Brainstem Encephalitis Caused by Listeria monocytogenes
Abstract
:1. Introduction
2. Epidemiology
3. Clinical Presentation
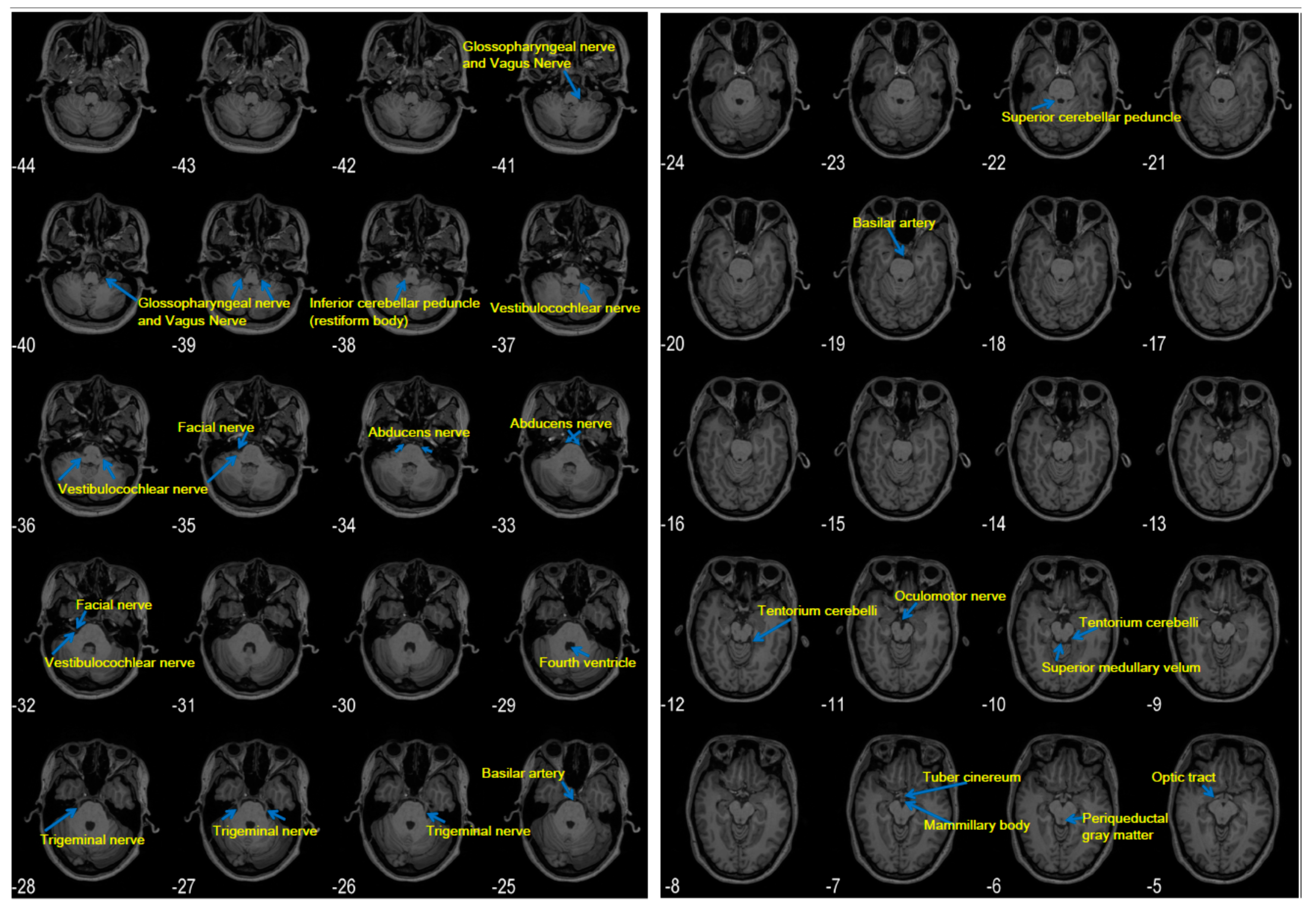
4. Anatomy and Physiology of the Brainstem
5. Pathology
6. Radiographic Features
7. Diagnosis, Treatment, and Prognosis
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vazquez-Boland, J.A.; Kuhn, M.; Berche, P.; Chakraborty, T.; Dominguez-Bernal, G.; Goebel, W.; Gonzalez-Zorn, B.; Wehland, J.; Kreft, J. Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 2001, 14, 584–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pagliano, P.; Ascione, T.; Boccia, G.; De Caro, F.; Esposito, S. Listeria monocytogenes meningitis in the elderly: Epidemiological, clinical and therapeutic findings. Infez Med. 2016, 24, 105–111. [Google Scholar] [PubMed]
- Desai, A.N.; Anyoha, A.; Madoff, L.C.; Lassmann, B. Changing epidemiology of Listeria monocytogenes outbreaks, sporadic cases, and recalls globally: A review of ProMED reports from 1996 to 2018. Int. J. Infect. Dis. 2019, 84, 48–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, I.L.; Mowry, E.M.; Steele, S.U.; Pardo, C.A.; McArthur, J.C.; Nath, A.; Venkatesan, A. Brainstem encephalitis: Etiologies, treatment, and predictors of outcome. J. Neurol. 2013, 260, 2312–2319. [Google Scholar] [CrossRef] [PubMed]
- Charlier, C.; Poiree, S.; Delavaud, C.; Khoury, G.; Richaud, C.; Leclercq, A.; Helenon, O.; Lecuit, M. Imaging of human neurolisteriosis: A prospective study of 71 cases. Clin. Infect. Dis. 2018, 67, 1419–1426. [Google Scholar] [CrossRef]
- Ramachandran, V.S. Encyclopedia of the Human Brain, 1st ed.; Academic Press: San Diego, CA, USA, 2002; p. 461. [Google Scholar]
- Jubelt, B.; Mihai, C.; Li, T.M.; Veerapaneni, P. Rhombencephalitis/brainstem encephalitis. Curr. Neurol. Neurosci. Rep. 2011, 11, 543–552. [Google Scholar] [CrossRef]
- Multistate Outbreak of Listeriosis Linked to Whole Cantaloupes from Jensen Farms, Colorado (FINAL UPDATE). Available online: https://www.cdc.gov/listeria/outbreaks/cantaloupes-jensen-farms/index.html (accessed on 12 May 2020).
- South Africa Declares End to Largest Ever Listeria Outbreak. Available online: https://www.foodsafetynews.com/2018/09/south-africa-declares-end-to-largest-ever-listeria-outbreak (accessed on 1 May 2020).
- Multi-Country Outbreak of Listeria Monocytogenes Infections Linked to Consumption of Salmon Products. Available online: https://www.ecdc.europa.eu/en/news-events/multi-country-outbreak-listeria-monocytogenes-infections-linked-consumption-salmon (accessed on 12 May 2020).
- Oevermann, A.; Zurbriggen, A.; Vandevelde, M. Rhombencephalitis caused by listeria monocytogenes in humans and ruminants: A zoonosis on the rise? Interdiscip. Perspect. Infect. Dis. 2010, 632513. [Google Scholar] [CrossRef] [Green Version]
