 74, 2360–2369. [Google Scholar] [CrossRef] [PubMed]
- Gibreel, T.M.; Dodgson, A.R.; Cheesbrough, J.; Fox, A.J.; Bolton, F.J.; Upton, M. Population structure, virulence potential and antibiotic susceptibility of uropathogenic Escherichia coli from Northwest England. J. Antimicrob. Chemother. 2012, 67, 346–356. [Google Scholar] [CrossRef] [PubMed]
- McNally, A.; Alhashash, F.; Collins, M.; Alqasim, A.; Paszckiewicz, K.; Weston, V.; Diggle, M. Genomic analysis of extra-intestinal pathogenic Escherichia coli urosepsis. Clin. Microbiol. Infect. 2013, 19, E328–E334. [Google Scholar] [CrossRef] [PubMed]
- Maluta, R.P.; Logue, C.M.; Casas, M.R.; Meng, T.; Guastalli, E.A.; Rojas, T.C.; Montelli, A.C.; Sadatsune, T.; de Carvalho Ramos, M.; Kay Nolan, L.; et al. Overlapped sequence types (STs) and serogroups of avian pathogenic (APEC) and human extra-intestinal pathogenic (ExPEC) Escherichia coli isolated in Brazil. PLoS ONE 2014, 9, e105016. [Google Scholar] [CrossRef]
- Durkalec, M.; Nawrocka, A.; Żmudzki, J.; Filipek, A.; Niemcewicz, M.; Posyniak, A. Concentration of mercury in the livers of small terrestrial rodents from rural areas in Poland. Molecules 2019, 24, 4108. [Google Scholar] [CrossRef]
- European Commission. Commission Implementing Decision 2013/652/EU of 12 November 2013 on the Monitoring and Reporting of Antimicrobial Resistance in Zoonotic and Commensal Bacteria. Off. J. Eur. Union 2013, 303, 26–39. [Google Scholar]
- Chen, J.; Griffiths, M.W. PCR differentiation of Escherichia coli from other Gram-negative bacteria using primers derived from the nucleotide sequences flanking the gene encoding the universal stress protein. Lett. Appl. Microbiol. 1998, 27, 369–371. [Google Scholar] [CrossRef]
- Wasyl, D.; Hoszowski, A.; Zając, M.; Szulowski, K. Antimicrobial resistance in commensal Escherichia coli isolated from animals at slaughter. Front. Microbiol. 2013, 4, 221. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Zankari, E.; Hasman, H.; Cosentino, S.; Vestergaard, M.; Rasmussen, S.; Lund, O.; Aarestrup, F.M.; Larsen, M.V. Identification of acquired antimicrobial resistance genes. J. Antimicrob. Chemother. 2012, 67, 2640–2644. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.V.; Cosentino, S.; Rasmussen, S.; Friis, C.; Hasman, H.; Marvig, R.L.; Jelsbak, L.; Sicheritz-Pontén, T.; Ussery, D.W.; Aarestrup, F.M.; et al. Multilocus sequence typing of total-genome-sequenced bacteria. J. Clin. Microbiol. 2012, 50, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Joensen, K.G.; Scheutz, F.; Lund, O.; Hasman, H.; Kaas, R.S.; Nielsen, E.M.; Aarestrup, F.M. Real-Time whole-genome sequencing for routine typing, surveillance, and outbreak detection of verotoxigenic Escherichia coli. J. Clin. Microbiol. 2014, 52, 1501. [Google Scholar] [CrossRef] [PubMed]