- Antal, E.-A.; Dietrichs, E.; Løberg, E.M.; Melby, K.K.; Mæhlen, J. Brain stem encephalitis in listeriosis. Scand. J. Infect. Dis. 2005, 37, 190–194. [Google Scholar] [CrossRef]
- Uldry, P.A.; Kuntzer, T.; Bogousslavsky, J.; Regli, F.; Miklossy, J.; Bille, J.; Francioli, P.; Janzer, R. Early symptoms and outcome of Listeria monocytogenes rhombencephalitis: 14 adult cases. J. Neurol. 1993, 240, 235–242. [Google Scholar] [CrossRef]
- Kampelmacher, E.H.; van Noorle Jansen, L.M. Isolation of Listeria monocytogenes from faeces of clinically healthy humans and animals. Zent. Bakteriol Orig A 1969, 211, 353–359. [Google Scholar]
- Kampelmacher, E.H.; Huysinga, W.T.; Van Noorle Jansen, L.M. The presence of Listeria monocytogenes in feces of pregnant women and neonates. Zent. Bakteriol Orig A 1972, 222, 258–262. [Google Scholar]
- Vazquez-Boland, J.A.; Dominguez-Bernal, G.; Gonzalez-Zorn, B.; Kreft, J.; Goebel, W. Pathogenicity islands and virulence evolution in Listeria. Microbes Infect. 2001, 3, 571–584. [Google Scholar] [CrossRef]
- Carrique-Mas, J.J.; Hokeberg, I.; Andersson, Y.; Arneborn, M.; Tham, W.; Danielsson-Tham, M.L.; Osterman, B.; Leffler, M.; Steen, M.; Eriksson, E.; et al. Febrile gastroenteritis after eating on-farm manufactured fresh cheese—An outbreak of listeriosis? Epidemiol. Infect. 2003, 130, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Maurella, C.; Gallina, S.; Ru, G.; Adriano, D.; Bellio, A.; Bianchi, D.M.; Chiavacci, L.; Crescio, M.I.; Croce, M.; D’Errico, V.; et al. Outbreak of febrile gastroenteritis caused by Listeria monocytogenes 1/2a in sliced cold beef ham, Italy, May 2016. Eurosurveillance 2018, 23, 17–00155. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, F.; Nasu, R. Listeria monocytogenes septicemia and meningoencephalitis associated with relapsed and refractory follicular lymphoma. J. Infect. Chemother. 2020, 26, 619–621. [Google Scholar] [CrossRef]
- Koopmans, M.M.; Bijlsma, M.W.; Brouwer, M.C.; van de Beek, D.; van der Ende, A. Listeria monocytogenes meningitis in the Netherlands, 1985-2014: A nationwide surveillance study. J. Infect. 2017, 75, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Mylonakis, E.; Hohmann, E.L.; Calderwood, S.B. Central nervous system infection with Listeria monocytogenes. 33 years’ experience at a general hospital and review of 776 episodes from the literature. Medicine (Baltimore) 1998, 77, 313–336. [Google Scholar] [CrossRef]
- Pagliano, P.; Attanasio, V.; Rossi, M.; Carleo, M.A.; Carannante, N.; Ascione, T.; Tuccillo, F.; Fraganza, F. Listeria monocytogenes meningitis in the elderly: Distinctive characteristics of the clinical and laboratory presentation. J. Infect. 2015, 71, 134–136. [Google Scholar] [CrossRef]
- ECK, H. Encephalomyelitis listeriaca apostematosa. Schweiz. Med. Wochenschr. 1957, 87, 210–214. [Google Scholar]
- Bartt, R. Listeria and atypical presentations of Listeria in the central nervous system. Semin. Neurol. 2000, 20, 361–373. [Google Scholar] [CrossRef]
- Malinverni, R.; Bille, J.; Perret, C.; Regli, F.; Tanner, F.; Glauser, M.P. Epidemic listeriosis report of 25 cases in 15 months at the Vaud University Hospital Center. Schweiz. Med. Wochenschr. 1985, 115, 2–10. [Google Scholar] [PubMed]
- Armstrong, R.W.; Fung, P.C. Brainstem encephalitis (rhombencephalitis) due to Listeria monocytogenes: Case report and review. Clin. Infect. Dis. 1993, 16, 689–702. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, P.Ö.; Mutlu, N.M.; Sertçelik, A.; Bastug, A.; Doğu, C.; Kışlak, S. Linezolid and dexamethasone experience in a serious case of listeria rhombencephalitis. J. Infect. Public Health 2016, 9, 670–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansbridge, C.T.; Grecu, I.; Chong, J.S.L.V.; Vandervelde, C.; Saeed, K. Two cases of listeria rhombencephalitis. IDCases 2017, 11, 22–25. [Google Scholar] [CrossRef]
- Bazooyar, B. Rhombencephalitis by Listeria monocytogens in two diabetic patients. Arch. Iran. Med. 2015, 18, 613–615. [Google Scholar]
- Antal, E.A.; Løberg, E.M.; Dietrichs, E.; Maehlen, J. Neuropathological findings in 9 cases of listeria monocytogenes brain stem encephalitis. Brain Pathol. 2005, 15, 187–191. [Google Scholar] [CrossRef]
- Karlsson, W.K.; Harboe, Z.B.; Roed, C.; Monrad, J.B.; Lindelof, M.; Larsen, V.A.; Kondziella, D. Early trigeminal nerve involvement in Listeria monocytogenes rhombencephalitis: Case series and systematic review. J. Neurol. 2017, 264, 1875–1884. [Google Scholar] [CrossRef]
- Tang, Y.; Sun, W.; Toga, A.W.; Ringman, J.M.; Shi, Y. A probabilistic atlas of human brainstem pathways based on connectome imaging data. Neuroimage 2018, 169, 227–239. [Google Scholar] [CrossRef]
- Beissner, F.; Baudrexel, S. Investigating the human brainstem with structural and functional MRI. Front. Hum. Neurosci. 2014, 8, 116. [Google Scholar] [CrossRef] [Green Version]
- Naidich, T.P.; Duvernoy, H.M.; Delman, B.N.; Sorensen, A.G.; Kollias, S.S.; Haacke, E.M. Duvernoy’s Atlas of the Human Brain Stem and Cerebellum, 1st ed.; Springer: Wien, Austria, 2009; pp. 1–871. [Google Scholar]
- Haines, D.E. Neuroanatomy: An Atlas of Structures, Sections, and Systems, 7th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007; pp. 1–319. [Google Scholar]
- Ramachandran, V.S. Encyclopedia of the Human Brain, 1st ed.; Academic Press: Newyork, NY, USA, 2002; pp. 543–906. [Google Scholar]
- Standring, S. Gray’s Anatomy, 39th ed.; Elsevier: Madrid, Spain, 2008; pp. 441–724. [Google Scholar]
- Baker, E.; Lui, F. Neuroanatomy, Vagal Nerve Nuclei (Nucleus Vagus). BTI—StatPearls; 2020. Available online: http://www.ncbi.nlm.nih.gov//entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=31424793 (accessed on 20 May 2020).