- Carattoli, A.; Zankari, E.; Garcia-Fernandez, A.; Voldby Larsen, M.; Lund, O.; Villa, L.; Aarestrup, F.M.; Hasman, H. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob. Agents. Chemother. 2014, 58, 3895–3903. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Alikhan, N.F.; Mohamed, K.; Fan, Y.; Agama Study Group; Achtman, M. The enterobase user’s guide, with case studies on salmonella transmissions, yersinia pestis phylogeny, and escherichia core genomic diversity. Genome Res. 2020, 30, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Kaas, R.S.; Leekitcharoenphon, P.; Aarestrup, F.M.; Lund, O. Solving the problem of comparing whole bacterial genomes across different sequencing platforms. PLoS ONE 2014, 9, e104984. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v4: Recent updates and new developments. Nucleic Acids Res. 2019, 47, W256–W259. [Google Scholar] [CrossRef]
- Hunter, P.R.; Gaston, M.A. Numerical index of the discriminatory ability of typing systems: An application of Simpson’s index of diversity. J. Clin. Microbiol. 1988, 26, 2465–2466. [Google Scholar] [CrossRef]

| Antimicrobial Name and Abbreviation | NWT | Minimal Inhibitory Concentration Value (mg/L) | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | ≤0.008 | 0.016 | 0.032 | 0.064 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | 512 | 1024 | >1024 | |
| Ampicillin (AMP) | 0 | 0.0% | 0 | 17 | 177 | 69 | 0 | 0 | 0 | 0 | 0 | ||||||||||
| Ceftazidime (TAZ) | 0 | 0.0% | 0 | 263 | 0 | 0 | 0 | 0 | 0 | ||||||||||||
| Cefotaxime (FOT) | 0 | 0.0% | 0 | 263 | 0 | 0 | 0 | 0 | 0 | ||||||||||||
| Meropenem (MERO) | 0 | 0.0% | 0 | 261 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||
| Gentamicin (GEN) | 0 | 0.0% | 0 | 116 | 127 | 20 | 0 | 0 | 0 | 0 | 0 | ||||||||||
| Nalidixic acid (NAL) | 0 | 0.0% | 0 | 262 | 1 | 0 | 0 | 0 | 0 | 0 | |||||||||||
| Ciprofloxacin (CIP) | 0 | 0.0% | 0 | 232 | 30 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||
| Sulfamethoxazole (SMX) | 0 | 0.0% | 0 | 74 | 65 | 56 | 68 | 0 | 0 | 0 | 0 | 0 | |||||||||
| Trimethoprim (TMP) | 0 | 0.0% | 0 | 233 | 28 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||||
| Colistin (COL) | 4 | 1.5% | 0 | 259 | 0 | 0 | 1 | 3 | 0 | 0 | 0 | ||||||||||
| Azithromycin (AZI) | NI | 0 | 127 | 69 | 61 | 6 | 0 | 0 | 0 | ||||||||||||
| Chloramphenicol (CHL) | 0 | 0.0% | 0 | 263 | 0 | 0 | 0 | 0 | 0 | ||||||||||||
| Tetracycline (TET) | 1 | 0.4% | 0 | 262 | 0 | 0 | 0 | 0 | 1 | 0 | |||||||||||
| Tigecycline (TIGECY) | 0 | 0.0% | 0 | 259 | 4 | 0 | 0 | 0 | 0 | 0 | |||||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skarżyńska, M.; Zając, M.; Kamińska, E.; Bomba, A.; Żmudzki, J.; Jabłoński, A.; Wasyl, D. Salmonella and Antimicrobial Resistance in Wild Rodents—True or False Threat? Pathogens 2020, 9, 771. https://doi.org/10.3390/pathogens9090771
Skarżyńska M, Zając M, Kamińska E, Bomba A, Żmudzki J, Jabłoński A, Wasyl D. Salmonella and Antimicrobial Resistance in Wild Rodents—True or False Threat? Pathogens. 2020; 9(9):771. https://doi.org/10.3390/pathogens9090771
Chicago/Turabian StyleSkarżyńska, Magdalena, Magdalena Zając, Ewelina Kamińska, Arkadiusz Bomba, Jacek Żmudzki, Artur Jabłoński, and Dariusz Wasyl. 2020. "Salmonella and Antimicrobial Resistance in Wild Rodents—True or False Threat?" Pathogens 9, no. 9: 771. https://doi.org/10.3390/pathogens9090771
APA StyleSkarżyńska, M., Zając, M., Kamińska, E., Bomba, A., Żmudzki, J., Jabłoński, A., & Wasyl, D. (2020). Salmonella and Antimicrobial Resistance in Wild Rodents—True or False Threat? Pathogens, 9(9), 771. https://doi.org/10.3390/pathogens9090771