- Patel, N.M.M.; Das, J. Neuroanatomy, Spinal Trigeminal Nucleus. BTI—StatPearls; 2020. Available online: http://www.ncbi.nlm.nih.gov//entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=30969551 (accessed on 20 May 2020).
- Henssen, D.J.H.A.; Derks, B.; van Doorn, M.; Verhoogt, N.; van Walsum, A.M.V.C.; Staats, P.; Vissers, K. Vagus nerve stimulation for primary headache disorders: An anatomical review to explain a clinical phenomenon. Cephalalgia 2019, 39, 1180–1194. [Google Scholar] [CrossRef] [Green Version]
- Frangos, E.; Ellrich, J.; Komisaruk, B.R. Non-invasive access to the vagus nerve central projections via electrical stimulation of the external ear: fMRI evidence in humans. Brain Stimul. 2015, 8, 624–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sörös, P.; Inamoto, Y.; Martin, R.E. Functional brain imaging of swallowing: An activation likelihood estimation meta-analysis. Hum. Brain Mapp. 2009, 30, 2426–2439. [Google Scholar] [CrossRef] [PubMed]
- Restivo, D.A.; Hamdy, S. Pharyngeal electrical stimulation device for the treatment of neurogenic dysphagia: Technology update. Med. Devices (Auckl) 2018, 11, 21–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dziewas, R.; Stellato, R.; van der Tweel, I.; Walther, E.; Werner, C.J.; Braun, T.; Citerio, G.; Jandl, M.; Friedrichs, M.; Nötzel, K.; et al. Pharyngeal electrical stimulation for early decannulation in tracheotomised patients with neurogenic dysphagia after stroke (PHAST-TRAC): A prospective, single-blinded, randomised trial. Lancet Neurol. 2018, 17, 849–859. [Google Scholar] [CrossRef]
- Sclocco, R.; Beissner, F.; Bianciardi, M.; Polimeni, J.R.; Napadow, V. Challenges and opportunities for brainstem neuroimaging with ultrahigh field MRI. Neuroimage 2018, 168, 412–426. [Google Scholar] [CrossRef]
- Caggiano, V.; Leiras, R.; Goni-Erro, H.; Masini, D.; Bellardita, C.; Bouvier, J.; Caldeira, V.; Fisone, G.; Kiehn, O. Midbrain circuits that set locomotor speed and gait selection. Nature 2018, 553, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Pinto, M.J.; Ruder, L.; Capelli, P.; Arber, S. Connecting circuits for supraspinal control of locomotion. Neuron 2018, 100, 361–374. [Google Scholar] [CrossRef] [Green Version]
- Paxinos, G.; Huang, X.F. Atlas of the Human Brainstem, 1st ed.; Academic Press: San Diego, CA, USA, 1995; pp. 1–146. [Google Scholar]
- Alam, M.; Schwabe, K.; Krauss, J.K. The pedunculopontine nucleus area: Critical evaluation of interspecies differences relevant for its use as a target for deep brain stimulation. Brain 2011, 134, 11–23. [Google Scholar] [CrossRef] [Green Version]
- Watson, T.C.; Koutsikou, S.; Cerminara, N.L.; Flavell, C.R.; Crook, J.J.; Lumb, B.M.; Apps, R. The olivo-cerebellar system and its relationship to survival circuits. Front. Neural Circuits 2013, 7, 72. [Google Scholar] [CrossRef] [Green Version]
- Vaaga, C.E.; Brown, S.T.; Raman, I.M. Cerebellar modulation of synaptic input to freezing-related neurons in the periaqueductal gray. Elife 2020, 24, e54302. [Google Scholar] [CrossRef]
- Koutsikou, S.; Watson, T.C.; Crook, J.J.; Leith, J.L.; Lawrenson, C.L.; Apps, R.; Lumb, B.M. The periaqueductal gray orchestrates sensory and motor circuits at multiple levels of the neuraxis. J. Neurosci. 2015, 35, 14132–14147. [Google Scholar] [CrossRef] [PubMed]
- Maury, M.M.; Tsai, Y.H.; Charlier, C.; Touchon, M.; Chenal-Francisque, V.; Leclercq, A.; Criscuolo, A.; Gaultier, C.; Roussel, S.; Brisabois, A.; et al. Uncovering Listeria monocytogenes hypervirulence by harnessing its biodiversity. Nat. Genet. 2016, 48, 308–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oevermann, A.; Di Palma, S.; Doherr, M.G.; Abril, C.; Zurbriggen, A.; Vandevelde, M. Neuropathogenesis of naturally occurring encephalitis caused by Listeria monocytogenes in ruminants. Brain Pathol. 2010, 20, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Rocha, P.R.; Lomonaco, S.; Bottero, M.T.; Dalmasso, A.; Dondo, A.; Grattarola, C.; Zuccon, F.; Iulini, B.; Knabel, S.J.; Capucchio, M.T.; et al. Ruminant rhombencephalitis-associated Listeria monocytogenes strains constitute a genetically homogeneous group related to human outbreak strains. Appl. Environ. Microbiol. 2013, 79, 3059–3066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farber, J.M.; Peterkin, P.I. Listeria monocytogenes, a food-borne pathogen. Microbiol. Rev. 1991, 55, 476–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, M.; Pohlenz, J.; Jaton, K.; Ninet, B.; Bille, J. Studies of the detection of Listeria monocytogenes by culture and PCR in cerebrospinal fluid samples from ruminants with listeric encephalitis. Zent. Vet. B 1995, 42, 84–88. [Google Scholar] [CrossRef]
- Antal, E.A.; Løberg, E.M.; Bracht, P.; Melby, K.K.; Maehlen, J. Evidence for intraaxonal spread of Listeria monocytogenes from the periphery to the central nervous system. Brain Pathol. 2001, 11, 432–438. [Google Scholar] [CrossRef]
- Revold, T.; Abayneh, T.; Brun-Hansen, H.; Kleppe, S.L.; Ropstad, E.O.; Hellings, R.A.; Sørum, H. Listeria monocytogenes associated kerato-conjunctivitis in four horses in Norway. Acta Vet. Scand. 2015, 57, 76. [Google Scholar] [CrossRef] [Green Version]
- Barlow, R.M.; McGorum, B. Ovine listerial encephalitis: Analysis, hypothesis and synthesis. Vet. Rec. 1985, 116, 233–236. [Google Scholar] [CrossRef]
- Kassebaum, N.J.; Smith, A.G.C.; Bernabé, E.; Fleming, T.D.; Reynolds, A.E.; Vos, T.; Murray, C.J.L.; Marcenes, W. Global, regional, and national prevalence, incidence, and disability-adjusted life years for oral conditions for 195 countries, 1990–2015: A systematic analysis for the global burden of diseases, injuries, and risk factors. J. Dent. Res. 2017, 96, 380–387. [Google Scholar] [CrossRef]
- Hammoud, K.; Lanfranchi, M.; Adams, D.; Bedi, H.S.; Mehan, W.A. Prevalence and reporting rates of incidental dental disease on head ct examinations. Acad. Radiol. 2018, 25, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- Svensson, E.; Horváth-Puhó, E.; Thomsen, R.W.; Djurhuus, J.C.; Pedersen, L.; Borghammer, P.; Sørensen, H.T. Vagotomy and subsequent risk of Parkinson’s disease. Ann. Neurol. 2015, 78, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Kaelberer, M.M.; Buchanan, K.L.; Klein, M.E.; Barth, B.B.; Montoya, M.M.; Shen, X.; Bohórquez, D.V. A gut-brain neural circuit for nutrient sensory transduction. Science 2018, 361, eaat5236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terakawa, Y.; Takami, T.; Yamagata, T.; Saito, T.; Nakanishi, N. Magnetic resonance imaging of brain abscess with hemorrhage: Implications for the mechanism of hemorrhage. Neurol. Med. Chir. (Tokyo) 2007, 47, 516–518. [Google Scholar] [CrossRef] [Green Version]
- Mogilner, A.; Jallo, G.I.; Zagzag, D.; Kelly, P.J. Nocardia abscess of the choroid plexus: Clinical and pathological case report. Neurosurgery 1998, 43, 949–952. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Bai, Y.; Duan, C.; Zhao, S.; Chen, X.; Yang, Q. Central nervous system Listeria monocytogenes infection mimicking central nervous system idiopathic inflammatory demyelinating disease. Infect. Drug Resist. 2019, 12, 255–259. [Google Scholar] [CrossRef] [Green Version]
- Popescu, G.A.; Saquepée, M.; Poisson, D.; Prazuck, T. Treatment difficulties of a listerial rhombencephalitis in an adult patient allergic to penicillins. J. Clin. Pathol. 2004, 57, 665–666. [Google Scholar] [CrossRef] [Green Version]
- Morosi, S.; Francisci, D.; Baldelli, F. A case of rhombencephalitis caused by Listeria monocytogenes successfully treated with linezolid. J. Infect. 2006, 52, e73–e75. [Google Scholar] [CrossRef]
- Amin, M.R.; Koufman, J.A. Vagal neuropathy after upper respiratory infection: A viral etiology? Am. J. Otolaryngol. 2001, 22, 251–256. [Google Scholar] [CrossRef] [Green Version]
- von Bartheld, C.S. Axonal transport and neuronal transcytosis of trophic factors, tracers, and pathogens. J. Neurobiol. 2004, 58, 295–314. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, P.; Bao, R.; Fan, Y. Brainstem Encephalitis Caused by Listeria monocytogenes. Pathogens 2020, 9, 715. https://doi.org/10.3390/pathogens9090715
Wei P, Bao R, Fan Y. Brainstem Encephalitis Caused by Listeria monocytogenes. Pathogens. 2020; 9(9):715. https://doi.org/10.3390/pathogens9090715
Chicago/Turabian StyleWei, Pengxu, Ruixue Bao, and Yubo Fan. 2020. "Brainstem Encephalitis Caused by Listeria monocytogenes" Pathogens 9, no. 9: 715. https://doi.org/10.3390/pathogens9090715
APA StyleWei, P., Bao, R., & Fan, Y. (2020). Brainstem Encephalitis Caused by Listeria monocytogenes. Pathogens, 9(9), 715. https://doi.org/10.3390/pathogens9090715





